Abstract
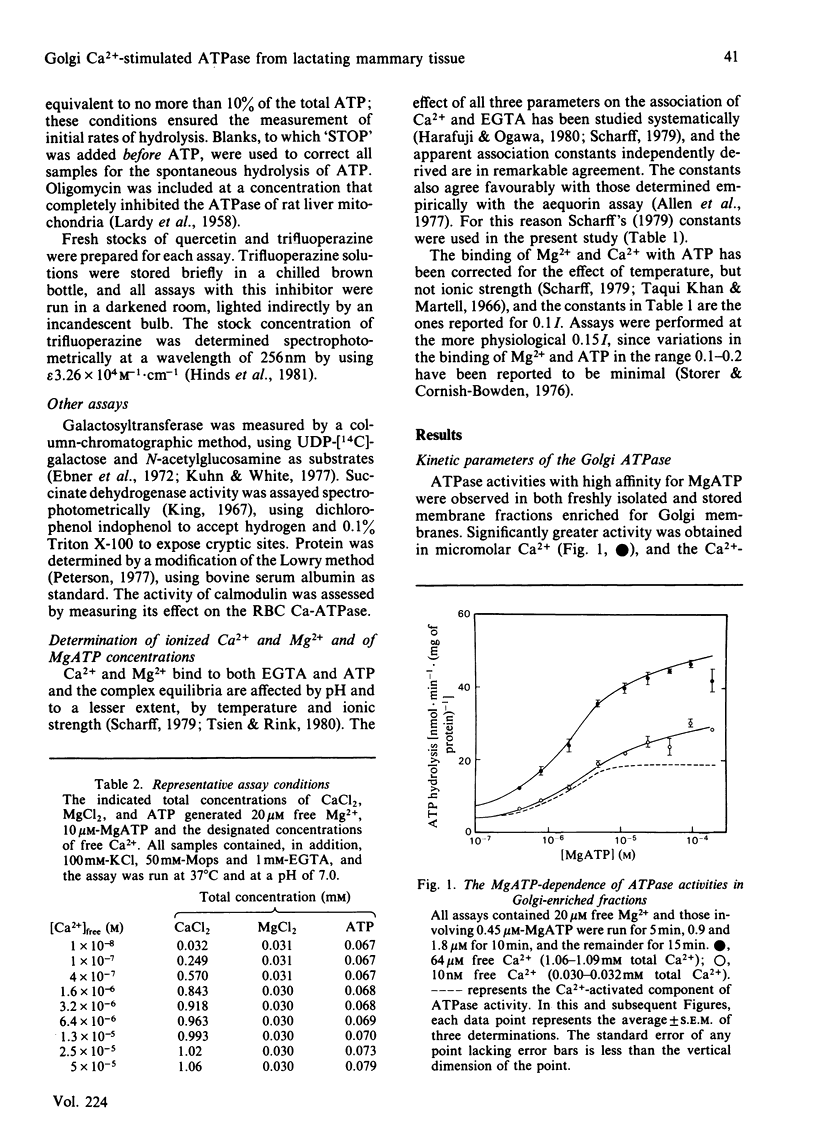
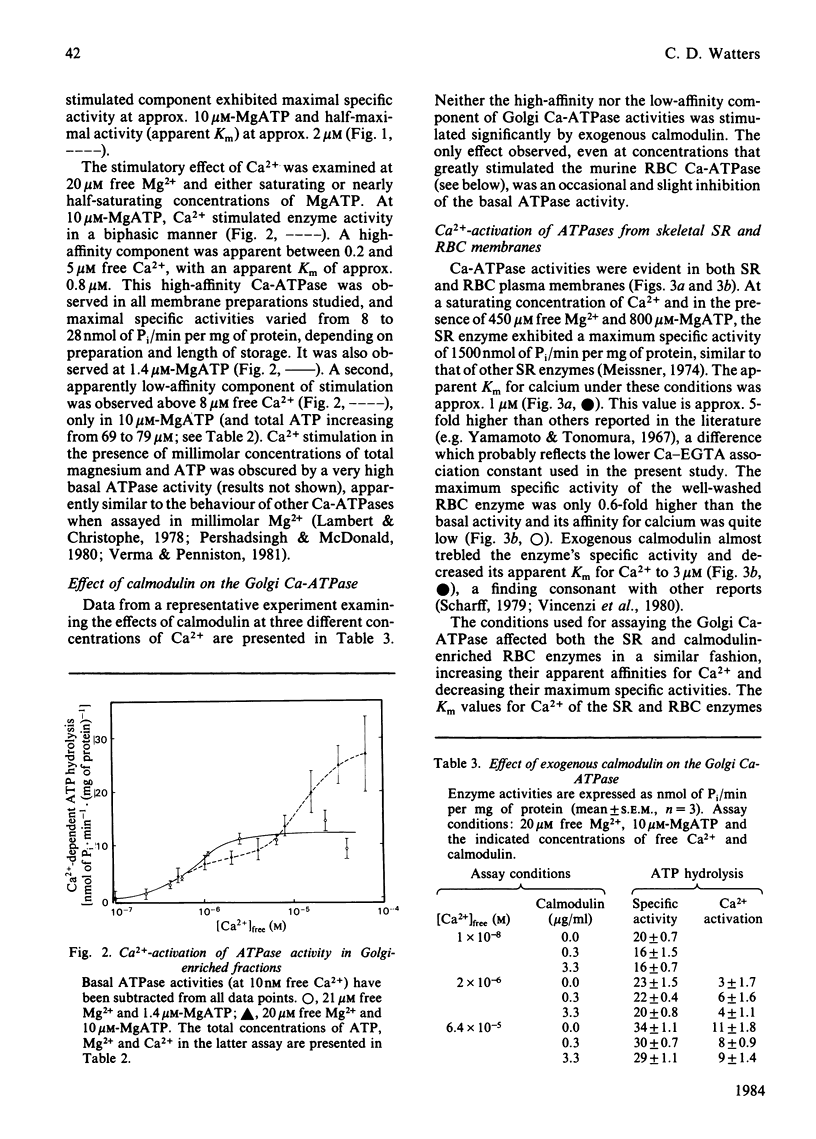
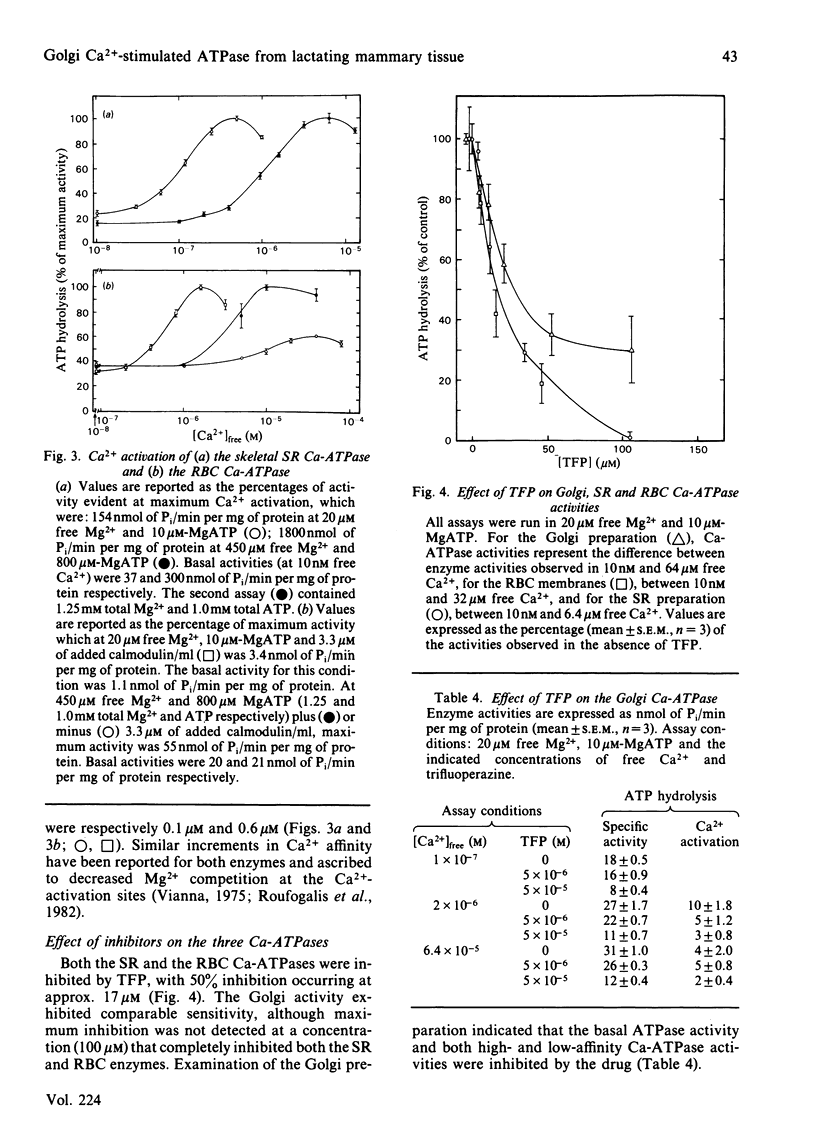
A membrane fraction isolated from lactating murine mammary tissue and enriched for the Golgi membrane marker enzyme galactosyltransferase exhibited Ca2+-stimulated ATPase activity (Ca-ATPase) in 20 microM-free Mg2+ and 10 microM-MgATP, with an apparent Km for Ca2+ of 0.8 microM. Exogenous calmodulin did not enhance Ca2+ stimulation, nor could Ca-ATPase activities be detected in millimolar total Mg2+ and ATP. When assayed with micromolar Mg2+ and MgATP the Ca-ATPases of skeletal-muscle sarcoplasmic reticulum and of calmodulin-enriched red blood cell plasma membranes were half-maximally activated by 0.1 microM- and 0.6 microM-Ca2+ respectively. All three Ca-ATPases were inhibited by similar micromolar concentrations of trifluoperazine, but the Golgi activity was unaffected by quercetin in concentrations which completely inhibited both the sarcoplasmic-reticulum and red-blood-cell enzymes. The results are consistent with the hypothesis that the high-affinity Ca-ATPase is responsible for the ATP-dependent Ca2+ transport exhibited by Golgi-enriched vesicles derived from lactating mammary gland [Neville, Selker, Semple & Watters (1981) J. Membr. Biol. 61, 97-105; West (1981) Biochim. Biophys. Acta 673, 374-386].
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Blinks J. R., Prendergast F. G. Aequorin luminescence: relation of light emission to calcium concentration--a calcium-independent component. Science. 1977 Mar 11;195(4282):996–998. doi: 10.1126/science.841325. [DOI] [PubMed] [Google Scholar]
- Baumrucker C. R., Keenan T. W. Membranes of mammary gland. X. Adenosine triphosphate dependent calcium accumulation by Golgi apparatus rich fractions from bovine mammary gland. Exp Cell Res. 1975 Feb;90(2):253–260. doi: 10.1016/0014-4827(75)90314-6. [DOI] [PubMed] [Google Scholar]
- Caroni P., Carafoli E. The Ca2+-pumping ATPase of heart sarcolemma. Characterization, calmodulin dependence, and partial purification. J Biol Chem. 1981 Apr 10;256(7):3263–3270. [PubMed] [Google Scholar]
- Harafuji H., Ogawa Y. Re-examination of the apparent binding constant of ethylene glycol bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid with calcium around neutral pH. J Biochem. 1980 May;87(5):1305–1312. doi: 10.1093/oxfordjournals.jbchem.a132868. [DOI] [PubMed] [Google Scholar]
- Hinds T. R., Raess B. U., Vincenzi F. F. Plasma membrane Ca2+ transport: antagonism by several potential inhibitors. J Membr Biol. 1981 Jan 30;58(1):57–65. doi: 10.1007/BF01871034. [DOI] [PubMed] [Google Scholar]
- Ikemoto N. Structure and function of the calcium pump protein of sarcoplasmic reticulum. Annu Rev Physiol. 1982;44:297–317. doi: 10.1146/annurev.ph.44.030182.001501. [DOI] [PubMed] [Google Scholar]
- Khan M. M., Martell A. E. Thermodynamic quantities associated with the interaction of adenosine triphosphate with metal ions. J Am Chem Soc. 1966 Feb 20;88(4):668–671. doi: 10.1021/ja00956a008. [DOI] [PubMed] [Google Scholar]
- Kuhn N. J., White A. The role of nucleoside diphosphatase in a uridine nucleotide cycle associated with lactose synthesis in rat mammary-gland Golgi apparatus. Biochem J. 1977 Dec 15;168(3):423–433. doi: 10.1042/bj1680423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARDY H. A., JOHNSON D., McMURRAY W. C. Antibiotics as tools for metabolic studies. I. A survey of toxic antibiotics in respiratory, phosphorylative and glycolytic systems. Arch Biochem Biophys. 1958 Dec;78(2):587–597. doi: 10.1016/0003-9861(58)90383-7. [DOI] [PubMed] [Google Scholar]
- Lambert M., Christophe J. Characterization of (Mg,Ca)-ATPase activity in rat pancreatic plasma membranes. Eur J Biochem. 1978 Nov 15;91(2):485–492. doi: 10.1111/j.1432-1033.1978.tb12701.x. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H. Purification and properties of an adenosine triphosphatase from sarcoplasmic reticulum. J Biol Chem. 1970 Sep 10;245(17):4508–4518. [PubMed] [Google Scholar]
- Marban E., Rink T. J., Tsien R. W., Tsien R. Y. Free calcium in heart muscle at rest and during contraction measured with Ca2+ -sensitive microelectrodes. Nature. 1980 Aug 28;286(5776):845–850. doi: 10.1038/286845a0. [DOI] [PubMed] [Google Scholar]
- McDonald J. M., Chan K. M., Goewert R. R., Mooney R. A., Pershadsingh H. A. The (Ca2+ +Mg2+)-ATPase of adipocyte plasma membrane: regulation by calmodulin and insulin. Ann N Y Acad Sci. 1982;402:381–401. doi: 10.1111/j.1749-6632.1982.tb25756.x. [DOI] [PubMed] [Google Scholar]
- Meissner G., Fleischer S. Characterization of sarcoplasmic reticulum from skeletal muscle. Biochim Biophys Acta. 1971 Aug 13;241(2):356–378. doi: 10.1016/0005-2736(71)90036-8. [DOI] [PubMed] [Google Scholar]
- Meissner G. Isolation of sarcoplasmic reticulum from skeletal muscle. Methods Enzymol. 1974;31:238–246. doi: 10.1016/0076-6879(74)31025-7. [DOI] [PubMed] [Google Scholar]
- Michaelis E. K., Michaelis M. L., Chang H. H., Kitos T. E. High affinity Ca2+-stimulated Mg2+-dependent ATPase in rat brain synaptosomes, synaptic membranes, and microsomes. J Biol Chem. 1983 May 25;258(10):6101–6108. [PubMed] [Google Scholar]
- Neville M. C., Peaker M. The secretion of calcium and phosphorus into milk. J Physiol. 1979 May;290(2):59–67. doi: 10.1113/jphysiol.1979.sp012759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville M. C., Selker F., Semple K., Watters C. ATP-dependent calcium transport by a Golgi-enriched membrane fraction from mouse mammary gland. J Membr Biol. 1981;61(2):97–105. doi: 10.1007/BF02007636. [DOI] [PubMed] [Google Scholar]
- Penniston J. T. Substrate specificity of the erythrocyte Ca2+-ATPase. Biochim Biophys Acta. 1982 Jun 28;688(3):735–739. doi: 10.1016/0005-2736(82)90286-3. [DOI] [PubMed] [Google Scholar]
- Pershadsingh H. A., McDonald J. M. A high affinity calcium-stimulated magnesium-dependent adenosine triphosphatase in rat adipocyte plasma membranes. J Biol Chem. 1980 May 10;255(9):4087–4093. [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Roufogalis B. D., Akyempon C. K., Al-Jobore A., Minocherhomjee A. M. Regulation of the Ca2+ pump of the erythrocyte membrane. Ann N Y Acad Sci. 1982;402:349–367. doi: 10.1111/j.1749-6632.1982.tb25754.x. [DOI] [PubMed] [Google Scholar]
- Saermark T., Vilhardt H. Isolation and partial characterization of magnesium ion- and calcium ion-dependent adenosine triphosphatase activity from bovine brain microsomal fraction. Biochem J. 1979 Aug 1;181(2):321–330. doi: 10.1042/bj1810321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkadi B. Active calcium transport in human red cells. Biochim Biophys Acta. 1980 Sep 30;604(2):159–190. doi: 10.1016/0005-2736(80)90573-8. [DOI] [PubMed] [Google Scholar]
- Scharff O. Stimulating effects of monovalent cations on activator-dissociated and activator-associated states of Ca2+-ATPase in human erythrocytes. Biochim Biophys Acta. 1978 Sep 22;512(2):309–317. doi: 10.1016/0005-2736(78)90255-9. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J., Bürgin H. Calcium in human red blood cells. Ann N Y Acad Sci. 1978 Apr 28;307:125–147. doi: 10.1111/j.1749-6632.1978.tb41939.x. [DOI] [PubMed] [Google Scholar]
- Schuurmans Stekhoven F., Bonting S. L. Transport adenosine triphosphatases: properties and functions. Physiol Rev. 1981 Jan;61(1):1–76. doi: 10.1152/physrev.1981.61.1.1. [DOI] [PubMed] [Google Scholar]
- Seals J. R., McDonald J. M., Bruns D., Jarett L. A sensitive and precise isotopic assay of ATPase activity. Anal Biochem. 1978 Oct 15;90(2):785–795. doi: 10.1016/0003-2697(78)90169-0. [DOI] [PubMed] [Google Scholar]
- Shigekawa M., Dougherty J. P., Katz A. M. Reaction mechanism of Ca2+-dependent ATP hydrolysis by skeletal muscle sarcoplasmic reticulum in the absence of added alkali metal salts. I. Characterization of steady state ATP hydrolysis and comparison with that in the presence of KCl. J Biol Chem. 1978 Mar 10;253(5):1442–1450. [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. Concentration of MgATP2- and other ions in solution. Calculation of the true concentrations of species present in mixtures of associating ions. Biochem J. 1976 Oct 1;159(1):1–5. doi: 10.1042/bj1590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M., Yamamoto T., Tonomura Y. Molecular mechanism of active calcium transport by sarcoplasmic reticulum. Physiol Rev. 1978 Jan;58(1):1–79. doi: 10.1152/physrev.1978.58.1.1. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim Biophys Acta. 1980 Jul;599(2):623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- Verma A. K., Penniston J. T. A high affinity Ca2+-stimulated and Mg2+-dependent ATPase in rat corpus luteum plasma membrane fractions. J Biol Chem. 1981 Feb 10;256(3):1269–1275. [PubMed] [Google Scholar]
- Vincenzi F. F., Hinds T. R., Raess B. U. Calmodulin and the plasma membrane calcium pump. Ann N Y Acad Sci. 1980;356:232–244. doi: 10.1111/j.1749-6632.1980.tb29614.x. [DOI] [PubMed] [Google Scholar]
- West D. W. Energy-dependent calcium sequestration activity in a Golgi apparatus fraction derived from lactating rat mammary glands. Biochim Biophys Acta. 1981 Apr 3;673(4):374–386. doi: 10.1016/0304-4165(81)90469-4. [DOI] [PubMed] [Google Scholar]
- Wooding F. B., Morgan G. Calcium localization in lactating rabbit mammary secretory cells. J Ultrastruct Res. 1978 Jun;63(3):323–333. doi: 10.1016/s0022-5320(78)80056-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Tonomura Y. Reaction mechanism of the Ca++ -dependent ATPase of sarcoplasmic reticulum from skeletal muscle. I. Kinetic studies. J Biochem. 1967 Nov;62(5):558–575. doi: 10.1093/oxfordjournals.jbchem.a128706. [DOI] [PubMed] [Google Scholar]


