Abstract
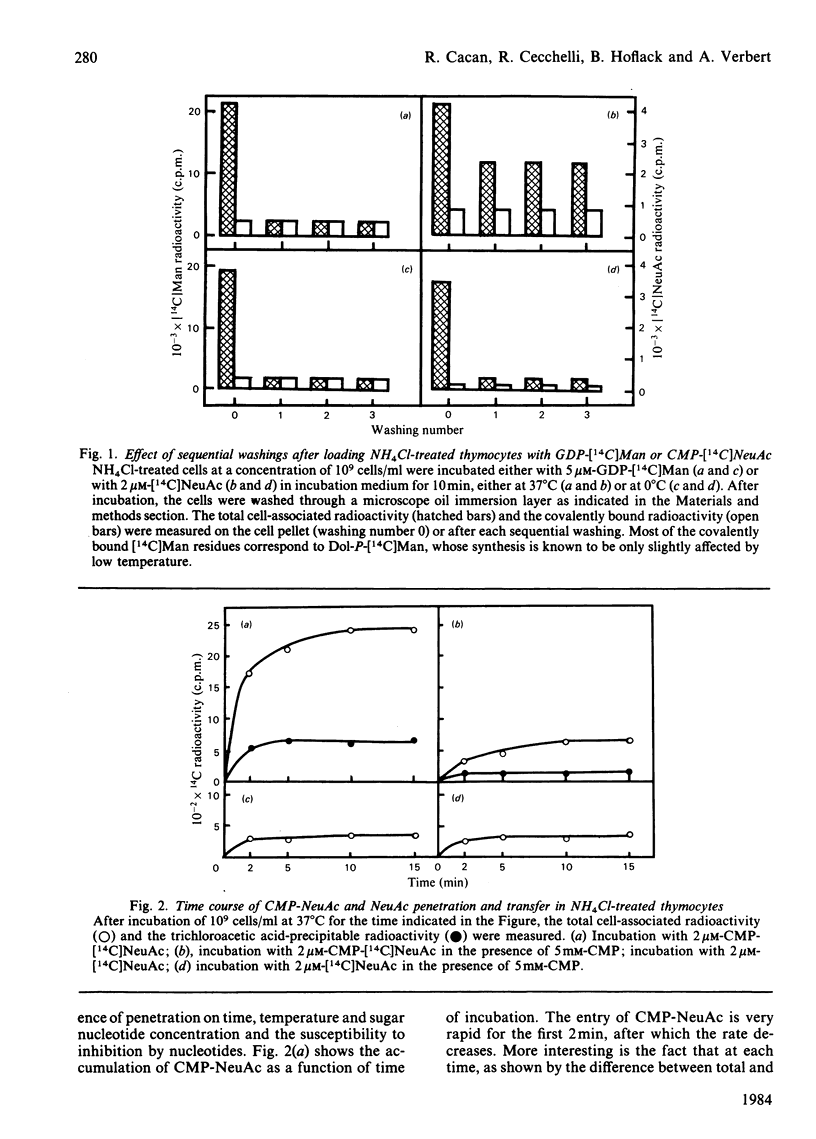
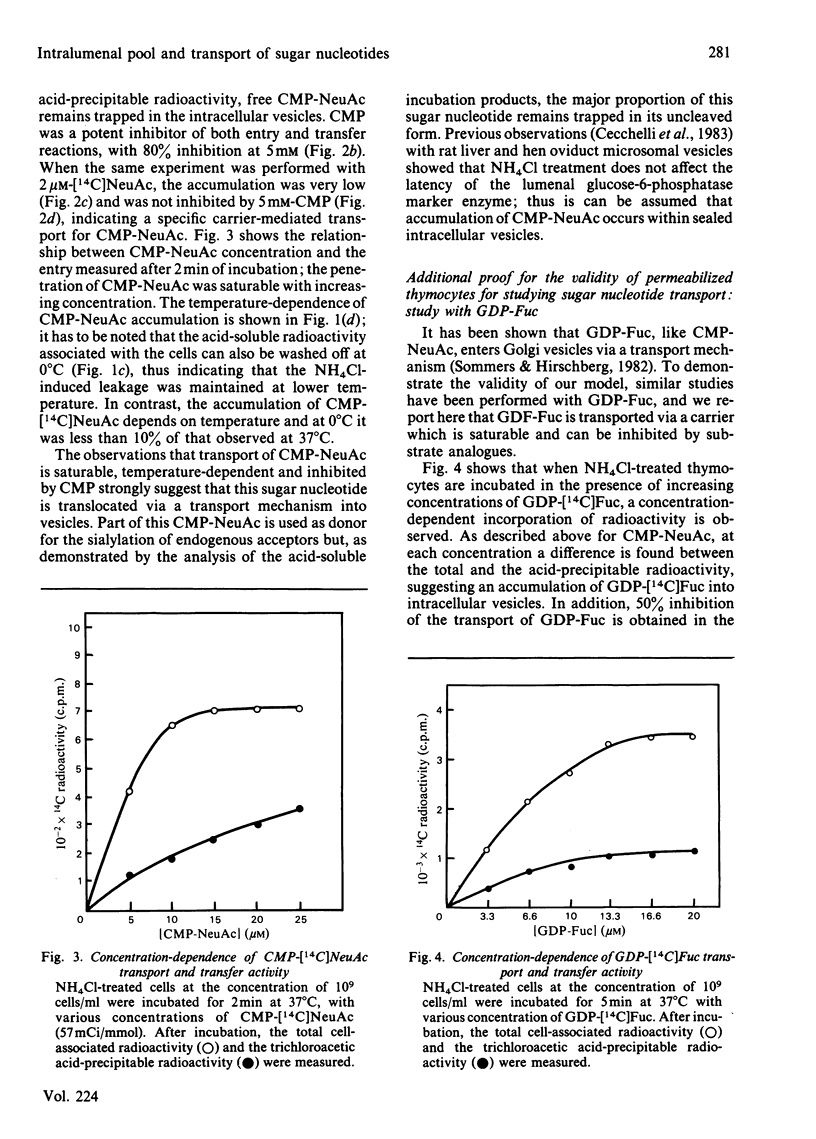
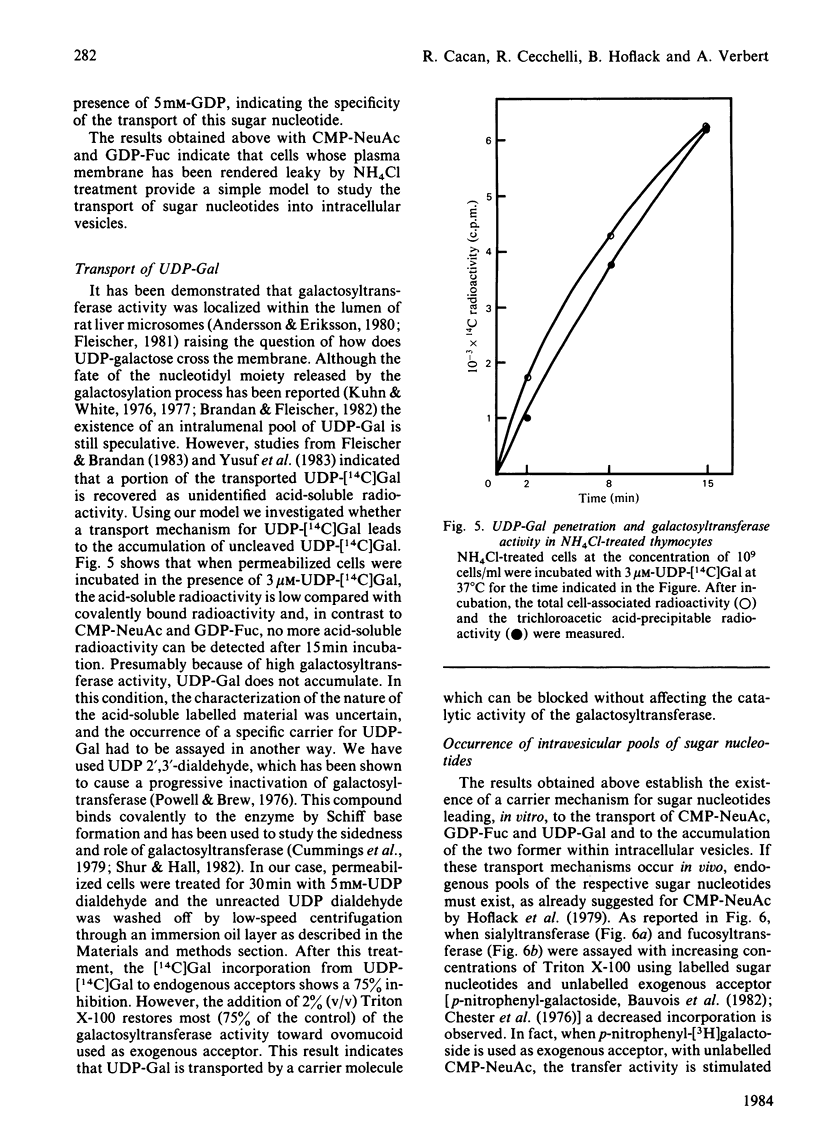
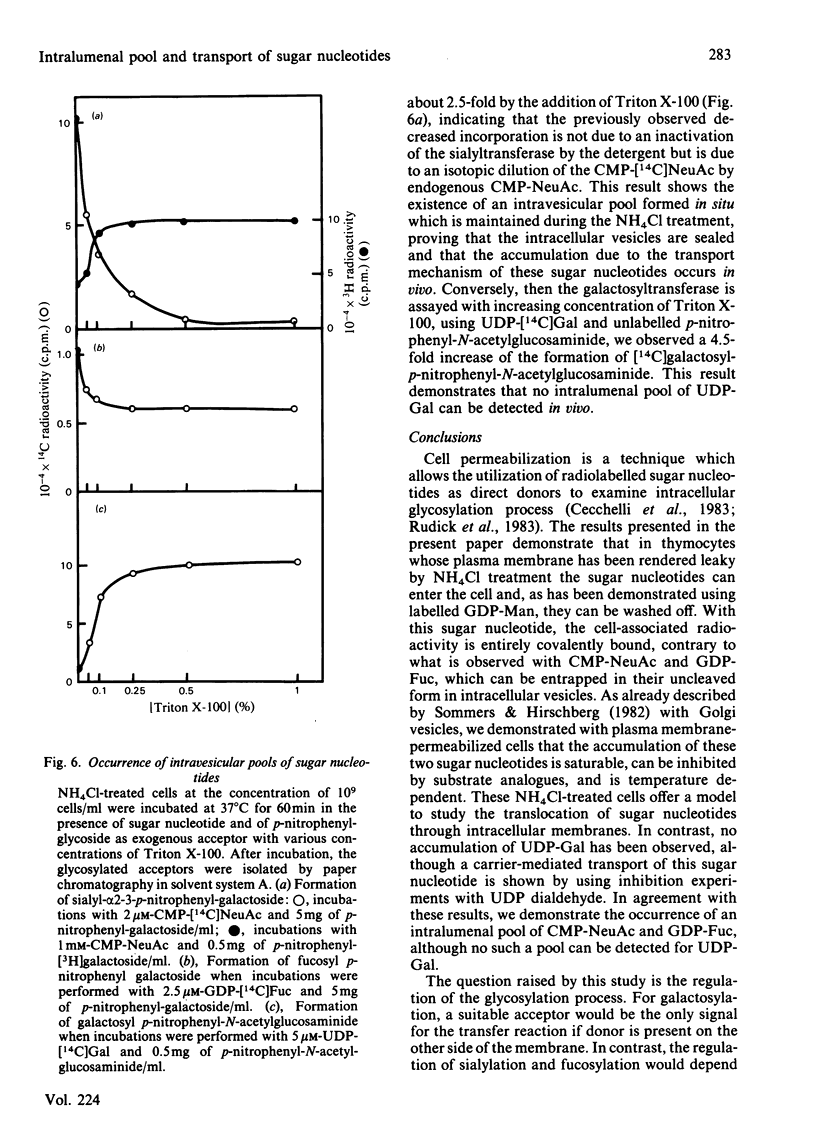
Treatment with NH4Cl of mouse thymocytes renders their plasma membrane permeable to sugar nucleotides both inwards and outwards. Using this model, we studied the entry and utilization of CMP-NeuAc, GDP-Fuc and UDP-Gal into intracellular vesicles in situ. It is shown that CMP-NeuAc and GDP-Fuc enter the vesicles in a manner indicating a carrier-mediated transport (substrate saturation curve, inhibition by substrate analogues, temperature dependence) and are entrapped in their uncleaved form. This leads to the formation of an intralumenal pool of these precursors which can be further utilized by the sialyltransferases and fucosyltransferases. The occurrence of an endogenous pool of CMP-NeuAc and GDP-Fuc is demonstrated by the fact that, when the vesicles are disrupted by detergent, the release of the endogenous sugar nucleotides causes an isotopic dilution of the labelled precursors added to measure the glycosyltransferase activities. In contrast, no accumulation of UDP-Gal has been detected, suggesting that transport and transfer reaction are simultaneous events. However, experiments with UDP 2',3'-dialdehyde indicate that UDP-Gal is not transported through the membrane by galactosyltransferase action but by a distinct carrier molecule.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson G. N., Eriksson L. C. Studies on the latency of UDP-galactose-asialo-mucin galactosyltransferase activity in microsomal and Golgi subfractions from rat liver. Biochim Biophys Acta. 1980 Aug 4;600(2):571–576. doi: 10.1016/0005-2736(80)90457-5. [DOI] [PubMed] [Google Scholar]
- Bauvois B., Cacan R., Fournet B., Caen J., Montreuil J., Verbert A. Discrimination between activity of (alpha 2-3)-sialyltransferase and (alpha 2-6)-sialyltransferase in human platelets using p-nitrophenyl-beta-D-galactoside as acceptor. Eur J Biochem. 1982 Jan;121(3):567–572. doi: 10.1111/j.1432-1033.1982.tb05824.x. [DOI] [PubMed] [Google Scholar]
- Brandan E., Fleischer B. Orientation and role of nucleosidediphosphatase and 5'-nucleotidase in Golgi vesicles from rat liver. Biochemistry. 1982 Sep 14;21(19):4640–4645. doi: 10.1021/bi00262a019. [DOI] [PubMed] [Google Scholar]
- Cacan R., Hoflack B., Verbert A. Effect of bis-(p-nitrophenyl) phosphate on the biosynthesis and the utilization of lipid-intermediates. Biochem J. 1982 Sep 1;205(3):603–609. doi: 10.1042/bj2050603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacan R., Verbert A., Hoflack B., Montreuil J. Occurrence of an intracellular inhibitor of ectosialyltransferase in lymphocytes. FEBS Lett. 1977 Sep 1;81(1):53–56. doi: 10.1016/0014-5793(77)80926-5. [DOI] [PubMed] [Google Scholar]
- Carey D. J., Hirschberg C. B. Topography of sialoglycoproteins and sialyltransferases in mouse and rat liver Golgi. J Biol Chem. 1981 Jan 25;256(2):989–993. [PubMed] [Google Scholar]
- Carey D. J., Sommers L. W., Hirschberg C. B. CMP-N-acetylneuraminic acid: isolation from and penetration into mouse liver microsomes. Cell. 1980 Mar;19(3):597–605. doi: 10.1016/s0092-8674(80)80036-5. [DOI] [PubMed] [Google Scholar]
- Cecchelli R., Cacan R., Hoflack B., Verbert A. Glycosylation of proteins from sugar nucleotides by whole cells. Effect of ammonium chloride treatment on mouse thymocytes. Biochem J. 1983 Dec 15;216(3):681–686. doi: 10.1042/bj2160681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox T. M., Peters T. J. The kinetics of iron uptake in vitro by human duodenal mucosa: studies in normal subjects. J Physiol. 1979 Apr;289:469–478. doi: 10.1113/jphysiol.1979.sp012747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings R. D., Cebula T. A., Roth S. Characterization of a galactosyltransferase in plasma membrane-enriched fractions from Balb/c 3T12 cells. J Biol Chem. 1979 Feb 25;254(4):1233–1240. [PubMed] [Google Scholar]
- Fleischer B. Orientation of glycoprotein galactosyltransferase and sialyltransferase enzymes in vesicles derived from rat liver Golgi apparatus. J Cell Biol. 1981 May;89(2):246–255. doi: 10.1083/jcb.89.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover J. A., Lennarz W. J. Transmembrane assembly of N-linked glycoproteins. Studies on the topology of saccharide synthesis. J Biol Chem. 1982 Mar 25;257(6):2787–2794. [PubMed] [Google Scholar]
- Hoflack B., Cacan R., Montreuil J., Verbert A. Detection of ectosiallyltransferase activity using whole cells. Correction of misleading results due to the release of intracellular CMP-N-acetylneuraminic acid. Biochim Biophys Acta. 1979 Jun 6;568(2):348–356. doi: 10.1016/0005-2744(79)90302-4. [DOI] [PubMed] [Google Scholar]
- Hoflack B., Cacan R., Verbert A. Occurrence of two fucosyltransferase activities at the outer surface of rat lymphocytes. Eur J Biochem. 1978 Jul 17;88(1):1–6. doi: 10.1111/j.1432-1033.1978.tb12416.x. [DOI] [PubMed] [Google Scholar]
- Kuhn N. J., White A. Evidence for specific transport of uridine diphosphate galactose across the Golgi membrane of rat mammary gland. Biochem J. 1976 Jan 15;154(1):243–244. doi: 10.1042/bj1540243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J., White A. The role of nucleoside diphosphatase in a uridine nucleotide cycle associated with lactose synthesis in rat mammary-gland Golgi apparatus. Biochem J. 1977 Dec 15;168(3):423–433. doi: 10.1042/bj1680423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J., Wooding F. B., White A. Properties of galactosyltransferase-enriched vesicles of Golgi membranes from lactating-rat mammary gland. Eur J Biochem. 1980 Jan;103(2):377–385. doi: 10.1111/j.1432-1033.1980.tb04324.x. [DOI] [PubMed] [Google Scholar]
- Powell J. T., Brew K. Affinity labeling of bovine colostrum galactosyltransferase with a uridine 5'-diphosphate derivative. Biochemistry. 1976 Aug 10;15(16):3499–3505. doi: 10.1021/bi00661a016. [DOI] [PubMed] [Google Scholar]
- Rudick V. L., Rudick M. J., Jones P. M. Glycosyl acceptors in intact and permeabilized normal and cystic fibrosis fibroblasts. J Cell Physiol. 1983 May;115(2):143–150. doi: 10.1002/jcp.1041150207. [DOI] [PubMed] [Google Scholar]
- Shur B. D., Hall N. G. A role for mouse sperm surface galactosyltransferase in sperm binding to the egg zona pellucida. J Cell Biol. 1982 Nov;95(2 Pt 1):574–579. doi: 10.1083/jcb.95.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider M. D., Sultzman L. A., Robbins P. W. Transmembrane location of oligosaccharide-lipid synthesis in microsomal vesicles. Cell. 1980 Sep;21(2):385–392. doi: 10.1016/0092-8674(80)90475-4. [DOI] [PubMed] [Google Scholar]
- Sommers L. W., Hirschberg C. B. Transport of sugar nucleotides into rat liver Golgi. A new Golgi marker activity. J Biol Chem. 1982 Sep 25;257(18):10811–10817. [PubMed] [Google Scholar]
- Verbert A., Cacan R., Montreuil J. Ectogalactosyltransferase. Presence of enzyme and acceptors on the rat lymphocyte cell surface. Eur J Biochem. 1976 Nov 1;70(1):49–53. doi: 10.1111/j.1432-1033.1976.tb10954.x. [DOI] [PubMed] [Google Scholar]
- Yusuf H. K., Pohlentz G., Sandhoff K. Tunicamycin inhibits ganglioside biosynthesis in rat liver Golgi apparatus by blocking sugar nucleotide transport across the membrane vesicles. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7075–7079. doi: 10.1073/pnas.80.23.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]


