Abstract
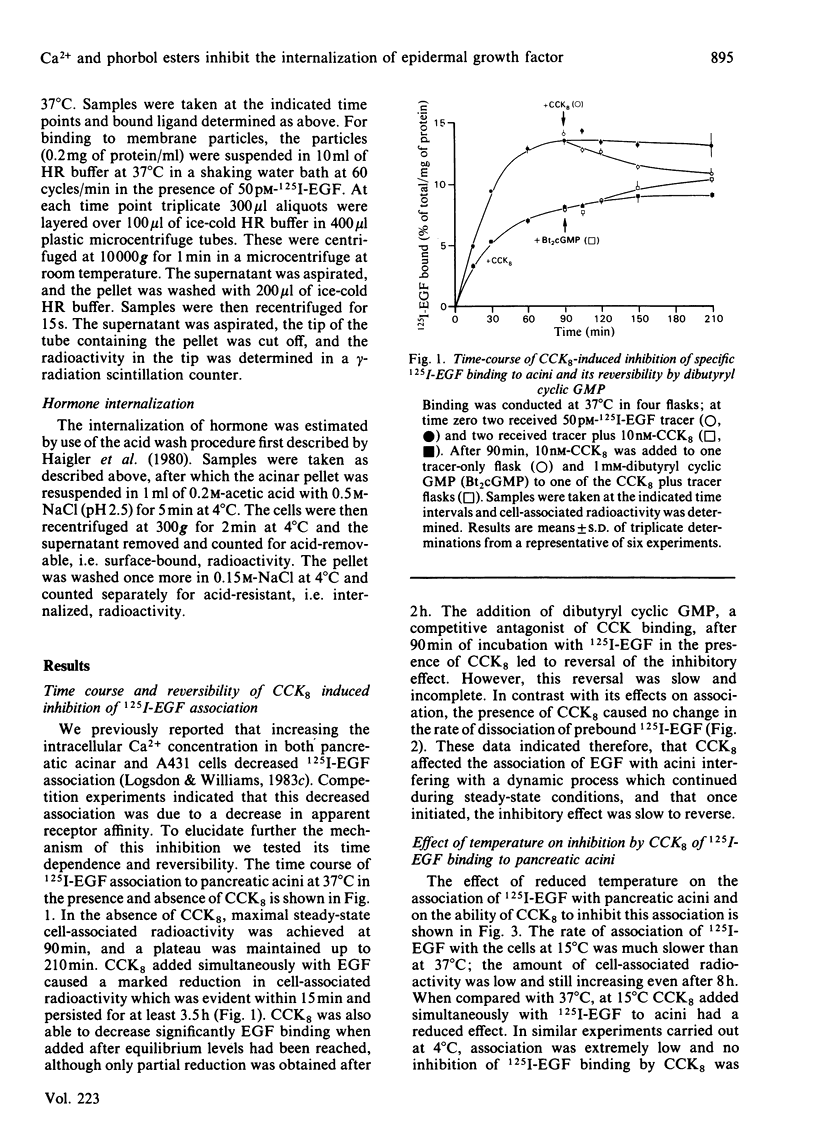
The association of 125I-labelled epidermal growth factor (125I-EGF) with mouse pancreatic acinar cells was inhibited by secretagogues which increase intracellular free Ca2+ concentrations. These agents included cholecystokinin-octapeptide (CCK8) and the Ca2+ ionophore A23187. Inhibition by CCK8 was blocked by lowering the incubation temperature from 37 degrees C to 15 degrees C. Moreover, in contrast with studies of intact acini, the binding of 125I-EGF to isolated acinar membrane particles was not affected either by CCK8, or by varying the level of Ca2+ in the incubation medium. These results indicated, therefore, that the inhibition of 125I-EGF association with acinar cells required intact cells that are metabolically active. Since intact cells at 37 degrees C are known to internalize bound EGF rapidly, acid washing was used to distinguish membrane-associated hormone from internalized hormone. Under steady-state conditions 86% of the 125I-EGF associated with the acini was found to be internalized by this technique. When agents that increased intracellular Ca2+ were tested they all markedly reduced the amount of internalized hormone, whereas surface binding was only minimally affected. The phorbol ester 12-O-tetradecanoyl-phorbol 13-acetate (TPA), which is known to activate protein kinase C, a Ca2+-regulated enzyme, also inhibited the association of EGF with acini. This inhibition was similar to that induced by elevated intracellular Ca2+. To test whether these two inhibitory phenomena were related, the effects of TPA in combination with the Ca2+ ionophore A23187 were examined. At low concentrations the effects were synergistic, whereas at high concentrations the maximal level of inhibition was not changed. We suggest therefore that elevated intracellular Ca2+ and phorbol esters may inhibit EGF internalization by a mechanism involving activation of protein kinase C.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown K. D., Dicker P., Rozengurt E. Inhibition of epidermal growth factor binding to surface receptors by tumor promotors. Biochem Biophys Res Commun. 1979 Feb 28;86(4):1037–1043. doi: 10.1016/0006-291x(79)90221-3. [DOI] [PubMed] [Google Scholar]
- Burnham D. B., Williams J. A. Activation of protein kinase activity in pancreatic acini by calcium and cAMP. Am J Physiol. 1984 May;246(5 Pt 1):G500–G508. doi: 10.1152/ajpgi.1984.246.5.G500. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Cochet C., Gill G. N., Meisenhelder J., Cooper J. A., Hunter T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Feb 25;259(4):2553–2558. [PubMed] [Google Scholar]
- De Larco J. E., Todaro G. J. Sarcoma growth factor (SGF): specific binding to epidermal growth factor (EGF) membrane receptors. J Cell Physiol. 1980 Feb;102(2):267–277. doi: 10.1002/jcp.1041020218. [DOI] [PubMed] [Google Scholar]
- Dembiński A., Gregory H., Konturek S. J., Polański M. Trophic action of epidermal growth factor on the pancreas and gastroduodenal mucosa in rats. J Physiol. 1982 Apr;325:35–42. doi: 10.1113/jphysiol.1982.sp014133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. F., Das M. Internalization and processing of the EGF receptor in the induction of DNA synthesis in cultured fibroblasts: the endocytic activation hypothesis. J Supramol Struct. 1979;10(2):199–214. doi: 10.1002/jss.400100210. [DOI] [PubMed] [Google Scholar]
- Froscio M., Guy G. R., Murray A. W. Calmodulin inhibitors modify cell surface changes triggered by a tumor promoter. Biochem Biophys Res Commun. 1981 Feb 12;98(3):829–835. doi: 10.1016/0006-291x(81)91186-4. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Smith G. J., Wong K. Y., Jones A. L. Cellular uptake and nuclear binding of insulin in human cultured lymphocytes: evidence for potential intracellular sites of insulin action. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1368–1372. doi: 10.1073/pnas.74.4.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther G. R. Effect of 12-O-tetradecanoyl-phorbol-13-acetate on Ca2+ efflux and protein discharge in pancreatic acini. J Biol Chem. 1981 Dec 10;256(23):12040–12045. [PubMed] [Google Scholar]
- Haigler H. T., Maxfield F. R., Willingham M. C., Pastan I. Dansylcadaverine inhibits internalization of 125I-epidermal growth factor in BALB 3T3 cells. J Biol Chem. 1980 Feb 25;255(4):1239–1241. [PubMed] [Google Scholar]
- Hems D. A., Whitton P. D. Control of hepatic glycogenolysis. Physiol Rev. 1980 Jan;60(1):1–50. doi: 10.1152/physrev.1980.60.1.1. [DOI] [PubMed] [Google Scholar]
- Iwashita S., Fox C. F. Epidermal growth factor and potent phorbol tumor promoters induce epidermal growth factor receptor phosphorylation in a similar but distinctively different manner in human epidermoid carcinoma A431 cells. J Biol Chem. 1984 Feb 25;259(4):2559–2567. [PubMed] [Google Scholar]
- Johnson L. R. Effects of gastrointestinal hormones on pancreatic growth. Cancer. 1981 Mar 15;47(6 Suppl):1640–1645. doi: 10.1002/1097-0142(19810315)47:6+<1640::aid-cncr2820471430>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- King A. C., Cuatrecasas P. Peptide hormone-induced receptor mobility, aggregation, and internalization. N Engl J Med. 1981 Jul 9;305(2):77–88. doi: 10.1056/NEJM198107093050206. [DOI] [PubMed] [Google Scholar]
- Korc M., Matrisian L. M., Magun B. E. Cytosolic calcium regulates epidermal growth factor endocytosis in rat pancreas and cultured fibroblasts. Proc Natl Acad Sci U S A. 1984 Jan;81(2):461–465. doi: 10.1073/pnas.81.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korc M., Matrisian L. M., Magun B. E. Direct modulation of epidermal growth factor binding by cholecystokinin. Life Sci. 1983 Aug 8;33(6):561–568. doi: 10.1016/0024-3205(83)90131-5. [DOI] [PubMed] [Google Scholar]
- Korc M., Matrisian L. M., Planck S. R., Magun B. E. Binding of epidermal growth factor in rat pancreatic acini. Biochem Biophys Res Commun. 1983 Mar 29;111(3):1066–1073. doi: 10.1016/0006-291x(83)91408-0. [DOI] [PubMed] [Google Scholar]
- Lee L. S., Weinstein I. B. Mechanism of tumor promoter inhibition of cellular binding of epidermal growth factor. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5168–5172. doi: 10.1073/pnas.76.10.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. S., Weinstein I. B. Tumor-promoting phorbol esters inhibit binding of epidermal growth factor to cellular receptors. Science. 1978 Oct 20;202(4365):313–315. doi: 10.1126/science.308698. [DOI] [PubMed] [Google Scholar]
- Logsdon C. D., Williams J. A. Epidermal growth factor binding and biologic effects on mouse pancreatic acini. Gastroenterology. 1983 Aug;85(2):339–345. [PubMed] [Google Scholar]
- Logsdon C. D., Williams J. A. Epidermal growth factor: intracellular Ca2+ inhibits its association with pancreatic acini and A431 cells. FEBS Lett. 1983 Dec 12;164(2):335–339. doi: 10.1016/0014-5793(83)80312-3. [DOI] [PubMed] [Google Scholar]
- Logsdon C. D., Williams J. A. Pancreatic acini in short-term culture: regulation by EGF, carbachol, insulin, and corticosterone. Am J Physiol. 1983 Jun;244(6):G675–G682. doi: 10.1152/ajpgi.1983.244.6.G675. [DOI] [PubMed] [Google Scholar]
- Magun B. E., Matrisian L. M., Bowden G. T. Epidermal growth factor. Ability of tumor promoter to alter its degradation, receptor affinity and receptor number. J Biol Chem. 1980 Jul 10;255(13):6373–6381. [PubMed] [Google Scholar]
- Murray A. W., Froscio M. Inhibition of epidermal growth factor binding to cultured mouse epidermal cells by tumor promoters. Carcinogenesis. 1980 Aug;1(8):681–684. doi: 10.1093/carcin/1.8.681. [DOI] [PubMed] [Google Scholar]
- Naka M., Nishikawa M., Adelstein R. S., Hidaka H. Phorbol ester-induced activation of human platelets is associated with protein kinase C phosphorylation of myosin light chains. Nature. 1983 Dec 1;306(5942):490–492. doi: 10.1038/306490a0. [DOI] [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs D. L., Korenbrot J. I., Williams J. A. Intracellular free calcium concentrations in isolated pancreatic acini; effects of secretagogues. Biochem Biophys Res Commun. 1983 Nov 30;117(1):122–128. doi: 10.1016/0006-291x(83)91549-8. [DOI] [PubMed] [Google Scholar]
- Ozanne B., Fulton R. J., Kaplan P. L. Kirsten murine sarcoma virus transformed cell lines and a spontaneously transformed rat cell-line produce transforming factors. J Cell Physiol. 1980 Oct;105(1):163–180. doi: 10.1002/jcp.1041050118. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. R., Drubin D. G., Kelly R. B. Identification of three coated vesicle components as alpha- and beta-tubulin linked to a phosphorylated 50,000-dalton polypeptide. J Cell Biol. 1983 Jul;97(1):40–47. doi: 10.1083/jcb.97.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink T. J., Sanchez A., Hallam T. J. Diacylglycerol and phorbol ester stimulate secretion without raising cytoplasmic free calcium in human platelets. Nature. 1983 Sep 22;305(5932):317–319. doi: 10.1038/305317a0. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Brown K. D., Pettican P. Vasopressin inhibition of epidermal growth factor binding to cultured mouse cells. J Biol Chem. 1981 Jan 25;256(2):716–722. [PubMed] [Google Scholar]
- Rozengurt E., Collins M., Brown K. D., Pettican P. Inhibition of epidermal growth factor binding to mouse cultured cells by fibroblast-derived growth factor. Evidence for an indirect mechanism. J Biol Chem. 1982 Apr 10;257(7):3680–3686. [PubMed] [Google Scholar]
- Salomon D. S. Inhibition of epidermal growth factor binding to mouse embryonal carcinoma cells by phorbol esters mediated by specific phorbol ester receptors. J Biol Chem. 1981 Aug 10;256(15):7958–7966. [PubMed] [Google Scholar]
- Savion N., Vlodavsky I., Gospodarowicz D. Role of the degradation process in the mitogenic effect of epidermal growth factor. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1466–1470. doi: 10.1073/pnas.77.3.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., De Larco J. E., Todaro G. J. Biologically active phorbol esters specifically alter affinity of epidermal growth factor membrane receptors. Nature. 1979 May 31;279(5712):387–391. doi: 10.1038/279387a0. [DOI] [PubMed] [Google Scholar]
- Steigerwalt R. W., Williams J. A. Characterization of cholecystokinin receptors on rat pancreatic membranes. Endocrinology. 1981 Nov;109(5):1746–1753. doi: 10.1210/endo-109-5-1746. [DOI] [PubMed] [Google Scholar]
- Williams J. A. Regulation of pancreatic acinar cell function by intracellular calcium. Am J Physiol. 1980 Apr;238(4):G269–G279. doi: 10.1152/ajpgi.1980.238.4.G269. [DOI] [PubMed] [Google Scholar]


