Abstract
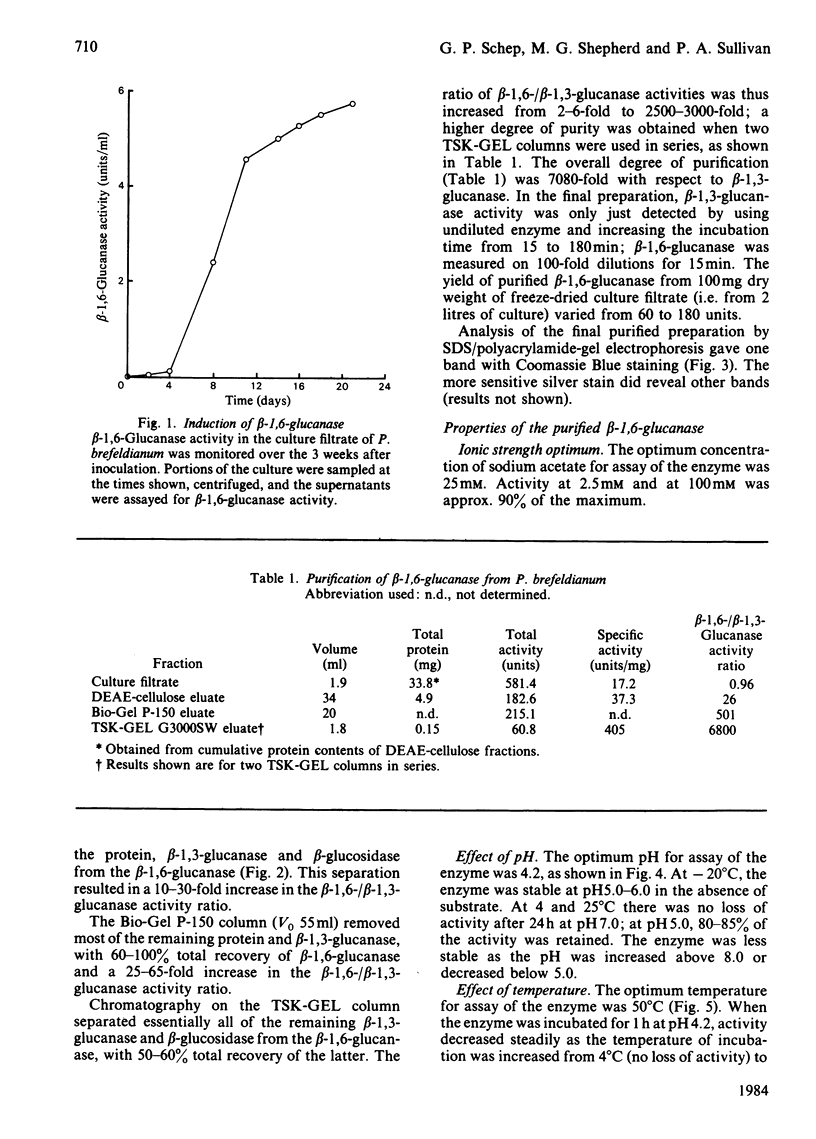
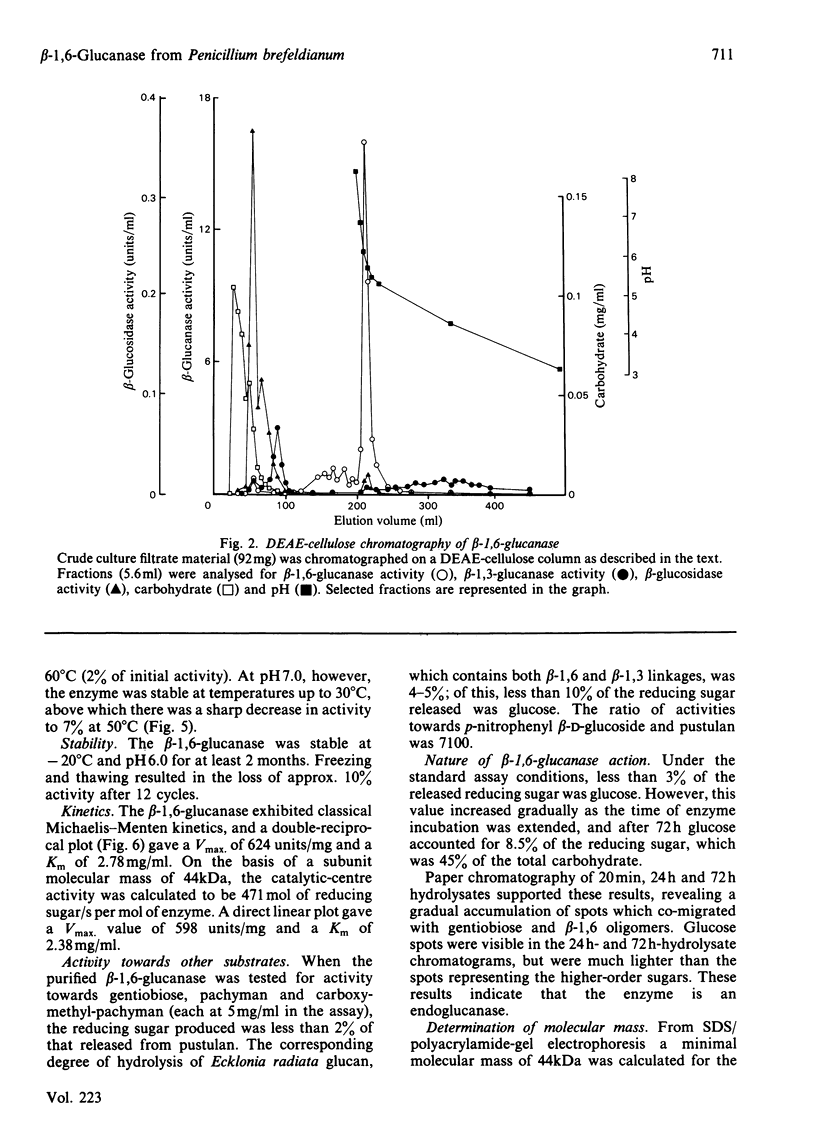
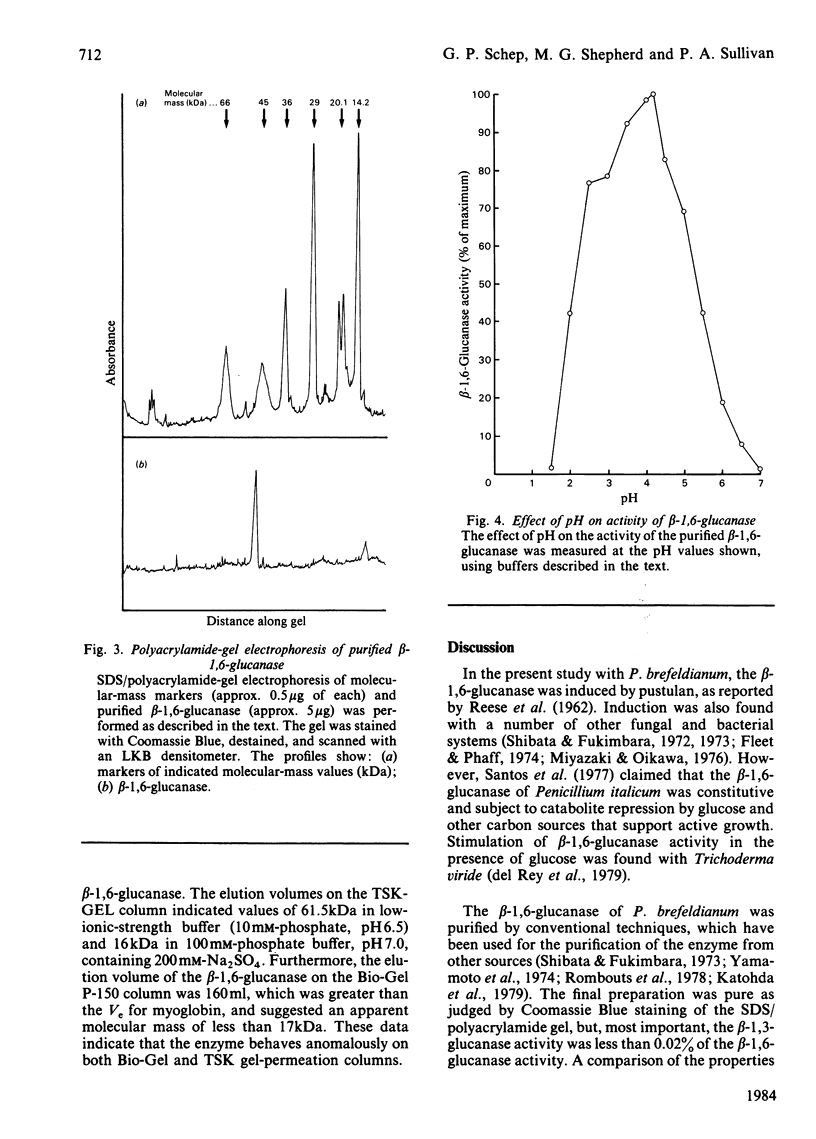
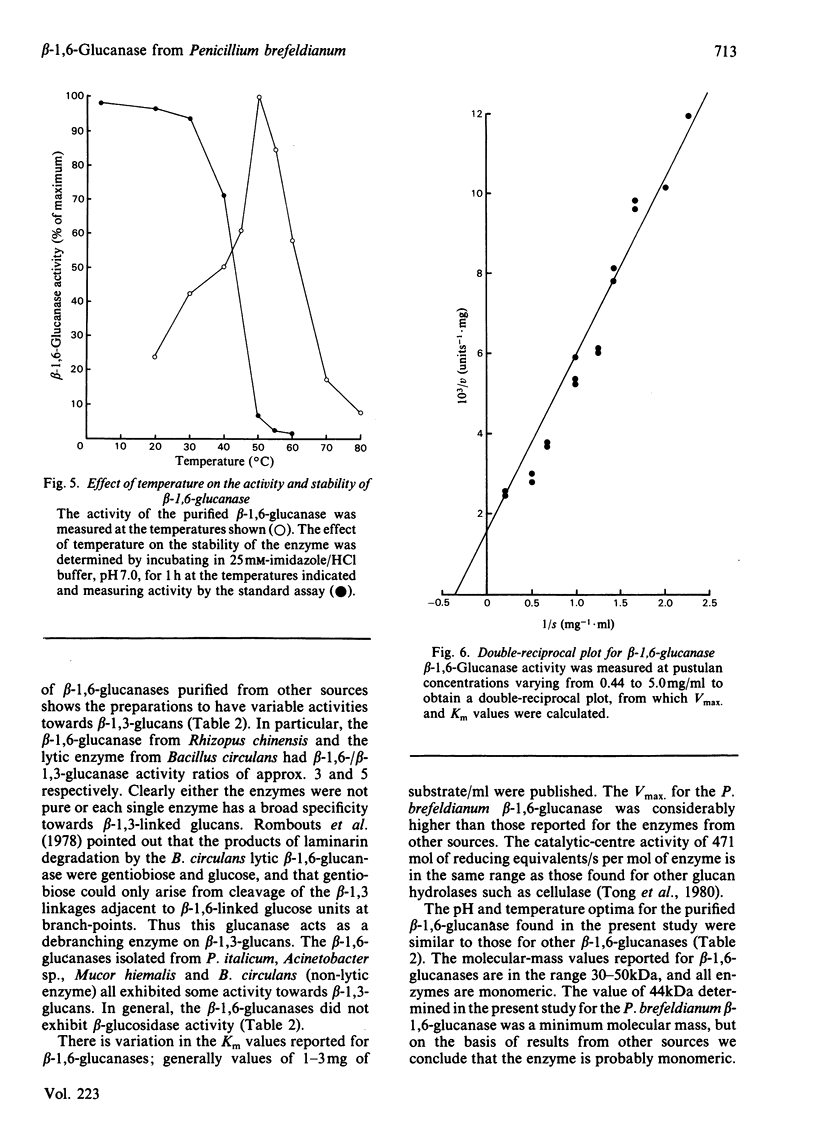
An inducible endo-beta-1,6-glucanase was purified from Penicillium brefeldianum by DEAE-cellulose, Bio-Gel P-150 and high-pressure liquid chromatography. The final preparation was essentially free from beta-1,3-glucanase and beta-glucosidase activities. Sodium dodecyl sulphate/polyacrylamide-gel electrophoresis revealed one protein band with an Mr of 44000. The Vmax. and Km values were calculated to be 624 units (mumol/min)/mg and 2.78 mg/ml respectively. The glucanase had lytic activity against mycelial cells of the yeast Candida albicans. The yield of purified beta-1,6-glucanase from 100 mg dry weight of freeze-dried culture filtrate varied from 60 to 180 units.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Duffus J. H., Levi C., Manners D. J. Yeast cell-wall glucans. Adv Microb Physiol. 1982;23:151–181. doi: 10.1016/s0065-2911(08)60337-9. [DOI] [PubMed] [Google Scholar]
- Fleet G. H., Phaff H. J. Lysis of yeast cell walls: glucanases from Bacillus circulans WL-12. J Bacteriol. 1974 Jul;119(1):207–219. doi: 10.1128/jb.119.1.207-219.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal P., Sullivan P. A., Shepherd M. G. Isolation and structure of glucan from regenerating spheroplasts of Candida albicans. J Gen Microbiol. 1984 May;130(5):1217–1225. doi: 10.1099/00221287-130-5-1217. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lever M. Colorimetric and fluorometric carbohydrate determination with p-hydroxybenzoic acid hydrazide. Biochem Med. 1973 Apr;7(2):274–281. doi: 10.1016/0006-2944(73)90083-5. [DOI] [PubMed] [Google Scholar]
- Lloyd J. B., Whelan W. J. An improved method for enzymic determination of glucose in the presence of maltose. Anal Biochem. 1969 Sep;30(3):467–470. doi: 10.1016/0003-2697(69)90143-2. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. SDS microslab linear gradient polyacrylamide gel electrophoresis. Anal Biochem. 1978 Jul 1;87(2):386–396. doi: 10.1016/0003-2697(78)90688-7. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Oikawa N. An endo-(1 lead to 6) -beta-D-glucanase from Mucor hiemalis. Carbohydr Res. 1976 Jun;48(2):209–216. doi: 10.1016/s0008-6215(00)83216-4. [DOI] [PubMed] [Google Scholar]
- REESE E. T., PARRISH F. W., MANDELS M. Beta-d-1, 6-Glucanases in fungi. Can J Microbiol. 1962 Jun;8:327–334. doi: 10.1139/m62-045. [DOI] [PubMed] [Google Scholar]
- Rombouts F. M., Phaff H. J. Lysis of yeast cell walls. Lytic beta-(1 leads to 6)-glucanase from Bacillus circulans WL-12. Eur J Biochem. 1976 Mar 16;63(1):109–120. doi: 10.1111/j.1432-1033.1976.tb10213.x. [DOI] [PubMed] [Google Scholar]
- Santos T., Nombela C., Villanueva J. R., Larriba G. Characterization and synthesis regulation of Penicillium italicum 1,6-beta-glucanase. Arch Microbiol. 1979 Jun;121(3):265–270. doi: 10.1007/BF00425066. [DOI] [PubMed] [Google Scholar]
- Santos T., Villanueva J. R., Nombela C. Production and catabolite repression of Penicillium italicum beta-glucanases. J Bacteriol. 1977 Jan;129(1):52–58. doi: 10.1128/jb.129.1.52-58.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Tong C. C., Cole A. L., Shepherd M. G. Purification and properties of the cellulases from the thermophilic fungus Thermoascus aurantiacus. Biochem J. 1980 Oct 1;191(1):83–94. doi: 10.1042/bj1910083. [DOI] [PMC free article] [PubMed] [Google Scholar]


