Abstract
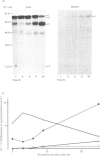
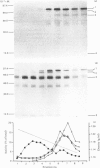
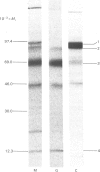
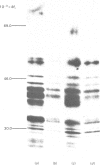
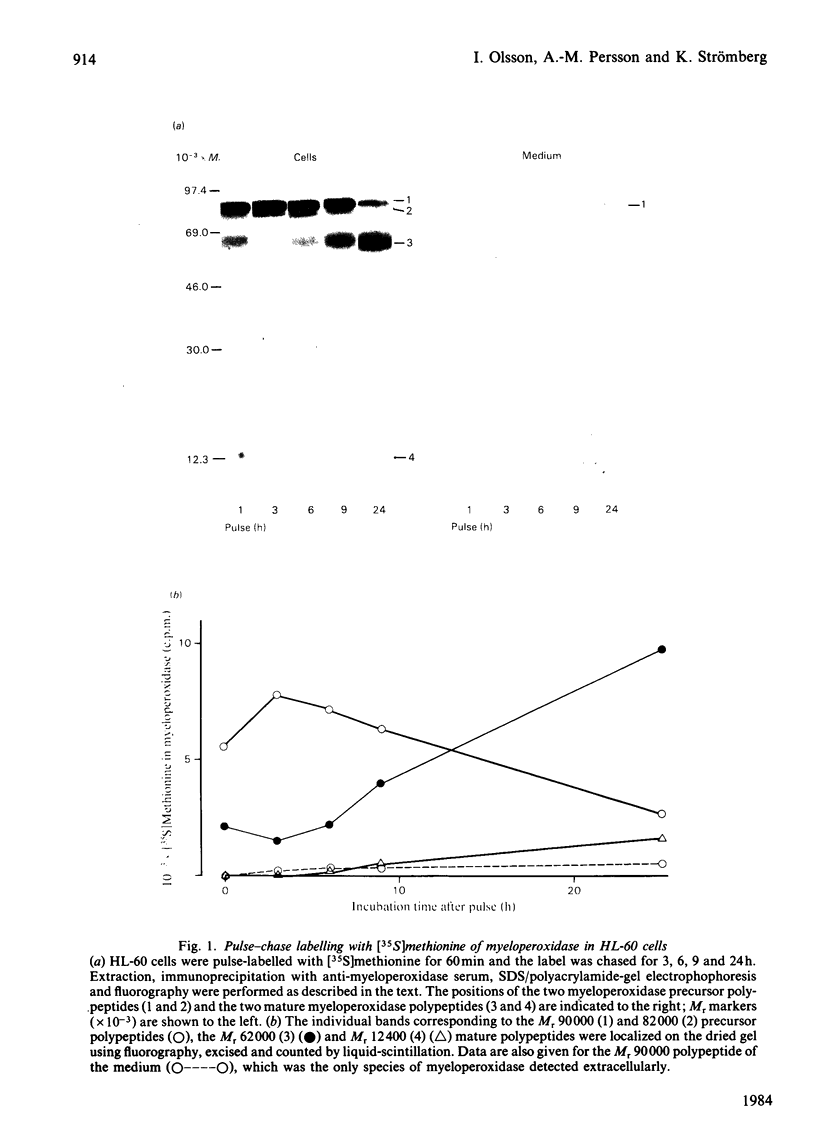
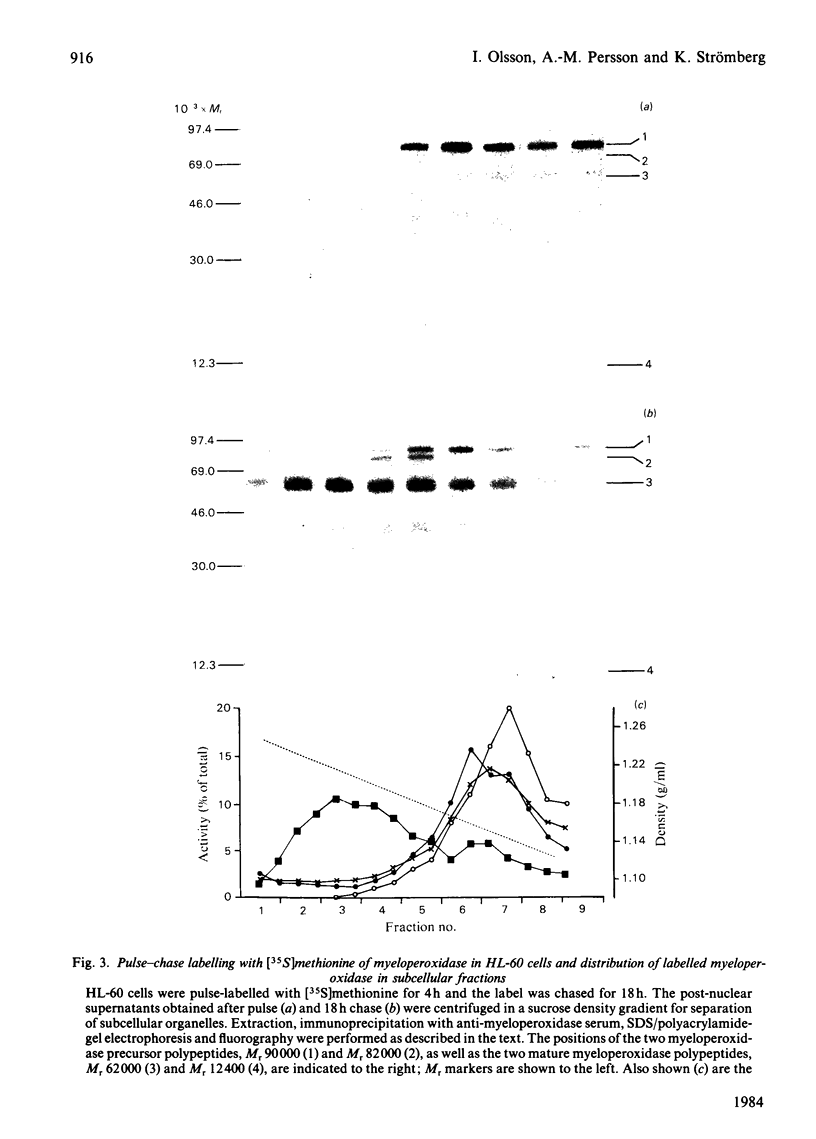
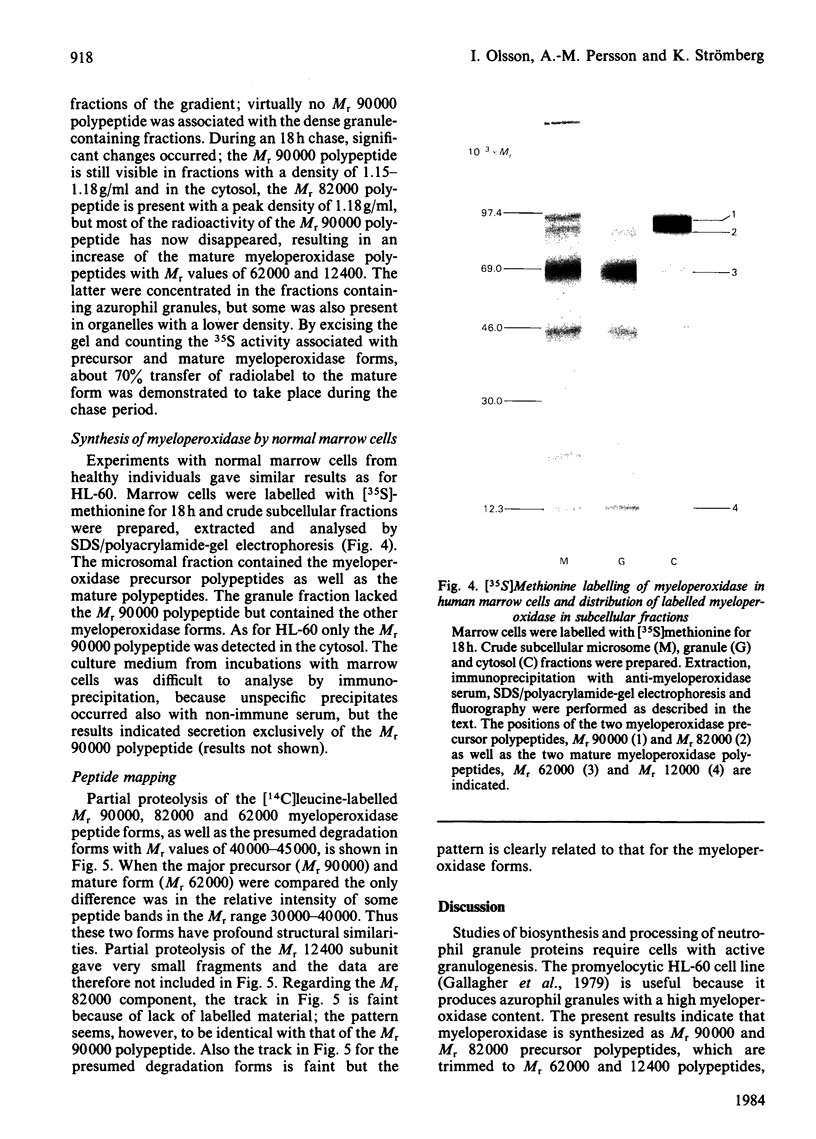
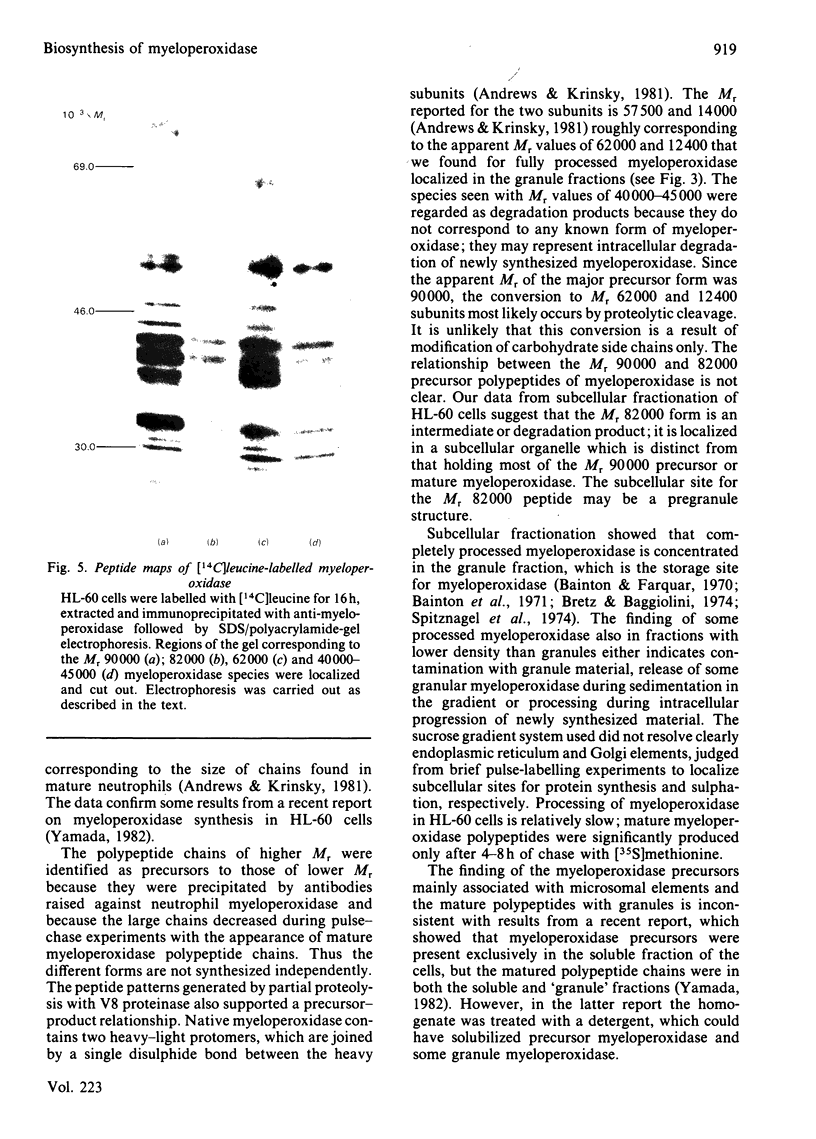
The processing and intracellular transport of myeloperoxidase were studied in the human promyelocytic leukaemia cell line HL-60 and in normal marrow cells labelled with [35S]methionine or [14C]leucine. Myeloperoxidase was precipitated with antimyeloperoxidase serum; the immunoprecipitates were subjected to sodium dodecyl sulphate/polyacrylamide-gel electrophoresis and radiolabelled myeloperoxidase visualized by fluorography. During a 1 h pulse, myeloperoxidase was labelled in a chain of apparent Mr 90 000. With a subsequent chase, the Mr 90 000 polypeptide disappeared and was replaced by chains of Mr 62 000 and 12 400 corresponding roughly to the size of neutrophil myeloperoxidase subunits. The identification of the radioactive polypeptides as different forms of myeloperoxidase was established also by the similarity in patterns generated by partial proteolysis with V8 proteinase from Staphylococcus aureus. Processing of myeloperoxidase in HL-60 was slow; mature polypeptides were significantly increased only after 6 h. Another myeloperoxidase chain of apparent Mr 82 000 was an intermediate precursor or degradation form. Pulse-chase experiments in combination with sucrose-density-gradient separations of homogenates showed that the Mr 90 000 precursor was located in light density organelles only and not in granule fractions, whereas the Mr 82 000 precursor was located only in intermediate density organelles, suggesting that the latter is a product of the former. Processed mature myeloperoxidase was concentrated in the granule fraction, but some occurred in lower density organelles, which may indicate processing during intracellular transport. Only the Mr 90 000 polypeptide was secreted into the culture medium; this was also the only form found in the cytosol fraction.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. C., Krinsky N. I. The reductive cleavage of myeloperoxidase in half, producing enzymically active hemi-myeloperoxidase. J Biol Chem. 1981 May 10;256(9):4211–4218. [PubMed] [Google Scholar]
- Bainton D. F., Farquhar M. G. Segregation and packaging of granule enzymes in eosinophilic leukocytes. J Cell Biol. 1970 Apr;45(1):54–73. doi: 10.1083/jcb.45.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton D. F., Ullyot J. L., Farquhar M. G. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J Exp Med. 1971 Oct 1;134(4):907–934. doi: 10.1084/jem.134.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretz U., Baggiolini M. Biochemical and morphological characterization of azurophil and specific granules of human neutrophilic polymorphonuclear leukocytes. J Cell Biol. 1974 Oct;63(1):251–269. doi: 10.1083/jcb.63.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Erickson A. H., Conner G. E., Blobel G. Biosynthesis of a lysosomal enzyme. Partial structure of two transient and functionally distinct NH2-terminal sequences in cathepsin D. J Biol Chem. 1981 Nov 10;256(21):11224–11231. [PubMed] [Google Scholar]
- Gallagher R., Collins S., Trujillo J., McCredie K., Ahearn M., Tsai S., Metzgar R., Aulakh G., Ting R., Ruscetti F. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979 Sep;54(3):713–733. [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Phosphorylation of mannose residues. J Biol Chem. 1980 May 25;255(10):4946–4950. [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Hultberg B., Lindsten J., Sjöblad S. Molecular forms and activities of glycosidases in cultures of amniotic-fluid cells. Biochem J. 1976 Jun 1;155(3):599–605. doi: 10.1042/bj1550599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A., Achord D. T., Sly W. S. Phosphohexosyl components of a lysosomal enzyme are recognized by pinocytosis receptors on human fibroblasts. Proc Natl Acad Sci U S A. 1977 May;74(5):2026–2030. doi: 10.1073/pnas.74.5.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Myerowitz R., Neufeld E. F. Maturation of alpha-L-iduronidase in cultured human fibroblasts. J Biol Chem. 1981 Mar 25;256(6):3044–3048. [PubMed] [Google Scholar]
- Olofsson T., Olsson I., Venge P., Elgefors B. Serum myeloperoxidase and lactoferrin in neutropenia. Scand J Haematol. 1977 Jan;18(1):73–80. doi: 10.1111/j.1600-0609.1977.tb01480.x. [DOI] [PubMed] [Google Scholar]
- Olsson I., Olofsson T., Odeberg H. Myeloperoxidase-mediated iodination in granulocytes. Scand J Haematol. 1972;9(5):483–491. doi: 10.1111/j.1600-0609.1972.tb00974.x. [DOI] [PubMed] [Google Scholar]
- Olsson I., Olofsson T., Ohlsson K., Gustavsson A. Serum and plasma myeloperoxidase, elastase and lactoferrin content in acute myeloid leukaemia. Scand J Haematol. 1979 May;22(5):397–406. doi: 10.1111/j.1600-0609.1979.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Olsson I. The intracellular transport of glycosaminoglycans (mucopolysaccharides) in human leukocytes. Exp Cell Res. 1969 Mar;54(3):318–325. doi: 10.1016/0014-4827(69)90209-2. [DOI] [PubMed] [Google Scholar]
- Olsson I., Venge P. The role of the human neutrophil in the inflammatory reaction. Allergy. 1980 Jan;35(1):1–13. doi: 10.1111/j.1398-9995.1980.tb01711.x. [DOI] [PubMed] [Google Scholar]
- SCHULTZ J., KAMINKER K. Myeloperoxidase of the leucocyte of normal human blood. I. Content and localization. Arch Biochem Biophys. 1962 Mar;96:465–467. doi: 10.1016/0003-9861(62)90321-1. [DOI] [PubMed] [Google Scholar]
- Sahagian G. G., Distler J., Jourdian G. W. Characterization of a membrane-associated receptor from bovine liver that binds phosphomannosyl residues of bovine testicular beta-galactosidase. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4289–4293. doi: 10.1073/pnas.78.7.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skudlarek M. D., Swank R. T. Turnover of two lysosomal enzymes in macrophages. J Biol Chem. 1981 Oct 10;256(19):10137–10144. [PubMed] [Google Scholar]
- Spitznagel J. K., Dalldorf F. G., Leffell M. S., Folds J. D., Welsh I. R., Cooney M. H., Martin L. E. Character of azurophil and specific granules purified from human polymorphonuclear leukocytes. Lab Invest. 1974 Jun;30(6):774–785. [PubMed] [Google Scholar]
- Yamada M. Myeloperoxidase precursors in human myeloid leukemia HL-60 cells. J Biol Chem. 1982 Jun 10;257(11):5980–5982. [PubMed] [Google Scholar]
- Young R. W. The role of the Golgi complex in sulfate metabolism. J Cell Biol. 1973 Apr;57(1):175–189. doi: 10.1083/jcb.57.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]