Abstract
Acute methadone toxicity is a major public health concern which has adverse effects on brain tissue and results in recurrent or delayed respiratory arrest. Our study aimed to investigate the time-dependent changes in several serum biochemical markers of brain damage, spatial working memory, and the brain tissue following acute methadone overdose. Adolescent male rats underwent an intraperitoneal (i.p.) injection of 15 mg/kg methadone. In case of apnea occurrence, resuscitation was performed by a ventilatory pump and administrating naloxone (2 mg/kg; i.p.). The animals were classified into groups of treated rats; methadone and naloxone-Apnea (M/N-Apnea), M/N-Sedate, Methadone, Naloxone, and control (saline) groups. The serum levels of S100B, neuron-specific enolase (NSE), myelin basic protein factors, and (Lactate/Pyruvate) L/P ratio were evaluated at the time-points of 6, 24, and 48 h (h). We found that the alterations of S100B and L/P ratio were considerable in the M/N-Apnea and Methadone groups from the early hours post-methadone overdose, while NSE serum levels elevation was observed only in M/N-Apnea group with a delay at 48 h. Further, we assessed the spatial working memory (Y-maze test), morphological changes, and neuronal loss. The impaired spontaneous alternation behavior was detected in the M/N-Apnea groups on days 5 and 10 post-methadone overdose. The morphological changes of neurons and the neuronal loss were detectable in the CA1, striatum, and cerebellum regions, which were pronounced in both M/N-Apnea and Methadone groups. Together, our findings suggest that alterations in the serum levels of S100B and NSE factors as well as L/P ratio could be induced by methadone overdose with the presence or absence of apnea before the memory impairment and tissue injury in adolescent male rats.
Keywords: Methadone, Overdose, Apnea, S100B, MBP, NSE, Lactate, Pyruvate
Introduction
Methadone (6-(dimethylamino)-4,4-diphenyl-3-heptanone) is a synthetic opioid, which is used for maintenance treatment of opiate addicts (Corkery et al. 2004). The high bioavailability of 40–99% (Foster et al. 2000, 2004) and approximate half-life of 24 h with a wide potential range of 5–130 h (Eap et al 2002) as well as long-acting effects on mu-opioid receptors has made it treatment of choice in the field of drug abuse therapy (Farid et al. 2008). However, acute methadone poisoning by intentional or accidental overdose can cause neurological morbidity and mortality (Shields et al. 2007; Jones et al. 2012).
The outcomes of methadone overdose can include loss of consciousness, respiratory depression, miosis, apnea, coma, and even death. (Taheri et al. 2013). An increasing body of evidence has reported the impairment of neuropsychological functions in methadone maintenance patients (Verdejo et al. 2005; Specka et al. 2000; Darke et al. 2000). We recently found that acute methadone overdose could give rise to apnea-induced impairment of motor and cognitive functions in rats (Ahmad-Molaei et al. 2018). On the other hand, the acute and non-chronic exposure to methadone could induce deficits in the performance and accuracy of memory retrieval tests in rodents (Cummins et al. 2012). Several publications have reported damage in several brain regions upon methadone intoxication in both children (Allameh et al. 2017; Winstanley and Stover 2019; Reisner et al. 2015; Haghighi-Morad et al. 2020) and adults (Haghighi-Morad et al. 2020) who urgently needed diagnosis and follow-up intervention to prevent subsequent complications. Most poisoning cases in children under 12 years old were due to methadone overdose in which 53.4% of cases were boys who experienced apnea (Gholami et al. 2017). Unfortunately, in our country, methadone is the frequently accounted opioid causing accidental intoxication and children hospitalization who admitted to emergency department (Mansori et al. 2016; Manouchehrifar et al. 2016).
Brain injuries that cause damage to neurons or glial cells lead to release of cell-type-specific proteins to the cerebro-spinal fluid (CSF) and blood (Petzold et al. 2008). Brain-derived proteins such as S100 calcium-binding protein B (S100B) and neuron-specific enolase (NSE) are widely studied as neurochemical markers that reflect injuries of the central nervous system (CNS) (Wunderlich et al. 2006; Schaf et al. 2005; Herrmann et al. 2000; Busnello et al. 2006). The increased levels of S100B and NSE have been described in the events of brain hypoxia, ischemic stroke, and head trauma (Gonçalves et al. 2008; Jauch et al. 2006). Meanwhile, myelin basic protein (MBP) is a myelin membrane proteolipid (Sternberger et al. 1978) and a potential marker of white matter damage (Brouns et al. 2010). Similarly, increased lactate to pyruvate (L/P) ratio suggests either a continuation of the hypoxic/ischemic state or an imbalance of the energy metabolism (Thoresen et al. 1998). The alteration of serum levels of S100B, NSE, MBP factors, and L/P ratio as brain damage markers has not been studied after acute methadone overdose with the presence or absence of apnea. It seems that peripheral assessment of these proteins along behavioral tests can represent a step forward in the early diagnosis of brain damage following the acute methadone overdose. Despite various reports presented a range of outcomes which include death to persistent neurological deficits or no residual impairments, there is lack of research regarding the evaluation of various subsequent neurological complications after methadone poisoning (Tiong et al. 2019). Thus, the purpose of the current study was to examine the hypothesis of serum levels alterations of S100B, NSE, MBP factors, and L/P ratio in serum, memory impairment, and morphological changes in neurons following the administration of an acute single toxic dose of methadone in adolescent male rats.
Material and Methods
Animals
The experiment was performed on male adolescent Wistar rats (37-days-old, 70–100 g), which were supplied by the Pasteur Institute, Tehran, Iran. Increasing rate of accidental methadone poisoning and apnea in children with most cases in boys (Alotaibi et al. 2012; Manouchehrifar et al. 2016; Mansori et al. 2016) led us to conduct the experiment in adolescent male rats. The animals were separated from their mothers at 30 days of age. The same experimenter continuously handled each animal gently everyday for 5 min during 7 days to reduce stress and acclimatize to a new environment. So, the exact age of animals was 37-days-old at the time of starting any procedure (Sengupta 2013). Our experimenter who was blind to the animal groups performed handling by using cup handling while quickly rats accustomed to the investigator. The animals were housed under the standard laboratory conditions (22 ± 2 °C, 12-h light/dark cycle), and free access to food and water ad libitum was provided. All research and animal care procedures were done based on the guidelines of the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 80-23, revised 1996). All procedures were ratified by the Research and Ethics Committee of School of Medicine, Shahid Beheshti University of Medical Sciences (IR.SBMU.MSP.REC.1395.33), Tehran, Iran.
Drugs
In our research, methadone hydrochloride in the concentration of 5 mg/mL (Darou-Pakhsh Pharmaceutical Company, Tehran, Iran), and naloxone 0.4 mg/mL (Tolid-Darou Pharmaceutical Company, Tehran, Iran) were used.
Experimental Design
A single toxic dose of 15 mg/kg methadone was administered intraperitoneally (i.p.) to induce the acute methadone overdose. The selected dose of methadone (15 mg/kg) was equal to 80% of the median lethal dose (LD50) (Chevillard et al. 2009). Following methadone overdose, we observed signs of drowsiness, motionlessness, cold and clammy skin, plantar cyanosis, muscular rigidity, straub tail, irritability, and slow and difficult breathing.
Apnea which is defined as cessation of breathing for 20 s (Gaspari and Paydarfar 2007) was observed in almost 35% of all rats, as reported in our previous study (Ahmad-Molaei et al. 2018). Since it would lead to death if they remained untreated, the intervention was performed immediately following the apnea occurrence including simultaneous injection of 2 mg/kg naloxone (i.p.), and resuscitation procedure by using a respiratory pump (Farahmandfar et al. 2010; Zamani et al. 2015). To prevent the possible effects of hypoxia, the animals which did not respond to the resuscitation procedure were excluded from the study. These rats were classified as M/N-Apnea groups, which received naloxone after the occurrence of apnea. The rest of the animals recovered from several period of bradypnea after a few hours without any intervention and then got a regular respiration rate. These subjects were selected randomly as the methadone group. In another group of rats without apnea, 2 mg/kg naloxone was administered 10 min after methadone injection at the onset of sedation state, which was considered as M/N-Sedate group. Additionally, naloxone group was provided by the administration of 2 mg/kg naloxone, and in saline group, an equal volume of saline 0.9% was administered. In Y-Maze test, three separate saline control groups were considered for each time-point of M/N-Apnea groups (M/N-Apnea Day 1, M/N-Apnea Day 5, M/N-Apnea Day 10, Table 1).
Table 1.
The effects of acute toxic dose of methadone on spatial working memory in the Y-maze test which was recorded during an 8-min trial
| Groups | Alternation behavior (%) | Number of total (n) arm entries |
|---|---|---|
| Saline | 61.49 ± 3.28 | 15.5 ± 2.12 |
| M/N-sedate | 59.77 ± 2.92 | 14.66 ± 1.38 |
| Methadone | 52.62 ± 2.57 | 13.66 ± 1.30 |
| Naloxone | 57.11 ± 3.69 | 15.83 ± 0.6 |
| Saline control Day 1 | 65.13 ± 5.06 | 15.16 ± 2.18 |
| M/N-apnea Day 1 | 49.40 ± 7.61 | 13.8 ± 1.53 |
| Saline control Day 5 | 62.13 ± 4.64 | 15.0 ± 0.77 |
| M/N-apnea Day 5 | 47.61 ± 2.84* | 14.16 ± 1.13 |
| Saline control Day 10 | 64.26 ± 4.78 | 14.16 ± 060 |
| M/N-Apnea Day 10 | 44.61 ± 7.12* | 12.66 ± 2.95 |
Data represent mean ± SEM for six rats
M Methadone, N Naloxone
*P < 0.05 as compared with its respective saline control groups
The blood samples were collected after methadone overdose to measure the changes of the neurobiochemical factors. Also, the possible effect of acute methadone overdose on short-term memory (spontaneous alternation behavior) was assessed by a behavioral Y-maze test. Then, the brains were removed for histological studies.
Assessment of S100B, NSE, MBP, Lactate, and Pyruvate Factors
The blood samples were obtained by orbital sinus extraction procedure under the light ether anesthesia 6, 24, and 48 h (h) to measure S100B (Raabe et al. 2003), NSE (Woertgen et al. 2002), MBP (Kurumatani et al. 1998) and L/P ratio (Chen et al. 2000) after administration of methadone (n = 3 per time). Data obtained from biochemical factors assays were compared with saline group and other multiple comparison were performed as well. Blood samples were incubated at 37 °C to allow clot formation and centrifuged according to manual instruction, then stored at − 80 °C for approximately two months before assay analysis. The serum levels of S100B, NSE, MBP, Lactate, and Pyruvate factors were quantified with the double antibody sandwich ELISA method according to the manufacturer’s instructions by commercially available ELISA kits (S100B; MyBiosource, NSE; MyBiosource, MBP; Cloud-Clone, Pyruvate; Abcam, and Lactate; Sigma-Aldrich).
Spontaneous Alternation Behavior Test (Y-maze)
The Y-maze test is a behavioral evaluation of the spatial working memory and the tendency to explore new environments in rodents. It was performed on days 1, 5, or 10 after apnea occurrence in the M/N-Apnea groups and their respective saline control groups; and day 1 in the rest of the groups which were compared to saline group (n = 6). The Y-maze apparatus was made of a Plexiglass Y-shaped maze with three arms (14 × 40 × 35 cm) designed at 120° from each other. The rat was allowed to explore the maze for an 8-min trial freely. Rodents typically prefer to examine a less recently visited arm rather than returning to one that was visited before. Consecutive entries into the three different arms were considered as an alternation behavior. The number of total arm entries and alternations were manually recorded. The following equation defined the percentage: (number of alternation/total number of arm entries − 2) × 100. Each subject with ≤ 8 arm entries during an 8 min-test was excluded from the study (Ma et al. 2007; Farhadinasab et al. 2009).
Histological Staining
The histological evaluation was done on days 2, 5, or 10 after apnea experience in the M/N-Apnea groups or 48 h after the drug administration in the rest of the groups. The animals were deeply anesthetized with diethyl ether and transcardially perfused with 0.1 M phosphate-buffered saline (PBS, pH 7.4) and 4% paraformaldehyde (PFA) solution (Sigma-Aldrich). The brains were harvested, fixed in 4% PFA for 24 h, and then processed for paraffin embedding. 5-μm-thick coronal sections were cut approximately passing through hippocampus (from 2.92 to 3.36, posterior to bregma) and striatum (0.48 mm to 1.44 mm, caudate and putamen, posterior to bregma), to investigate CA1 pyramidal neurons in the hippocampus as well as neurons in caudate-putamen, respectively. In addition, serial coronal sections were cut through cerebellum to investigate purkinje cells.
The morphological changes in neurons and cell damage were assessed. Further, the number of intact neurons of the hippocampal CA1 region and the striatum were calculated in 6 brain sections of each rat, and 6 randomly selected subfields under a light microscope (magnification, ×400). Neurons with round and pale stained nuclei were known as live neurons, whereas shrunken neurons with pyknotic nuclei were defined as not surviving. Only neurons with an evident nucleolus were counted to prevent recounting the same neuron. Images were taken using a digital camera (DS-Ri2) and analyzed by two independent researchers, blinded to the groups.
Hematoxylin and Eosin (H&E) Staining
The tissues were hydrated in ethanol baths (100%, 95%, 70%) and water. Then, stained with Mayer’s hematoxylin for 3 min and rinsed quickly in the running tap water. Next, the sections were soaked in a 1% eosin solution for 2 min and followed by a rinse in tap water. Finally, they were dehydrated by passing through an increasing concentration of ethanol (95%, 100%), cleared in xylene, and mounted in Entellan (Cardiff et al. 2014).
Cresyl Violet (CV) Staining
Tissue sections were stained with cresyl violet acetate (Nissl staining) (Ahn et al. 2016). This stain identifies the somas of neurons in purple/dark blue. Briefly, the sections were dewaxed by xylene and hydrated with graded ethanol and water. The sections were immersed in 0.1% cresyl violet for 20–30 min at 60 °C and rinsed in tap water and dehydrated. The stained sections were covered with Entellan. Data were expressed as the number of surviving cells/field.
Additionally, luxol fast blue-cresyl violet staining was used to stain the myelin of cerebellar white matter blue to green. Following deparaffinization and hydration, sections were stained with luxol fast blue solution at 57 °C overnight. Then, sections were washed in ethanol and tap water. Lithium carbonate and 70% ethanol solution were used to distinguish the gray and white matter, respectively. Staining with cresyl violet solution was performed 5–10 min. Lastly, the sections were dehydrated in absolute alcohol, cleared in xylene, and mounted. The luxol fast blue stains myelin sheaths blue, while the cresyl violet counterstains the nissl substance violet (Wegener et al. 2006).
Statistical Analysis
All data were reported as Mean ± SEM (Standard error of mean), and analysis was performed by using commercially available software Graphpad Prism 5.0. Data analysis in biochemical factors measurements was done by two-way repeated ANOVA (analysis of variance) followed by the post hoc Bonferroni's test for multiple comparison. Behavioral data analyzed by independent samples Student 's t-test (when two groups were considered) or one-way ANOVA followed by Tukey's post hoc test for multiple comparisons. For statistical analysis of the number of live neurons, the Kruskal Wallis test used to compare the differences in appropriate groups. In addition, the statistical significance level was set at P < 0.05.
Results
The Effects of a Single Acute Toxic Dose of Methadone Administration on Serum Levels of S100B, NSE, MBP Factors, and Lactate/Pyruvate Ratio After 6, 24, and 48 h
Two-way repeated-measures ANOVA indicated significant main effects of time [F (2, 20) = 18.09; P < 0.001], treatment [F (4, 10) = 4.702; P = 0.021] and treatment × time interaction [F (8, 20) = 2.716; P < 0.033] on serum levels of S100B at the time-points of 6, 24, and 48 h after methadone overdose. As illustrated in Fig. 1, the mean serum S100B value was significantly higher (P < 0.001) in the M/N-Apnea group at 6 h (67.67 pg/ml) compared to the observed level in the saline group (19.61 pg/ml). On the other hand, the mean difference between M/N-Apnea and saline groups (MD = − 48.06, 95% CI − 79.85 to − 16.27, P < 0.001) was significant and it remained meaningfully elevated until 24 h (MD = − 33.31, 95% CI − 65.10 to − 1.522, P < 0.05). However, this value then declined toward normal amount at 48 h, (MD = 1.102, 95% CI − 30.69 to 32.89, ns) compared with the saline group. The mean serum levels of S100B in the Methadone (without apnea) (35.00 pg/ml) and M/N-Sedate (48.46 pg/ml) groups did not reveal significant increases at 6 h post-methadone overdose when compared to the saline group (MD = − 15.40, 95% CI − 47.18 to 16.39, ns and MD = − 28.86, 95% CI − 60.64 to 2.933, ns, respectively). In the Methadone group, the mean values of serum S100B showed a significant decrease at the 24 h (9.88 pg/ml) versus 6 h in the same animals (35.00 pg/ml) (MD = 25.11, 95% CI 2.217 to 48.01, P < 0.05). Similar finding was observed in the M/N-Sedate group regarding to the considerable time-dependent decrease occurred at 24 h (23.79 pg/ml) and 48 h (19.73 pg/ml) post-methadone overdose compared to 6 h in the same animals (48.46 pg/ml) (MD = 24.67, 95% CI 1.774 to 47.57, P < 0.05 and MD = 28.73, 95% CI 5.831 to 51.62, P < 0.05, respectively). In the M/N-Apnea group, higher levels of S100B were observed at 6 and 24 h compared with the Methadone group (without apnea) (P < 0.05 and P < 0.01, respectively).
Fig. 1.
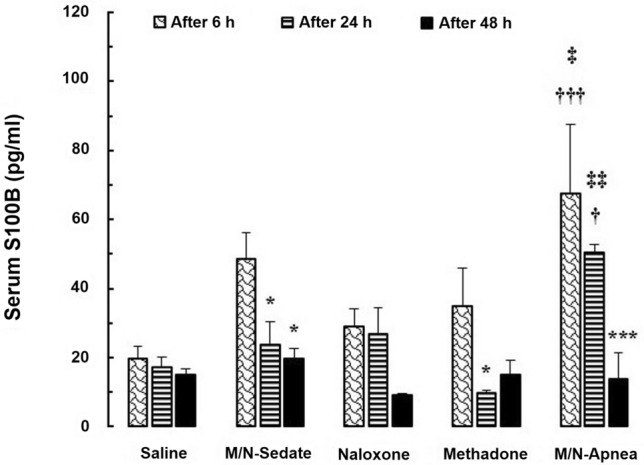
The changes of the serum S100B level in the treated and saline groups during the different time-points (6, 24, and 48 h) after methadone overdose (15 mg/kg; i.p.). In the M/N-Apnea group, a single dose of naloxone (2 mg/kg; i.p.) was administered following methadone overdose immediately after the occurrence of apnea. In the M/N-Sedate group, a single dose of naloxone (2 mg/kg; i.p.) was administered immediately in the initiation of sedation state following methadone overdose (10 min after methadone administration). In each saline, methadone and naloxone groups, saline, methadone, or naloxone were administered alone, respectively. Data presented as mean ± SEM from 3 rats per group. * P < 0.05; *** P < 0.001 as compared with the 6 h group in the same animals. †P < 0.05; †††P < 0.001 as compared with the saline group. ‡P < 0.05; ‡‡P < 0.01 as compared with the methadone group
Two-way repeated-measures ANOVA revealed the significant main effect of treatment [F (4, 10) = 3.432; P = 0.050] but not time or their interaction in the serum level of NSE in the different groups [F (2, 20) = 0.523; P = 0.600 and F (8, 20) = 1.114, P = 0.395, respectively, Fig. 2]. The mean serum value of NSE was higher in the M/N-Apnea group (0.597 µg/ml) when compared with the saline group (0.418 µg/ml) at 48 h post-methadone overdose (MD = − 0.178, 95% CI − 0.330 to − 0.027, P < 0.05).
Fig. 2.
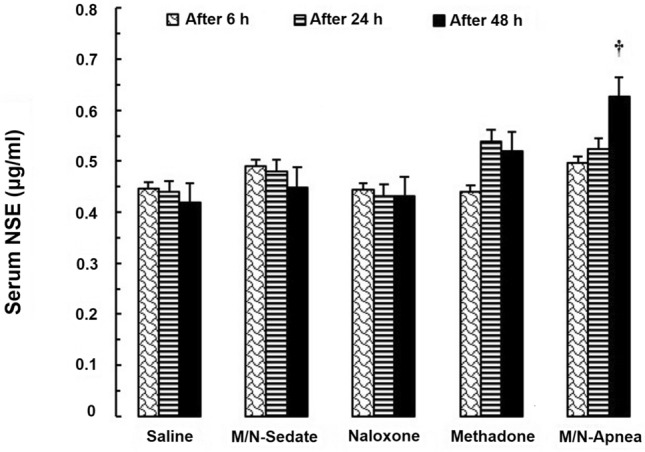
The changes of the serum NSE level in the treated and saline groups during the different time-points (6, 24, and 48 h) following methadone overdose (15 mg/kg; i.p.). In the M/N-Apnea group, a single dose of naloxone (2 mg/kg; i.p.) was administered following methadone overdose immediately after the occurrence of apnea. In the M/N-Sedate group, a single dose of naloxone (2 mg/kg; i.p.) was administered immediately in the initiation of sedation state following methadone overdose (10 min after methadone administration). In each saline, methadone and naloxone groups, saline, methadone, or naloxone were administered alone, respectively. Data presented as mean ± SEM from 3 rats per group. †P < 0.05 as compared with the saline group
Two-way repeated-measures ANOVA indicated significant differences in the main effects of time [F (2, 20) = 4.02; P = 0.034] and treatment [F (4, 10) = 5.135; P = 0.016] but not time × treatment interaction [F (8, 20) = 0.427; P = 0.890] in the serum level of MBP in each treated groups. Moreover, Bonferroni post hoc test revealed that there was not any remarkable alteration on 6, 24, and 48 h in MBP levels among treated groups (Fig. 3).
Fig. 3.
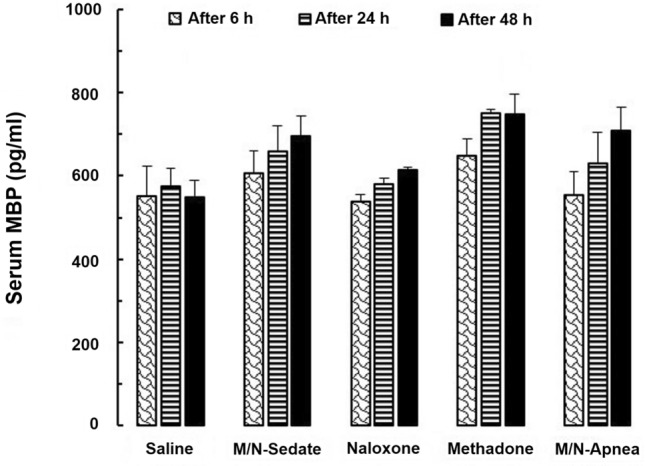
The changes in the serum MBP level in the treated and saline groups during the different time-points (6, 24, and 48 h) following methadone overdose (15 mg/kg; i.p.). In the M/N-Apnea group, a single dose of naloxone (2 mg/kg; i.p.) was administered following methadone overdose immediately after the occurrence of apnea. In the M/N-Sedate group, a single dose of naloxone (2 mg/kg; i.p.) was administered immediately in the initiation of sedation state following methadone overdose (10 min after methadone administration). In each saline, methadone and naloxone groups, saline, methadone, or naloxone were administered alone, respectively. Data presented as mean ± SEM from 3 rats per group
Two-way repeated-measures ANOVA indicated significant effects of time [F (2, 20) = 9.01; P = 0.001] and interaction of time × treatment [F (8, 20) = 5.065; P = 0.001] but not the treatment [F (4, 10) = 1.977; P = 0.174] on the serum value of the L/P ratio in different groups at time-points of 6, 24 and 48 h post-methadone administration. According to the Bonferroni post hoc test, the serum level of L/P ratio rose significantly, peaking at 6 h after methadone overdose in the M/N-Apnea (1.170) and methadone (1.183) groups compared to the saline group (0.4351) (MD = − 0.735, 95% CI − 1.282 to − 0.188, P < 0.01 and MD = − 0.747, 95% CI − 1.294 to − 0.200, P < 0.01, respectively, Fig. 4). Then, the ratio of L/P was significantly decreased at the time-points of 24 and 48 h versus 6 h in the same animals in the methadone (P < 0.001, P < 0.001, respectively) and the M/N-Apnea (P < 0.001, P < 0.001, respectively) groups. Notably, we found that the serum levels of L/P ratio at 6 h post-methadone overdose was higher in the M/N-Apnea (1.170) and the methadone (1.183) groups compared with the M/N-Sedate group (0.364) (MD = − 0.806, 95% CI − 1.353 to − 0.259, P < 0.01 and MD = − 0.818, 95% CI − 1.365 to − 0.271, P < 0.001, respectively).
Fig. 4.
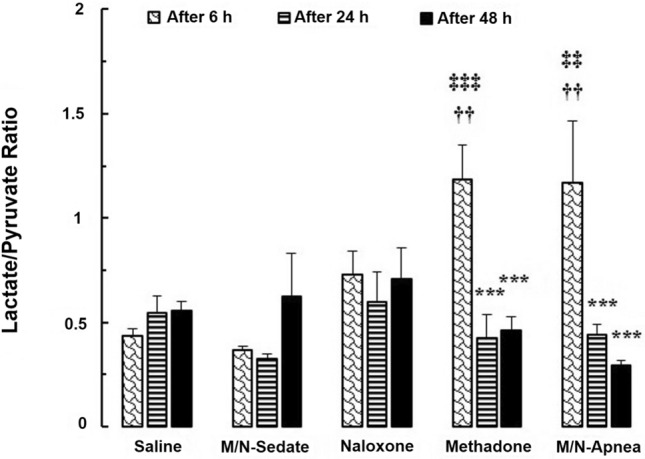
The changes of the L/P ratio in the treated and saline groups during the different time-points (6, 24, and 48 h) following methadone overdose (15 mg/kg; i.p.). In the M/N-Apnea group, a single dose of naloxone (2 mg/kg; i.p.) was administered immediately after the occurrence of apnea following the methadone overdose. In the M/N-Sedate group, a single dose of naloxone (2 mg/kg; i.p.) was administered immediately in the initiation of sedation state following methadone overdose (10 min after methadone administration). In each saline, methadone and naloxone groups, saline, methadone, or naloxone were administered alone, respectively. Data presented as mean ± SEM from 3 rats per group. *** P < 0.001 as compared with the 6 h group in the same animals. ††P < 0.01 as compared with the saline group. ‡‡P < 0.01; ‡‡‡P < 0.001 as compared with the M/N-Sedate group
The Effects of a Single Acute Toxic Dose of Methadone Administration on the Spatial Working Memory in the Y-maze Test
The spontaneous alternation behavior was studied to evaluate the spatial working memory. An unpaired t-test analysis showed that the percentage of spontaneous alternation behavior was not significantly impaired in the M/N-Apnea group on day one after apnea occurrence when compared with its respective saline control group [t (10) = 1.720, P = 0.116, Table 1]. However, the percentage of spontaneous alternation decreased in the M/N-Apnea group on days 5 [t (10) = 2.666, P = 0.023] and 10 [t (10) = 2.289, P = 0.045] after occurrence of apnea, compared with their respective saline control groups. One-way ANOVA followed by Tukey's post hoc analysis shown that the alternation behavior did not significantly decrease in the M/N-sedate, methadone, or naloxone groups (Table 1) compared to the saline group [F (6, 35) = 1.841; P = 0.119].
The number of total arm entries was also measured (Table 1). The unpaired t-test analysis revealed that the number of arm entries was not significantly different in the M/N-Apnea groups on days 1 [t (10) = 0.499, P = 0.628; ns], 5 [t (10) = 0.605, P = 0.558; ns] or 10 [t (10) = 0.656, P = 0.526; ns] post-apnea occurrence as compared with their respective saline control groups (Table 1). Similarly, One-way ANOVA followed by Tukey's post hoc test revealed that there was no significant decrease in the number of total arm entries in the M/N-sedate, methadone, or naloxone groups (Table 1) when compared with the saline group [F (6, 35) = 0.403, P = 0.871].
The Effects of a Single Acute Toxic Dose of Methadone Administration on the Histological Changes of the Brain Tissue
Hematoxylin and Eosin Staining
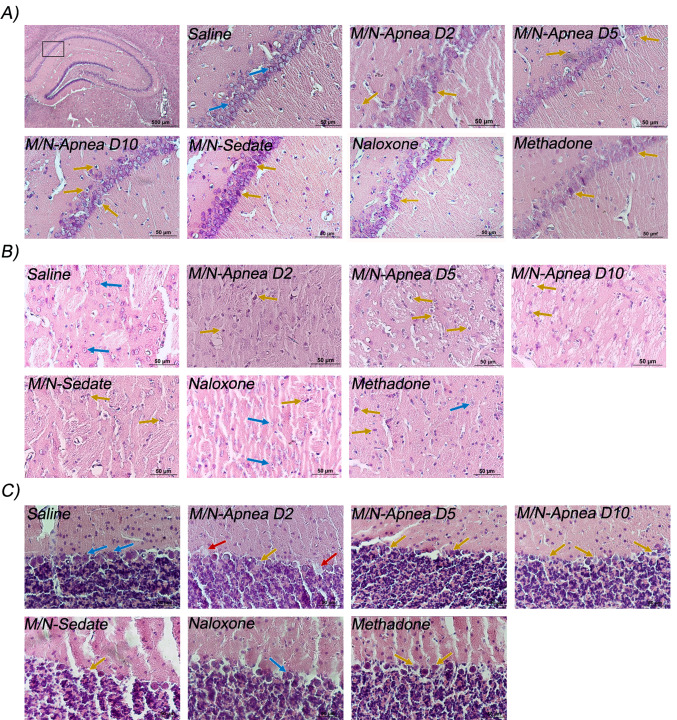
Animals in the saline group exhibited normal neurons with intact cell structures, and nuclear membrane integrity in the hippocampal CA1 region. Signs of early neuronal injury and mild cytoplasmic swelling were distinct in the neurons of the M/N-Apnea group on day 2. Further degenerative changes were observed on day 5 in the M/N-Apnea group, and the nuclei of some neurons were pyknotic. On day 10, neurons were shrunken with irregular morphology and condensed and darker nuclei. In the M/N-Sedate group, neurons were exhibited marked damage and irregular shapes in the CA1 region compared to the saline group. Swelling, degeneration, and necrosis were found in the neuronal cells of the methadone group. In the Naloxone-treated rats, CA1 neurons showed a darker cytoplasm in some cells (Fig. 5a).
Fig. 5.
Representative pictures of H&E staining of the CA1, striatum, and cerebellum regions following methadone overdose (15 mg/kg; i.p.). In the M/N-Apnea group, a single dose of naloxone (2 mg/kg; i.p.) was administered immediately after the occurrence of apnea following the methadone overdose and evaluated on days 2, 5, or 10 after apnea (D2, D5, D10). In the M/N-Sedate group, a single dose of naloxone (2 mg/kg; i.p.) was administered immediately in the initiation of sedation state (10 min after methadone administration) following the methadone overdose. In each saline, methadone and naloxone groups, saline, methadone, or naloxone were administered alone, respectively. a hippocampal CA1 region, b Striatum, and c Cerebellum. Normal neurons (blue arrows), damaged neurons (brown arrows), and Purkinje cell loss (red arrows) were indicated. Scale bar = 50 μm. Data represent mean ± SEM for 4 rats. Scale bar = 50 μm
Histological analysis of the striatum showed that in the saline group, neurons are intact with normal contour, and clear stained nuclei and cytoplasm. Extensive morphological alterations were detectable in the M/N-Apnea groups. On days 2 and 5, nuclei and cytoplasm were not distinguishable as a result of neuronal loss. Necrotic changes were detected on day 10. In the M/N-Sedate group, the neurons were shrunken, and no nucleolus was apparent. Neurons of the methadone group seemed deformed and shrunken. In the Naloxone group, neurons showed normal morphology. (Fig. 5b).
The neurons of the cerebellum tissue in the saline group were morphologically normal, which were arranged uniformly and compactly. In the M/N-Apnea, M/N-sedate, and methadone groups, the neurons were mostly distorted, angulated, wrinkled, and leaving vacuoles in intercellular spaces. After methadone overdose on days 2 and 5 in the M/N-Apnea groups, the tissue damage was significantly aggravated. At day 10, the injury of the cerebellum tissue was severer, and degenerative and necrotic changes were detected in Purkinje cells (Fig. 5c).
Cresyl Violet Staining
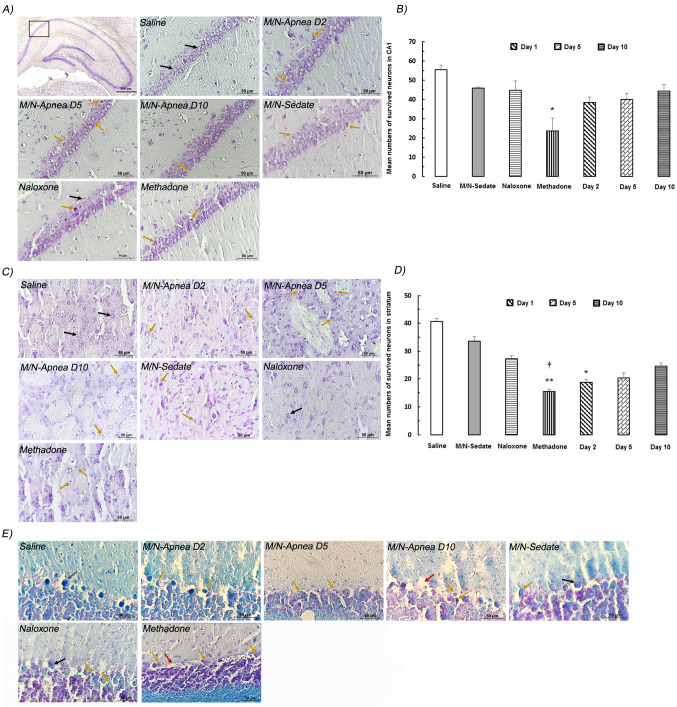
As it can be inferred from Fig. 6, the live neurons in the CA1 and striatum regions were quantified after methadone overdose. In the CA1 area of the rat in the saline group, typical CV positive cells were easily found. Statistical analysis indicated a major dead/dying pattern in the methadone group compared with the saline group (P = 0.018, Fig. 6a, b).
Fig. 6.
Representative pictures of Nissl staining of the CA1, striatum, and cerebellum regions following methadone overdose (15 mg/kg; i.p.). In the M/N-Apnea group, a single dose of naloxone (2 mg/kg; i.p.) was administered immediately after the occurrence of apnea following the methadone overdose and evaluated on days 2, 5, or 10 after apnea (D2, D5, D10). In the M/N-Sedate group, a single dose of naloxone (2 mg/kg; i.p.) was administered immediately in the initiation of sedation state (10 min after methadone administration) following the methadone overdose. In each saline, methadone and naloxone groups, saline, methadone, or naloxone were administered alone, respectively. a, b represents the hippocampal CA1 region and the mean numbers of the survived neurons in groups. c, d depicts the striatum region and the mean numbers of the live neurons in the striatum area. d is the luxol fast blue-cresyl violet staining of the cerebellum. Normal neurons (black arrows), damaged neurons (brown arrows), and Purkinje cell loss (red arrows) were indicated. Data represent mean ± SEM for 4 rats. Scale bar = 50 μm. *P < 0.05; **P < 0.001 as compared with the saline group. †P < 0.05 as compared with the M/N-Sedate group
In the striatum, neurons of the saline group were mostly alive and well-stained with CV. Live neurons were significantly decreased in the striatum of the methadone (P < 0.01) and M/N-Apnea on day 2 (P < 0.05) groups as compared to the saline group. Furthermore, the reduction in the number of live neuronal cells per view was also most pronounced in the methadone group versus M/N-Sedate group (P < 0.05, Fig. 6c, d).
By luxol fast blue-cresyl violet staining, degeneration of Purkinje cells and the molecular layer was found in the M/N-Apnea group on days 5 and 10, and the methadone group without apnea (Fig. 6e).
Discussion
The goal of the current experiment was to evaluate the brain injury through Y-maze test, histological evaluation, and biochemical studies of some neurobiochemical factors in the blood at the early hours following the acute overdose of methadone in adolescent male rats. The main findings demonstrated that (1) Serum levels of S100B increased at 6 and 24 h in the rats which experienced apnea after acute methadone overdose, (2) NSE marker was elevated with delay at 48 h post-acute methadone overdose in the rats-experienced Apnea, (3) MBP levels did not change significantly up to 48 h post-acute methadone overdose, (4) At 6 h post-acute methadone overdose L/P ratio increased in the M/N-Apnea and methadone groups, (5) The alternation behavior but not the number of entries was impaired in the Y-maze test in the M/N-Apnea groups after administration of an acute toxic dose of methadone, (6) The extended morphological changes were obvious in the CA1, striatum, and cerebellum regions in some days post-methadone overdose, (7) Neuronal death in the CA1 and striatum areas occurred on day 2 in the M/N-Apnea and methadone groups.
Methadone acts as a mu-opioid receptor agonist and an N-methyl-d-aspartate receptor (NMDA) antagonist (Doi et al. 2016). Mu-opioid receptors are mostly located in the limbic system and the cerebellum (Li et al. 2016) and contribute to the effects of opioids via facilitating long-term potentiation (Klenowski et al. 2015). The hippocampus, basal ganglia, and globous pallidus seem particularly susceptible to hypoxia, by leading to short-term memory loss and changes in cognitive and physical functioning (Barash et al. 2017; Betts et al. 2012). Hypoxia-related neurological consequences include seizures, nerve damage, temporary motor paralysis, and stroke which have been reported using fentanyl, heroine or morphine (Somerville et al. 2017; Betts et al. 2012). Further, respiratory depression is a primary complication induced by opioid overdose (King et al. 2015) via the activation of the mu-opioid receptors which are expressed on the neurons located in respiratory centers of brainstem (Chevillard et al. 2009). It could return to normal breathing activity either on its own or with the use of an opioid overdose reversal medication (Wermeling 2015). Naloxone, a short-acting opioid receptor antagonist, acts as a competitive antagonist at all three opioid receptors (mu, kappa and delta) and antagonizes respiratory and CNS depression caused by opiates (van Dorp et al. 2007). Availability of mu-opioid receptors in multiple brain regions are affected by sex or age-related differences (Kantonen et al. 2020), could result in individual differences in response to methadone (Kantonen et al. 2020. Methadone is principally metabolized by cytochrome P450 3A4 (CYP3A4), CYP2B6, CYP2C19 and to a lesser extent, by CYP2C9 and CYP2D6 (Villegas and Thielet 2020). Although developmental processes can influence drug disposition, it has been described that clearance of methadone in neonates and children is similar to adults (Sharma et al. 2011; Thigpen et al. 2019). Additionally, several number of studies have reported the impact of sex-related variabilities in metabolism rates of methadone. Therefore, there might be some possible factors influencing both therapeutic or toxic doses of methadone between individuals especially in patients who take the methadone for the first time (Laycock and Bantel 2019). In this regard, more prevalence of methadone poisoning has been reported in boys under 4 years old (Manouchehrifar et al. 2016). In contrast, a previous study on children under 18 years old indicated no sex differences in the outcomes or subsequent complications related to methadone toxicity (Atighi et al. 2018). It has been demonstrated that in subjects of 10–14 years of age, concomitant use of drugs, history of addiction in family or psychiatric disease as well as female gender are several risk factors which might cause increasing incidence of methadone poisoning and probability of intentional overdose (Manouchehrifar et al. 2016).
In recent years, brain-specific proteins have been widely investigated as global tools for prognostic evaluation of structural brain damage in adults (Thelin et al. 2016) and children (Žurek and Fedora 2012). S100B, a calcium-binding protein, is physiologically produced and released principally by astrocytes (Gonçalves et al. 2008) but its serum level rises in CNS disorders such as cerebral ischemia and injury (Yilmaz et al. 2018). The S100B was detected in the injured cardiomyocytes of the subjects who died from the drug overdose, while it was not found in the heart of subjects who died of other causes (Faa et al. 2012). Also, patients with acute benzodiazepine overdose showed an increased level of S100B (Ambrožič et al. 2008). Our findings revealed higher level of S100B at 6 h in rats of M/N-Apnea group while reaching to baseline at 48 h which was significantly associated with methadone intoxication. It was probably indicating damage to the astrocytes (Abdul-Khaliq et al. 2000), disruption of blood–brain barrier (BBB) or functional secretion of S100B by astrocytes after methadone poisoning (Pinto et al. 2000). Higher level of S100B in M/N-Apnea group possibly has occurred due to cessation of respiration. However, significant increase of S100B level was observed in the M/N-Sedate group which did not experience hypoxia. It might partly happen due to overstimulation of opioid receptors, which would lead to cellular energy loss. Other clinical and experimental studies described kinetic of S100B after different models of brain injury with the peak around 27 (Ercole et al. 2016) and 6 h (Tanaka et al. 2009), respectively. Additionally, the concentration of S100B increased after heroin overdose in adult patients at emergency department (Brvar et al. 2005). It has been reported that S100B at high concentration activates inflammatory processes in astrocytes and promotes microglia migration participating in inflammatory responses (Kozlyuk et al. 2019). Meanwhile, previous studies have shown that mean serum level of S100B is an age-dependent factor with higher concentration under 3-year-old children (Bouvier et al. 2011). In addition, conflicting data of literatures have been described sex-related effects on serum S100B levels in pediatrics (Bouvier et al. 2011; Gazzolo et al. 2003). Therefore, age-and sex-matched control patients may be required to evaluate distinct profile patterns of biochemical factors to appropriately compare differences.
NSE is a dimeric γ-isoenzyme in the axoplasm of the neurons, which is involved in the glycolysis pathway (Haque et al. 2018) and upregulates when axons are damaged (Wu et al. 2004). NSE has not been extensively studied in the context of an opioid overdose (Tramullas et al. 2007). Higher levels of NSE have been reported in severe traumatic brain injury (TBI; Shahim et al. 2014; Böhmer et al. 2011) and stroke patients compared with the healthy group (González-García et al. 2012). According to our results, higher serum NSE levels were detected only in M/N-Apnea group at 48 h, indicating possible neuronal damage with a delay in adolescent male rats. We did not find any association between NSE serum levels in different time-points during the study period. On the other hand, the current findings showed significant neuronal loss in CA1 and striatum. In line with our result, NSE level in CSF was increased with a delay peak at 48 h post-TBI in children, and lower NSE concentration was correlated with better clinical outcomes (Chiaretti et al. 2009). On the other hand, age dependency of NSE serum level has been previously reported with higher baseline NSE level in 1-week-old rat pups than maturated adult rats (Sankar et al. 1997), so further investigation is needed with large-scale studies in older pups and aged matched controls to obtain accurate reference of NSE values.
Impairment of white matter integrity has been reported in adult patients and children with acute overdose or chronic methadone maintenance treatment (Haghighi-Morad et al. 2020). Chronic hypoxia may contribute to the methadone-related demyelination, impairment of the axon, and altered MBP level (Li et al. 2016). Our results showed a non-significant but increasing pattern of MBP during a period of 48-h assessment. Possibly, the white matter integrity disruption occurred slowly (Fan et al. 2018) and could not be detected from the early time-points following the acute methadone overdose. Therefore, evaluating the extended time-course of MBP levels might be needed to reveal the alteration of myelination processes to anticipate possible white matter impairments (Fan et al. 2018). Furthermore, several reports have described white matter disruption following the acute methadone overdose in children (Anselmo et al. 2006; Salgado et al. 2010; Reisner et al. 2015) or a chronic consumption of opioids (Li et al. 2016). Regardless of the dose, it has been shown that exposure to opioid can alter normal early or late myelination processes in neonates or adolescent rats (Vestal-Laborde et al. 2014). Apart from MBP alteration, axonal injury might be exist in white matter structural changes due to methadone toxicity as well. Then, further research is needed to assess the specific indicator of axonal changes following methadone intoxication (Li et al. 2016). The findings of the current study argue against MBP and white matter damage in the early hours post-methadone overdose.
Cerebral hypoxia causes changes of the anaerobic metabolism and raises the L/P ratio. L/P ratio is a consistent indicator of anaerobic metabolism associated with unfavorable outcomes and mortality during the first 72 h of monitoring in adult patients with TBI (Timofeev et al. 2011). In our current study, there were significant elevation of L/P ratio both in methadone and M/N-Apnea groups with highest level at 6 h while it reached to baseline at 48 h, therefore, it could indicate metabolic impairment at early hours after methadone poisoning. It was concluded as the high glycolytic activity occurring in the hypoxia or mitochondrial dysfunction (Timofeev et al. 2011). Further, measurement of pyruvate level may be used to discriminate the contribution of anerobic/hypoxic condition to the increased level of L/P ratio (Sahuquillo et al. 2014). Additionally, it has been reported that elevation of lactate concentration in a continuous mode is significantly associated with worse prognosis and even fatalities after acute opioid overdose in adult patients, but in children this relation has not been elucidated yet (Fox et al. 2018; Wardi et al. 2020). Likewise, early lactate clearance in opioid drug overdose patients has been associated with better outcome in emergency department (Cheung et al. 2018). Altogether, there are very limited research focused on the characterizing the specific biomarkers of brain injury following acute opioid overdose in adults or children. In addition, further studies are needed to investigate the correlation between the aforementioned biochemical factors at the early hours following methadone intoxication and neurological outcomes to provide prognostic information.
Recently, the deficits of working memory have been reported following methadone maintenance therapy (Rapeli et al. 2009). In our study, impaired alternation behavior was observed on days 5 and 10 after methadone overdose with apnea occurrence. These findings are in agreement with the results of our previous study, which revealed the impaired cognitive function after acute methadone overdose (Ahmad-Molaei et al. 2018). It has been suggested that one mechanism contributing to memory impairment is in part due to antagonist properties of methadone on NMDA receptors (Finke et al. 2011; Hepner et al. 2002). In line with our results, administration of toxic dose of methadone in animal model significantly impaired the spatial memory in Morris water maze (Hepner et al. 2002) suggesting the possible decreasing level of BDNF protein may impair learning and memory (Nouri et al. 2019). There is evidence that learning of spatial task in 30-day-old mice is physiologicaly lower than adult which may be due to differences in protein signaling between weaning and adulthood (McCormick and Mathews 2010). On the other hand, the influence of gonadal hormones on alterations of spine density in the CA1 region are noticeable in female in proestrus cycle which may partly contribute to sex differences on the regulation of physiology and molecular signaling (Hyer et al. 2018).
Studies have come to the conclusion that the limbic system, cerebellum (Schadrack et al. 1999), and the CA1 region (Lupica et al. 1992) contain the great density of opioid receptors. It seems that overactivation of opioid receptors in these regions, in addition to their high sensitivity to hypoxia (Cervos-Navarro and Diemer 1991) implicated in the toxicological effects of methadone overdose. On the other hand, methadone cross the BBB and reach to the frontal cortex, cerebellum, basal ganglia, and hippocampus (Wehner et al. 2000; Haghighi-Morad et al. 2020). The current morphological results showed altered neurons with the apoptotic features such as apoptotic bodies and impaired survival, chromatin condensation, and neuronal pyknosis in the CA1, striatum, and cerebellum regions of the rats in the M/N-Apnea groups. According to the morphological changes of neurons and significant reduction of live neurons in the methadone and M/N-Sedate groups, this possibility is raised that the toxic effects of methadone have an obvious role along with its side effects including respiratory depression in neurological complications following methadone overdose.
Prediction of outcomes is questionable following opioid/methadone poisoning likely due to a broad range of neurological impairments in adult and especially in children. The relationship between dose of methadone and efficacy or toxicity is complicated regarding to genetic polymorphism, difference of the opioid receptors availability, various metabolism and elimination. On the other hand, there is not a single test to detect ongoing brain damage. Invasive procedures or doing repeated brain computed tomography (CT) can be difficult or being incursive for pediatrics in comparison with blood sampling by calculating the wide range of biomarkers. It also provides valuable information for accurate diagnostic assessment of clinical outcomes to anticipate possible brain damage following opioid intoxications. Therefore, it would be favaorable by designating diagnostic tools to develop reference values for serum biochemical factors indicating brain injury in patients who are at risks of secondary neurological impairments.
Despite the several limitation, this is the first study measured some biochemical factors in which the elevation of S100B and NSE markers, as well as L/P ratio were observed following methadone overdose and subsequent apnea in male adolescent rats. Immunohistochemical approaches are required to reveal the presence of apoptosis-related proteins in different brain areas including hippocampus, prefrontal cortex, cerebellum, and globus pallidus. Thus, regarding the high incidence of methadone toxicity, especially in children, future studies must be done to differentiate direct (apoptosis-related to opioid exposure) and indirect effects (hypoxia in the brain) of methadone induced-toxicity. More evaluation is also needed to compare the outcomes of methadone overdose on a larger sample size cross gender as well as in different developmental stages to track the influence of both sex and developmental changes in the vulnerability to methadone-related intoxication. Likewise, further follow-up with extended time-points is essential to assess the relationship between the alterations of the aforementioned biochemical factors and long-term cognitive deficits following the acute methadone overdose in adolescent rats.
Acknowledgment
This study was carried out as a part of the Ph.D. thesis and supported by funding from the School of Medicine, Shahid Beheshti University of Medical Sciences (Grant No. 70), Tehran, Iran. This work was also supported by the Vice-Chancellor for Research & Technology of Shahid Beheshti University of Medical Sciences (Grant No. 98-21486-1398-11-29). The authors extend appreciation to the Neuroscience Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran for valuable cooperation.
Author Contributions
Abbas Haghparast was responsible for the study concept and design. Leila Ahmad-Molaei and Mahsa Pourhamzeh conducted the working memory test, histological and molecular studies. Abbas Haghparast, Fariba Khodagholi, Reza Ahadi, Hassanian-Moghaddam and Leila Ahmad-Molaei assisted with data analysis and interpretation of findings. Mahsa Pourhamzeh and Leila Ahmad-Molaei drafted the manuscript. All authors critically revised and approved final version for publication.
Funding
This study was supported by funding from the School of Medicine, Shahid Beheshti University of Medical Sciences (Grant No. 98-21486-1398-11-29), Tehran, Iran. This funding source had no role in the design of this study, execution, analysis, and interpretation of the data; in the writing of the report; and in the decision to submit the paper for publication.
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdel-Salam OM, Sleem AA, Youness ER, Omara EA (2019) Identification of biomarkers for the detection of subtle brain injury after cannabis and/or tramadol administration. Egyp J Foren Sci 9(1):58. 10.1186/s41935-019-0165-z [Google Scholar]
- Abdul-Khaliq H, Schubert S, Stoltenburg-Didinger G et al (2000) Protein S-100β in brain and serum after deep hypothermic circulatory arrest in rabbits: relationship to perivascular astrocytic swelling. Clin Chem Lab Med 38:1169–1172. 10.1515/CCLM.2000.180 [DOI] [PubMed] [Google Scholar]
- Abdul-Muneer PM, Chandra N, Haorah J (2015) Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol Neurobiol 51(3):966–979. 10.1007/s12035-014-8752-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad-Molaei L, Hassnian-Moghaddam H, Farnaghi F et al (2018) Delay-dependent impairments in memory and motor functions after acute methadone overdose in rats. Front Pharmacol 9:1023. 10.3389/fphar.2018.01023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn JH, Shin BN, Park JH et al (2016) Long-term observation of neuronal degeneration and microgliosis in the gerbil dentate gyrus after transient cerebral ischemia. J Neurol Sci 363:21–26. 10.1016/j.jns.2016.02.015 [DOI] [PubMed] [Google Scholar]
- Allameh Y, Akrami FS, Mohammadi G, Molavi N, Babakhanian M (2017) Methadone poisoning in children: a systematic review and meta-analysis in Iran. J Pediatr Rev 5(2):1–8. 10.5812/jpr.10133 [Google Scholar]
- Alotaibi N, Sammons H, Choonara I (2012) Methadone toxicity in children. Arch Dis Child 97(5):e1–e1. 10.1136/archdischild-2012-301728.2 [Google Scholar]
- Ambrožič J, Bunc M, Osredkar J et al (2008) S100B protein in benzodiazepine overdose. Emerg Med J 25:90–92. 10.1136/emj.2006.044222 [DOI] [PubMed] [Google Scholar]
- Anselmo M, Rainho AC, do Carmo Vale M, Estrada J, Valente R, Correia M et al (2006) Methadone intoxication in a child: toxic encephalopathy? J Child Neurol 21(7):618–620. 10.1177/08830738060210071101 [DOI] [PubMed] [Google Scholar]
- Atighi Y, Eizadi-Mood N, Mansourian M, Zamani A, Saffaei A, Sabzghabaee AM (2018) Predictive factors of treatment outcomes for hospital care in children with acute methadone poisoning. J Res Pharmacy Pract 7(4):200. 10.4103/jrpp.JRPP_16_141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barash JA, Somerville N, DeMaria A (2017) Cluster of an unusual amnestic syndrome—Massachusetts. MMWR Morbid Mortal Weekly Rep 66(3):76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts AM, Ritter JL, Kubal WS (2012) Reversible delayed posthypoxic leukoencephalopathy after drug overdose: MRI findings in a collection of patients. Emerg Radiol 19(2):165–173. 10.1007/s10140-011-1013- [DOI] [PubMed] [Google Scholar]
- Bouvier D, Castellani C, Fournier M, Dauphin JB, Ughetto S, Breton M et al (2011) Reference ranges for serum S100B protein during the first three years of life. Clin Biochem 44(10–11):927–929. 10.1016/j.clinbiochem.2011.05.004 [DOI] [PubMed] [Google Scholar]
- Böhmer AE, Oses JP, Schmidt AP et al (2011) Neuron-specific enolase, S100B, and glial fibrillary acidic protein levels as outcome predictors in patients with severe traumatic brain injury. Neurosurgery 68:1624–1631. 10.1227/NEU.0b013e318214a81f [DOI] [PubMed] [Google Scholar]
- Brvar M, Ambrozic J, Osredkar J, Mozina M, Bunc M (2005) S100B protein in heroin overdose: a pilot study. Crit Care 9(1):1–1. 10.1186/cc3353 [Google Scholar]
- Brouns R, De Vil B, Cras P et al (2010) Neurobiochemical markers of brain damage in cerebrospinal fluid of acute ischemic stroke patients. Clin Chem 56:451–458. 10.1373/clinchem.2009.134122 [DOI] [PubMed] [Google Scholar]
- Bunten H, Liang WJ, Pounder DJ, Seneviratne C, Osselton D (2011) Interindividual variability in the prevalence of OPRM1 and CYP2B6 gene variations may identify drug-susceptible populations. J Anal Toxicol 35(7):431–437. 10.1093/anatox/35.7.431 [DOI] [PubMed] [Google Scholar]
- Busnello JV, Leke R, Oses JP et al (2006) Acute and chronic electroconvulsive shock in rats: effects on peripheral markers of neuronal injury and glial activity. J Neuroinflammation 78:3013–3017. 10.1016/j.lfs.2005.11.028 [DOI] [PubMed] [Google Scholar]
- Cardiff RD, Miller CH, Munn RJ (2014) Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb Proto. 10.1101/pdb.prot073411 [DOI] [PubMed] [Google Scholar]
- Cervos-Navarro J, Diemer NH (1991) Selective vulnerability in brain hypoxia. Crit Rev Neurobiol 6:149–182 [PubMed] [Google Scholar]
- Chen C-J, Cheng F-C, Liao S-L et al (2000) Effects of naloxone on lactate, pyruvate metabolism and antioxidant enzyme activity in rat cerebral ischemia/reperfusion. Neurosci Lett 287:113–116. 10.1016/S0304-3940(00)01151-4 [DOI] [PubMed] [Google Scholar]
- Cheung R, Hoffman RS, Vlahov D, Manini AF (2018) Prognostic utility of initial lactate in patients with acute drug overdose: a validation cohort. Ann Emerg Med 72(1):16–23. 10.1016/j.annemergmed.2018.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevillard L, Mégarbane B, Risède P et al (2009) Characteristics and comparative severity of respiratory response to toxic doses of fentanyl, methadone, morphine, and buprenorphine in rats. Toxicol Lett 191:327–340. 10.1016/j.toxlet.2009.09.017 [DOI] [PubMed] [Google Scholar]
- Chiaretti A, Barone G, Riccardi R, Antonelli A, Pezzotti P, Genovese O et al (2009) NGF, DCX, and NSE upregulation correlates with severity and outcome of head trauma in children. Neurology 72(7):609–616. 10.1212/01.wnl.0000342462.51073.06 [DOI] [PubMed] [Google Scholar]
- Corkery JM, Schifano F, Ghodse AH et al (2004) The effects of methadone and its role in fatalities. Hum Psychopharmacol 19:565–576. 10.1002/hup.630 [DOI] [PubMed] [Google Scholar]
- Cummins E, Allen CP, Ricchetti A et al (2012) The effects of acute and chronic steady state methadone on memory retrieval in rats. Psychopharmacology 222:225–235. 10.1007/s00213-012-2638-8 [DOI] [PubMed] [Google Scholar]
- Darke S, Sims J, McDonald S et al (2000) Cognitive impairment among methadone maintenance patients. J Urban Health 95:687–695. 10.1046/j.1360-0443.2000.9556874.x [DOI] [PubMed] [Google Scholar]
- Doi S, Mori T, Uzawa N et al (2016) Characterization of methadone as a β-arrestin-biased μ-opioid receptor agonist. Mol Pain 12:1744806916654146. 10.1177/1744806916654146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eap CB, Buclin T, Baumann P (2002) Interindividual variability of the clinical pharmacokinetics of methadone. Clin Pharmacokinet 41(14):1153–1193. 10.2165/00003088-200241140-00003 [DOI] [PubMed] [Google Scholar]
- Ercole A, Thelin EP, Holst A, Bellander BM, Nelson DW (2016) Kinetic modelling of serum S100b after traumatic brain injury. BMC Neurol 16(1):1–8. 10.1186/s12883-016-0614-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faa A, Senes G, Locci A et al (2012) S100B protein expression in the heart of deceased individuals by overdose: a new forensic marker? Clinics (Sao Paulo) 67:821–826. 10.6061/clinics/2012(07)19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan R, Schrott LM, Arnold T, Snelling S, Rao M, Graham D et al (2018) Chronic oxycodone induces axonal degeneration in rat brain. BMC Neurosci 19(1):15. 10.1186/s12868-018-0417-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahmandfar M, Karimian SM, Naghdi N et al (2010) Morphine-induced impairment of spatial memory acquisition reversed by morphine sensitization in rats. Behav Brain Res 211:156–163. 10.1016/j.bbr.2010.03.013 [DOI] [PubMed] [Google Scholar]
- Farhadinasab A, Shahidi S, Najafi A et al (2009) Role of naloxone as an exogenous opioid receptor antagonist in spatial learning and memory of female rats during the estrous cycle. Brain Res 1257:65–74. 10.1016/j.brainres.2008.12.043 [DOI] [PubMed] [Google Scholar]
- Farid W, Dunlop S, Tait R et al (2008) The effects of maternally administered methadone, buprenorphine and naltrexone on offspring: review of human and animal data. Curr Neuropharmacol 6:125–150. 10.2174/157015908784533842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finke C, Kopp UA, Prüss H (2011) Cognitive deficits following anti-NMDA receptor encephalitis. Neurol Neurosurg Psychatry 83:195–198. 10.1136/jnnp-2011-300411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DJ, Somogyi AA, Dyer KR et al (2000) Steady-state pharmacokinetics of (R)-and (S)-methadone in methadone maintenance patients. Br J Clin Pharmacol 50:427–440. 10.1046/j.1365-2125.2000.00272.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DJ, Somogyi AA, White JM et al (2004) Population pharmacokinetics of (R)-,(S)-and rac-methadone in methadone maintenance patients. Br J Clin Pharmacol 57:742–755. 10.1111/j.1365-2125.2004.02079.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox LM, Hoffman RS, Vlahov D, Manini AF (2018) Risk factors for severe respiratory depression from prescription opioid overdose. Addiction 113(1):59–66. 10.1111/add.13925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspari RJ, Paydarfar DJN (2007) Pathophysiology of respiratory failure following acute dichlorvos poisoning in a rodent model. NeuroToxicology 28:664–671. 10.1016/j.neuro.2007.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzolo D, Michetti F, Bruschettini M, Marchese N, Lituania M, Mangraviti S et al (2003) Pediatric concentrations of S100B protein in blood: age-and sex-related changes. Clin Chem 49(6):967–970. 10.1373/49.6.967 [DOI] [PubMed] [Google Scholar]
- Gholami N, Alwasabi F, Farnaghi F (2017) Drug-induced apnea in children admitted to loghman hakim hospital, Tehran. Iran. Iranian J Child Neurol 11(3):15 [PMC free article] [PubMed] [Google Scholar]
- Gonçalves C-A, Leite MC, Nardin P (2008) Biological and methodological features of the measurement of S100B, a putative marker of brain injury. Clin Biochem 41:755–763. 10.1016/j.clinbiochem.2008.04.003 [DOI] [PubMed] [Google Scholar]
- González-García S, González-Quevedo A, Fernández-Concepción O et al (2012) Short-term prognostic value of serum neuron specific enolase and S100B in acute stroke patients. Clin Biochem 45:1302–1307. 10.1016/j.clinbiochem.2012.07.094 [DOI] [PubMed] [Google Scholar]
- Graziani M, Nisticò R (2015) Gender differences in pharmacokinetics and pharmacodynamics of methadone substitution therapy. Front Pharmacol 6:122. 10.3389/fphar.2015.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi-Morad M, Naseri Z, Jamshidi N et al (2020) Methadone-induced encephalopathy: a case series and literature review. BMC Med Imaging 20:6. 10.1186/s12880-020-0410-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque A, Polcyn R, Matzelle D et al (2018) New insights into the role of neuron-specific enolase in neuro-inflammation, neurodegeneration, and neuroprotection. Brain Sci 8:33. 10.3390/brainsci8020033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepner IJ, Homewood J, Taylor AJ (2002) Methadone disrupts performance on the working memory version of the Morris water task. Physiol Behav 76(1):41–49. 10.1016/S0031-9384(02)00695-9 [DOI] [PubMed] [Google Scholar]
- Herrmann M, Ebert AD, Galazky I et al (2000) Neurobehavioral outcome prediction after cardiac surgery: role of neurobiochemical markers of damage to neuronal and glial brain tissue. Stroke 31:645–650. 10.1161/01.STR.31.3.645 [DOI] [PubMed] [Google Scholar]
- Hyer MM, Phillips LL, Neigh GN (2018) Sex differences in synaptic plasticity: hormones and beyond. Frontiers Mole Neurosci 11:266. 10.3389/fnmol.2018.00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauch EC, Lindsell C, Broderick J et al (2006) Association of serial biochemical markers with acute ischemic stroke: the National Institute of Neurological Disorders and Stroke recombinant tissue plasminogen activator Stroke Study. Stroke 37:2508–2513. 10.1161/01.STR.0000242290.01174.9e [DOI] [PubMed] [Google Scholar]
- Jones JD, Mogali S, Comer SDJD et al (2012) Polydrug abuse: a review of opioid and benzodiazepine combination use. Drug Alcohol Depend 125:8–18. 10.1016/j.drugalcdep.2012.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantonen T, Karjalainen T, Isojärvi J, Nuutila P, Tuisku J, Rinne J et al (2020) Interindividual variability and lateralization of μ-opioid receptors in the human brain. Neuroimage. 10.1016/j.neuroimage.2020.116922 [DOI] [PubMed] [Google Scholar]
- King F, Morris NA, Schmahmann JD (2015) Delayed posthypoxic leukoencephalopathy: improvement with antioxidant therapy. Case Rep Neurol 7(3):242–246. 10.1159/000441892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenowski P, Morgan M, Bartlett SE (2015) The role of δ-opioid receptors in learning and memory underlying the development of addiction. Br J Pharmacol 172:297–310. 10.1111/bph.12618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlyuk N, Monteith AJ, Garcia V, Damo SM, Skaar EP, Chazin WJ (2019) S100 proteins in the innate immune response to pathogens. In: Calcium-binding proteins of the EF-hand superfamily. Humana Press, New York, pp 275–290. 10.1007/978-1-4939-9030-6_18 [DOI] [PMC free article] [PubMed]
- Kurumatani T, Kudo T, Ikura Y et al (1998) White matter changes in the gerbil brain under chronic cerebral hypoperfusion. Stroke 29:1058–1061. 10.1007/bf00227672 [DOI] [PubMed] [Google Scholar]
- Laycock H, Bantel C (2019) Opioid mechanisms and opioid drugs. Anaesth Intens Care Med. 10.1016/j.mpaic.2019.05.009 [Google Scholar]
- Li W, Li Q, Wang Y et al (2016) Methadone-induced damage to white matter integrity in methadone maintenance patients: a longitudinal self-control DTI study. Sci Rep 6:19662. 10.1038/srep19662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupica CR, Proctor WR, Dunwiddie TV (1992) Dissociation of μ and δ opioid receptor-mediated reductions in evoked and spontaneous synaptic inhibition in the rat hippocampus in vitro. Brain Res 593:226–238. 10.1016/0006-8993(92)91312-3 [DOI] [PubMed] [Google Scholar]
- Ma M, Chen Y, He J, et al. (2007) Effects of morphine and its withdrawal on Y-maze spatial recognition memory in mice. Neuroscience 147: 1059–1065. 10.1016/j.neuroscience.2007.05.020 [DOI] [PubMed]
- McCormick CM, Mathews IZ (2010) Adolescent development, hypothalamic-pituitary-adrenal function, and programming of adult learning and memory. Prog Neuropsychopharm Biol Psychiatry 34(5):756–765. 10.1016/j.pnpbp.2009.09.019 [DOI] [PubMed] [Google Scholar]
- Manouchehrifar M, Derakhshandeh N, Shojaee M, Sabzghabaei A, Farnaghi F (2016) An epidemiologic study of pediatric poisoning; a six-month cross-sectional study. Emergency 4(1):21 [PMC free article] [PubMed] [Google Scholar]
- Mansori K, Soori H, Farnaghi F, Khodakarim S (2016) A case-control study on risk factors for unintentional childhood poisoning in Tehran. Med J Islamic Repub Iran 30:355 [PMC free article] [PubMed] [Google Scholar]
- Nouri F, Afarinesh MR, Sheibani V, Foroumadi A, Mahani SE, Mahmoudi M, Rohani E (2019) Concomitant abuse of methadone and methamphetamine could impair spatial learning and memory in male rats. Learn Motiv 65:43–51. 10.1016/j.lmot.2019.01.001 [Google Scholar]
- Petzold A, Michel P, Stock M et al (2008) Glial and axonal body fluid biomarkers are related to infarct volume, severity, and outcome. J Stroke Cerebrovasc Dis 17:196–203. 10.1016/j.jstrokecerebrovasdis.2008.02.002 [DOI] [PubMed] [Google Scholar]
- Raabe, A., Kopetsch, O., Woszczyk, A., Lang, J., Gerlach, R., Zimmermann, M., & Seifert, V. (2003). Serum S-100B protein as a molecular marker in severe traumatic brain injury. Restorative neurology and neuroscience, 21(3, 4), 159–169. [PubMed]
- Rady JJ, Portoghese PS, Fujimoto JM (2002) Methadone and heroin antinociception: predominant δ-opioid-receptor responses in methadone-tolerant mice. Jpn J Pharmacol 88:319–331. 10.1254/jjp.88.319 [DOI] [PubMed] [Google Scholar]
- Rapeli P, Fabritius C, Kalska H et al (2009) Memory function in opioid-dependent patients treated with methadone or buprenorphine along with benzodiazepine: longitudinal change in comparison to healthy individuals. Subst Abuse Treat Prev Policy 4:6. 10.1186/1747-597X-4-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisner A, Hayes LL, Holland CM, Wrubel DM, Kebriaei MA, Geller RJ et al (2015) Opioid overdose in a child: case report and discussion with emphasis on neurosurgical implications. J Neurosurg 16(6):752–757. 10.3171/2015.4.PEDS14667 [DOI] [PubMed] [Google Scholar]
- Sahuquillo J, Merino MA, Sanchez-Guerrero A, Arikan F, Vidal-Jorge M, Martínez-Valverde T et al (2014) Lactate and the lactate-to-pyruvate molar ratio cannot be used as independent biomarkers for monitoring brain energetic metabolism: a microdialysis study in patients with traumatic brain injuries. PLoS ONE. 10.1371/journal.pone.0102540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado RA, Jorens PG, Baar I, Cras P, Hans G, Parizel PM (2010) Methadone-induced toxic leukoencephalopathy: MR imaging and MR proton spectroscopy findings. Am J Neuroradiol 31(3):565–566. 10.3174/ajnr.A1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar R, Shin DH, Wasterlain CG (1997) Serum neuron-specific enolase is a marker for neuronal damage following status epilepticus in the rat. Epilepsy Res 28(2):129–136. 10.1016/S0920-1211(97)00040-5 [DOI] [PubMed] [Google Scholar]
- Schadrack J, Willoch F, Platzer S et al (1999) Opioid receptors in the human cerebellum: evidence from [11C] diprenorphine PET, mRNA expression and autoradiography. NeuroReport 10:619–624. 10.1097/00001756-199902250-00032 [DOI] [PubMed] [Google Scholar]
- Schaf DV, Tort AB, Fricke D et al (2005) S100B and NSE serum levels in patients with Parkinson's disease. Parkinsonism Relat Disord 11:39–43. 10.1016/j.parkreldis.2004.07.002 [DOI] [PubMed] [Google Scholar]
- Sengupta P (2013) The laboratory rat: relating its age with human's. Int J Preven Med 4(6):624. 10.1038/s41598-020-65722-6 [PMC free article] [PubMed] [Google Scholar]
- Shahim P, Tegner Y, Wilson DH et al (2014) Blood biomarkers for brain injury in concussed professional ice hockey players. JAMA Neurol 71:684–692. 10.1001/jamaneurol.2014.367 [DOI] [PubMed] [Google Scholar]
- Sharma A, Tallchief D, Blood J, Kim T, London A, Kharasch ED (2011) Perioperative pharmacokinetics of methadone in adolescents. Anesthesiology 115(6):1153–1161. 10.1097/ALN.0b013e318238fec5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields LB, Hunsaker JC III, Corey TS et al (2007) Methadone toxicity fatalities: a review of medical examiner cases in a large metropolitan area. J Forensic Sci 52:1389–1395. 10.1111/j.1556-4029.2007.00565.x [DOI] [PubMed] [Google Scholar]
- Somerville NJ, O’Donnell J, Gladden RM, Zibbell JE, Green TC, Younkin M et al (2017) Characteristics of fentanyl overdose—Massachusetts, 2014–2016. MMWR Morbid Mortal Weekly Rep 66(14):382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specka M, Finkbeiner T, Lodemann E et al (2000) Cognitive-motor performance of methadone-maintained patients. Eur Addict Res 6:8–19. 10.1159/000019004 [DOI] [PubMed] [Google Scholar]
- Sternberger NH, Itoyama Y, Kies MW et al (1978) Myelin basic protein demonstrated immunocytochemically in oligodendroglia prior to myelin sheath formation. PNAS. 10.1073/pnas.75.5.2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri F, Yaraghi A, Sabzghabaee AM et al (2013) Methadone toxicity in a poisoning referral center. J Res Pharm Pract 2:130. 10.4103/2279-042X.122387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Marumo T, Shibuta H, Omura T, Yoshida S (2009) Serum S100B, brain edema, and hematoma formation in a rat model of collagenase-induced hemorrhagic stroke. Brain Res Bull 78(4–5):158–163. 10.1016/j.brainresbull.2008.10.012 [DOI] [PubMed] [Google Scholar]
- Thelin EP, Jeppsson E, Frostell A et al (2016) Utility of neuron-specific enolase in traumatic brain injury; relations to S100B levels, outcome, and extracranial injury severity. Critical Care Crit Care 20:285. 10.1186/s13054-016-1450-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thigpen JC, Odle BL, Harirforoosh S (2019) Opioids: a review of pharmacokinetics and pharmacodynamics in neonates, infants, and children. Eur J Drug Metab Pharmacokinet. 10.1007/s13318-019-00552-0 [DOI] [PubMed] [Google Scholar]
- Tiong SC, Chieng JSL, Khoo HW, Ng CH (2019) Methadone-induced toxic encephalopathy in pediatric patients: two case reports. J Radiol Case Rep 13(5):1. 10.3941/jrcr.v13i5.3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoresen M, Hallström Å, Whitelaw A et al (1998) Lactate and pyruvate changes in the cerebral gray and white matter during posthypoxic seizures in newborn pigs. Pediatr Res 44:746–754. 10.1203/00006450-199811000-00018 [DOI] [PubMed] [Google Scholar]
- Timofeev I, Carpenter KL, Nortje J et al (2011) Cerebral extracellular chemistry and outcome following traumatic brain injury: a microdialysis study of 223 patients. Brain 134:484–494. 10.1093/brain/awq353 [DOI] [PubMed] [Google Scholar]
- Tramullas M, Martínez-Cué C, Hurlé MAJP (2007) Chronic methadone treatment and repeated withdrawal impair cognition and increase the expression of apoptosis-related proteins in mouse brain. Psychopharmacology 193:107–120. 10.1007/s00213-007-0751-x [DOI] [PubMed] [Google Scholar]
- Van Dorp EL, Yassen A, Dahan AJ (2007) Naloxone treatment in opioid addiction: the risks and benefits. Expert Opin Drug Saf 6:125–132. 10.1517/14740338.6.2.125 [DOI] [PubMed] [Google Scholar]
- Verdejo A, Toribio I, Orozco C et al (2005) Neuropsychological functioning in methadone maintenance patients versus abstinent heroin abusers. Drug Alcohol Depend 78:283–288. 10.1016/j.drugalcdep.2004.11.006 [DOI] [PubMed] [Google Scholar]
- Vestal-Laborde AA, Eschenroeder AC, Bigbee JW et al (2014) The opioid system and brain development: effects of methadone on the oligodendrocyte lineage and the early stages of myelination. Dev Neurosci 36:409–421. 10.1159/000365074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas SC, Thielet N (2020) Methadone prescribing caveats for chronic pain and opioid use disorder. J Nurse Practition 16(3):176–180. 10.1016/j.nurpra.2019.12.017 [Google Scholar]
- Wardi G, Brice J, Correia M, Liu D, Self M, Tainter C (2020) Demystifying lactate in the emergency department. Ann Emerg Med 75(2):287–298. 10.1016/j.annemergmed.2019.06.027 [DOI] [PubMed] [Google Scholar]
- Wegener S, Weber R, Ramos-Cabrer P et al (2006) Temporal profile of T 2-weighted MRI distinguishes between pannecrosis and selective neuronal death after transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab 26:38–47. 10.1038/sj.jcbfm.9600166 [DOI] [PubMed] [Google Scholar]
- Wehner F, Wehner H-D, Schieffer MC et al (2000) Immunohistochemical detection of methadone in the human brain. Front Pharmacol 112:11–16. 10.3389/fphar.2018.01023 [DOI] [PubMed] [Google Scholar]
- Wermeling DP (2015) Review of naloxone safety for opioid overdose: practical considerations for new technology and expanded public access. Therapeut Adv Drug Safety 6(1):20–31. 10.1177/2042098614564776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley EL, Stover AN (2019) The impact of the opioid epidemic on children and adolescents. Clin Ther 41(9):1655–1662. 10.1016/j.clinthera.2019.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woertgen C, Rothoerl RD, Wiesmann M, Missler U, Brawanski A (2002) Glial and neuronal serum markers after controlled cortical impact injury in the rat. In: Intracranial pressure and brain biochemical monitoring. Springer, Vienna, pp 205–207. 10.1007/978-3-7091-6738-0_53 [DOI] [PubMed]
- Wu H-M, Huang S-C, Hattori N et al (2004) Selective metabolic reduction in gray matter acutely following human traumatic brain injury. J Neurotrauma 21:149–161. 10.1089/089771504322778613 [DOI] [PubMed] [Google Scholar]
- Wunderlich M, Wallesch C, Goertler MJ (2006) Release of glial fibrillary acidic protein is related to the neurovascular status in acute ischemic stroke. Eur J Neurol 13:1118–1123. 10.1111/j.1468-1331.2006.01435.x [DOI] [PubMed] [Google Scholar]
- Yilmaz S, Karakayali O, Kale E et al (2018) Could serum S100B be a predictor of neuronal damage and clinical poor outcomes associated with the use of synthetic cannabinoids? S100B to predict neuronal damage of SC in the ED. Am J Emerg Med 36:435–441. 10.1016/j.ajem.2017.08.053 [DOI] [PubMed] [Google Scholar]
- Zamani N, Hassanian-Moghaddam H, Bayat AH et al (2015) Reversal of opioid overdose syndrome in morphine-dependent rats using buprenorphine. Toxicol Lett 232:590–594. 10.1016/j.toxlet.2014.12.007 [DOI] [PubMed] [Google Scholar]
- Žurek J, Fedora M (2012) The usefulness of S100B, NSE, GFAP, NF-H, secretagogin and Hsp70 as a predictive biomarker of outcome in children with traumatic brain injury. Acta Neurochir 154(1):93–103. 10.1007/s00701-011-1175-2 [DOI] [PubMed] [Google Scholar]