Abstract
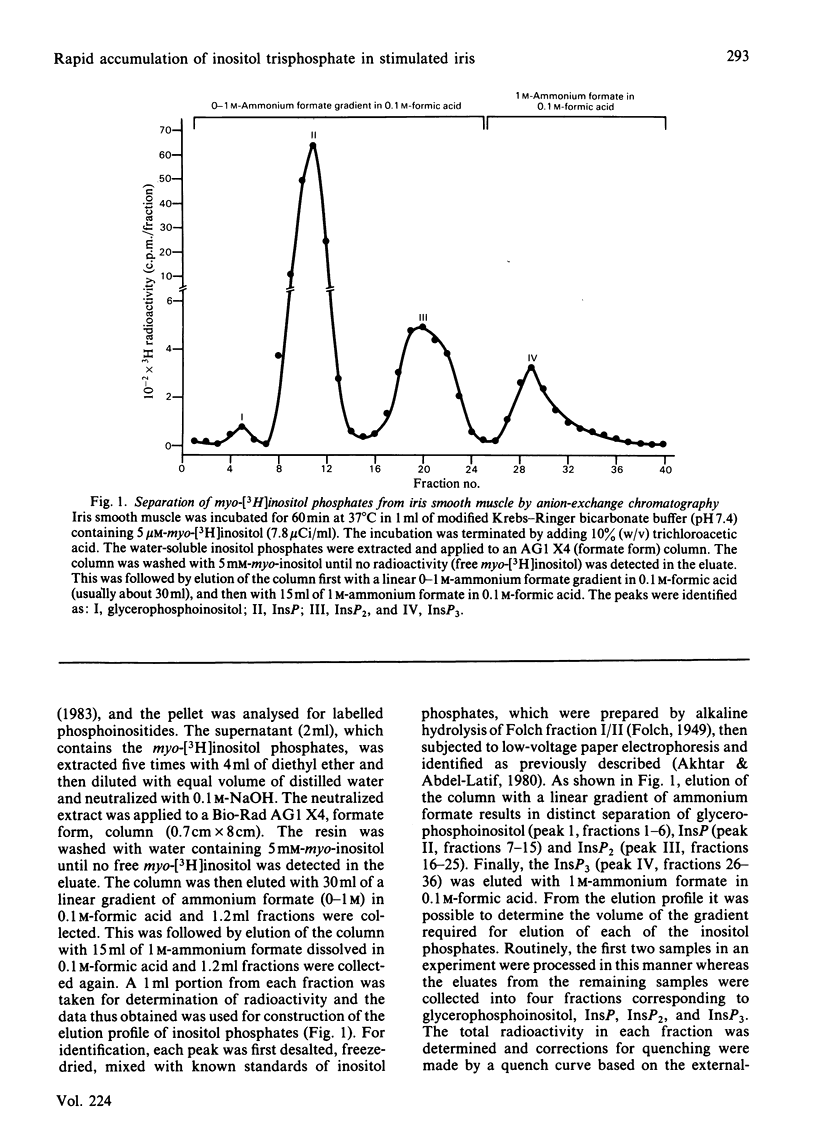
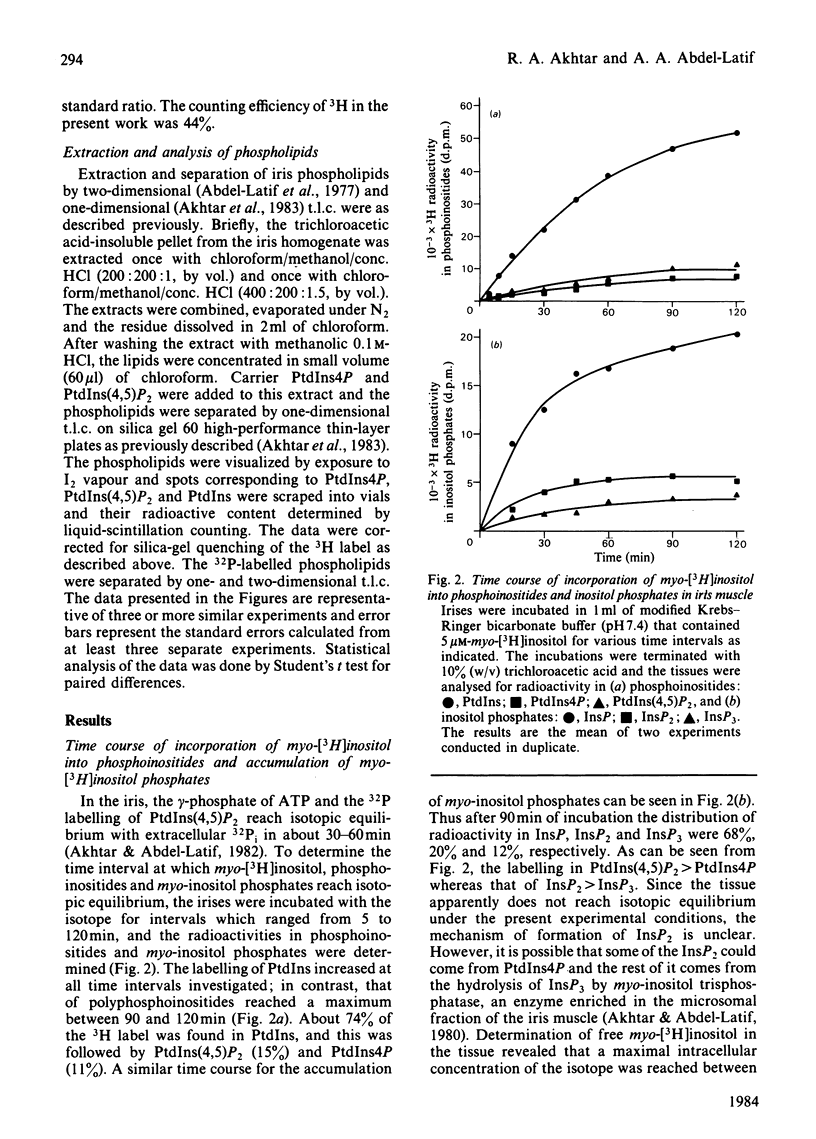
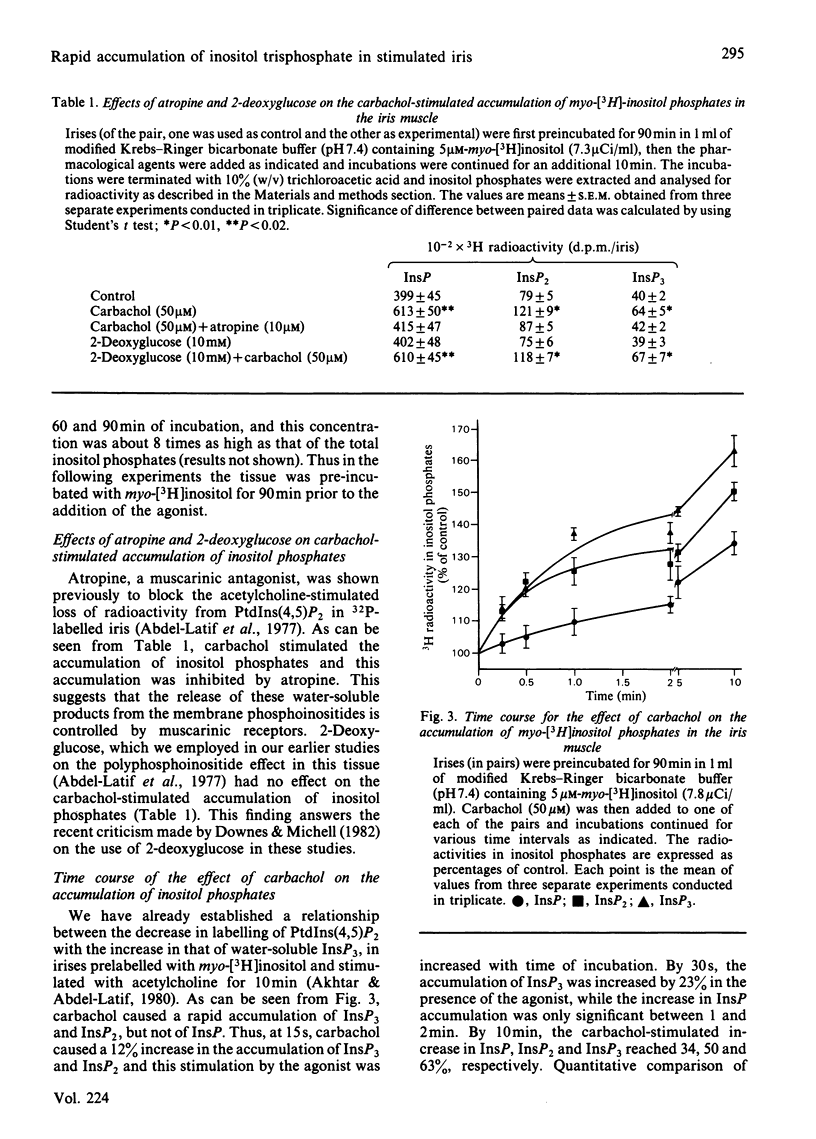
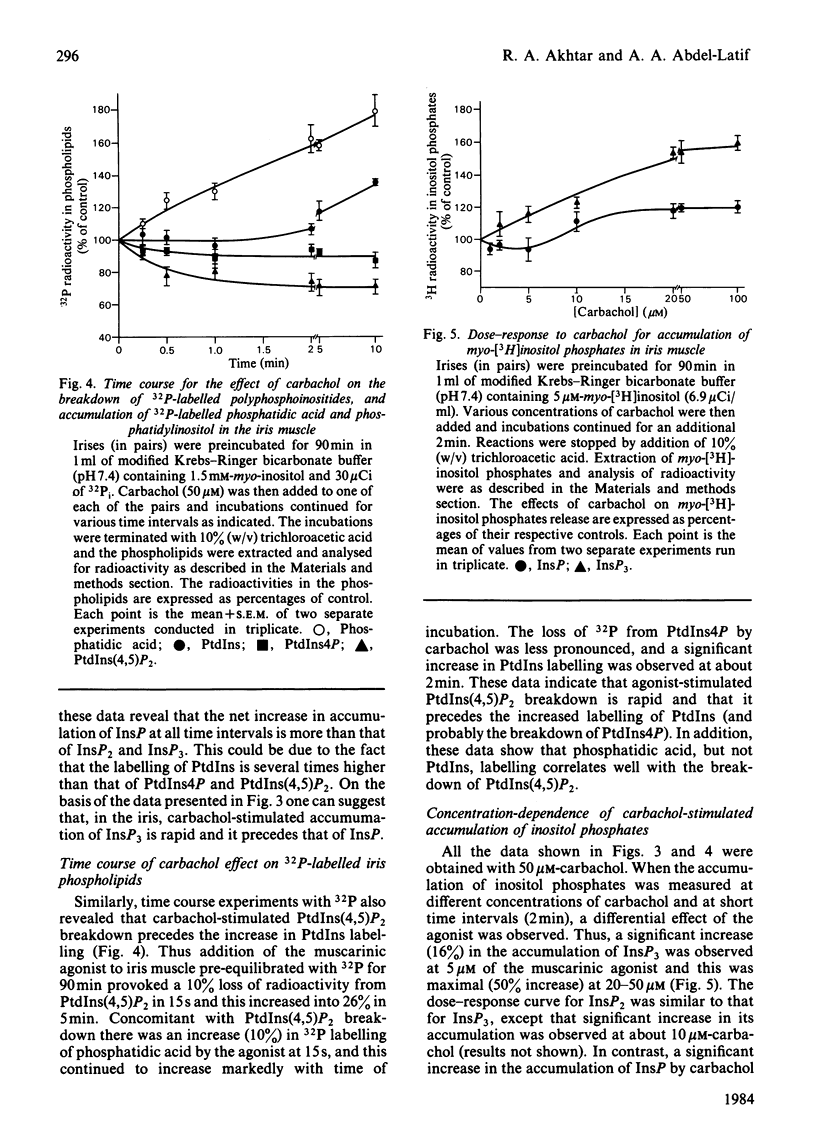
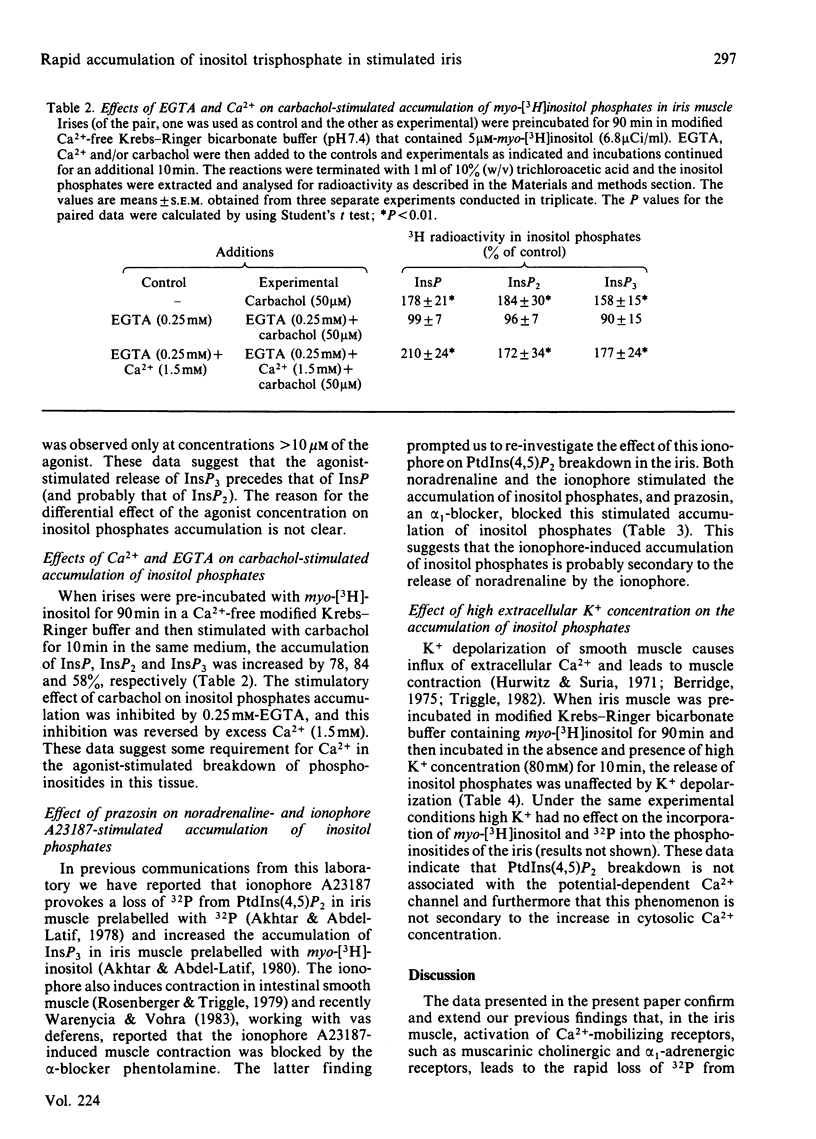
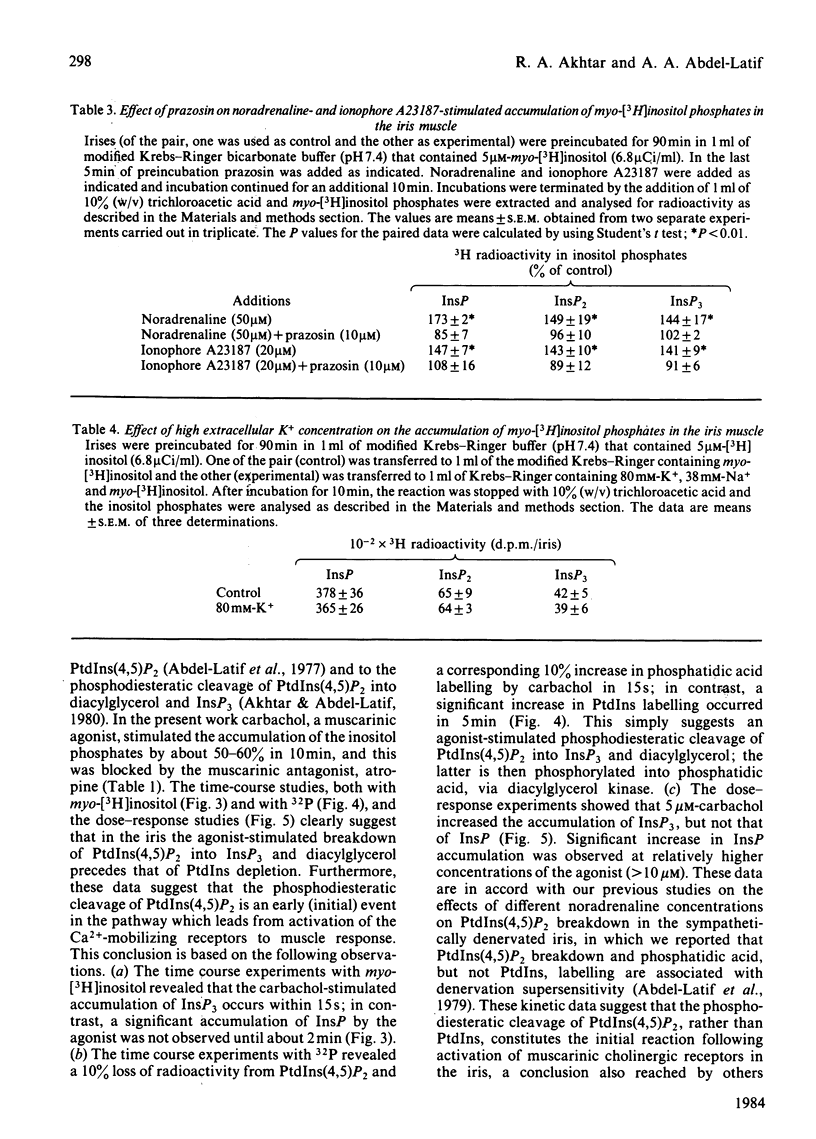
Rabbit iris smooth muscle was prelabelled with myo-[3H]inositol for 90 min and the effect of carbachol on the accumulation of inositol phosphates from phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2], phosphatidylinositol 4-phosphate (PtdIns4P) and phosphatidylinositol (PtdIns) was monitored with anion-exchange chromatography. Carbachol stimulated the accumulation of inositol phosphates and this was blocked by atropine, a muscarinic antagonist, and it was unaffected by 2-deoxyglucose. The data presented demonstrate that, in the iris, carbachol (50 microM) stimulates the rapid breakdown of PtdIns(4,5)P2 into [3H]inositol trisphosphate (InsP3) and diacylglycerol, measured as phosphatidate, and that the accumulation of InsP3 precedes that of [3H]inositol bisphosphate (InsP2) and [3H]inositol phosphate (InsP). This conclusion is based on the following findings. Time course experiments with myo-[3H]inositol revealed that carbachol increased the accumulation of InsP3 by 12% in 15s and by 23% in 30s; in contrast, a significant increase in InsP release was not observed until about 2 min. Time-course experiments with 32P revealed a 10% loss of radioactivity from PtdIns(4,5)P2 and a corresponding 10% increase in phosphatidate labelling by carbachol in 15s; in contrast a significant increase in PtdIns labelling occurred in 5 min. Dose-response studies revealed that 5 microM-carbachol significantly increased (16%) the accumulation of InsP3 whereas a significant increase in accumulation of InsP2 and InsP was observed only at agonist concentrations greater than 10 microM. Studies on the involvement of Ca2+ in the agonist-stimulated breakdown of PtdIns(4,5)P2 in the iris revealed the following. Marked stimulation (58-78%) of inositol phosphates accumulation by carbachol in 10 min was observed in the absence of extracellular Ca2+. Like the stimulatory effect of noradrenaline, the ionophore A23187-stimulated accumulation of InsP3 was inhibited by prazosin, an alpha 1-adrenergic blocker, thus suggesting that the ionophore stimulation of PtdIns(4,5)P2 breakdown we reported previously [Akhtar & Abdel-Latif (1978) J. Pharmacol. Exp. Ther. 204, 655-688; Akhtar & Abdel-Latif (1980) Biochem. J. 192, 783-791] was secondary to the release of noradrenaline by the ionophore. The carbachol-stimulated accumulation of inositol phosphates was inhibited by EGTA (0.25 mM) and this inhibition was reversed by excess Ca2+ (1.5 mM), suggesting that EGTA treatment of the tissue chelates extracellular Ca2+ required for polyphosphoinositide phosphodiesterase activity. K+ depolarization, which causes influx of extracellular Ca2+ in smooth muscle, did not change the level of InsP3.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A., Akhtar R. A. Acetylcholine causes an increase in the hydrolysis of triphosphoinositide pre-labelled with [32P]phosphate or [3H]myo-inositol and a corresponding increase in the labelling of phosphatidylinositol and phosphatidic acid in rabbit iris muscle. Biochem Soc Trans. 1976;4(2):317–321. doi: 10.1042/bst0040317. [DOI] [PubMed] [Google Scholar]
- Abdel-Latif A. A., Akhtar R. A., Hawthorne J. N. Acetylcholine increases the breakdown of triphosphoinositide of rabbit iris muscle prelabelled with [32P] phosphate. Biochem J. 1977 Jan 15;162(1):61–73. doi: 10.1042/bj1620061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Latif A. A., Green K., Smith J. P. Sympathetic denervation and the triphosphoinositide effect in the iris smooth muscle: a biochemical method for the determination of alpha-adrenergic receptor denervation supersensitivity. J Neurochem. 1979 Jan;32(1):225–228. doi: 10.1111/j.1471-4159.1979.tb04532.x. [DOI] [PubMed] [Google Scholar]
- Agranoff B. W., Murthy P., Seguin E. B. Thrombin-induced phosphodiesteratic cleavage of phosphatidylinositol bisphosphate in human platelets. J Biol Chem. 1983 Feb 25;258(4):2076–2078. [PubMed] [Google Scholar]
- Akhtar R. A., Abdel-Latif A. A. Calcium ion requirement for acetylcholine-stimulated breakdown of triphosphoinositide in rabbit iris smooth muscle. J Pharmacol Exp Ther. 1978 Mar;204(3):655–668. [PubMed] [Google Scholar]
- Akhtar R. A., Abdel-Latif A. A. Effects of Na+, Ca2+, and acetylcholine on phosphoinositide- and ATP-phosphate turnover in 32P-labeled rabbit iris smooth muscle. J Neurochem. 1982 Nov;39(5):1374–1380. doi: 10.1111/j.1471-4159.1982.tb12580.x. [DOI] [PubMed] [Google Scholar]
- Akhtar R. A., Abdel-Latif A. A. Requirement for calcium ions in acetylcholine-stimulated phosphodiesteratic cleavage of phosphatidyl-myo-inositol 4,5-bisphosphate in rabbit iris smooth muscle. Biochem J. 1980 Dec 15;192(3):783–791. doi: 10.1042/bj1920783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar R. A., Taft W. C., Abdel-Latif A. A. Effects of corticotropin-(1-24)-tetracosapeptide on polyphosphoinositide metabolism and protein phosphorylation in rabbit iris subcellular fractions. J Neurochem. 1983 Nov;41(5):1460–1468. doi: 10.1111/j.1471-4159.1983.tb00846.x. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983 Jun 15;212(3):849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. The interaction of cyclic nucleotides and calcium in the control of cellular activity. Adv Cyclic Nucleotide Res. 1975;6:1–98. [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Evidence for multiple metabolic pools of phosphatidylinositol in stimulated platelets. J Biol Chem. 1982 Oct 25;257(20):11856–11859. [PubMed] [Google Scholar]
- Bradford P. G., Marinetti G. V., Abood L. G. Stimulation of phospholipase A2 and secretion of catecholamines from brain synaptosomes by potassium and A23187. J Neurochem. 1983 Dec;41(6):1684–1693. doi: 10.1111/j.1471-4159.1983.tb00881.x. [DOI] [PubMed] [Google Scholar]
- Cohen M. L., Landry A. S., Perry K. W., Fuller R. W. Ionophore (A23187)-induced efflux of [3H]norepinephrine and endogenous norepinephrine in the rat vas deferens. Eur J Pharmacol. 1981 Sep 11;74(2-3):157–165. doi: 10.1016/0014-2999(81)90526-4. [DOI] [PubMed] [Google Scholar]
- Creba J. A., Downes C. P., Hawkins P. T., Brewster G., Michell R. H., Kirk C. J. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. Biochem J. 1983 Jun 15;212(3):733–747. doi: 10.1042/bj2120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes P., Michell R. H. Phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate: lipids in search of a function. Cell Calcium. 1982 Oct;3(4-5):467–502. doi: 10.1016/0143-4160(82)90031-8. [DOI] [PubMed] [Google Scholar]
- HENDRICKSON H. S., BALLOU C. E. ION EXCHANGE CHROMATOGRAPHY OF INTACT BRAIN PHOSPHOINOSITIDES ON DIETHYLAMINOETHYL CELLULOSE BY GRADIENT SALT ELUTION IN A MIXED SOLVENT SYSTEM. J Biol Chem. 1964 May;239:1369–1373. [PubMed] [Google Scholar]
- Hawthorne J. N. Polyphosphoinositide metabolism in excitable membranes. Review. Biosci Rep. 1983 Oct;3(10):887–904. doi: 10.1007/BF01140658. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Kirk C. J., Creba J. A., Downes C. P., Michell R. H. Hormone-stimulated metabolism of inositol lipids and its relationship to hepatic receptor function. Biochem Soc Trans. 1981 Oct;9(5):377–379. doi: 10.1042/bst0090377. [DOI] [PubMed] [Google Scholar]
- Mangel A. W., Nelson D. O., Rabovsky J. L., Prosser C. L., Connor J. A. Depolarization-induced contractile activity of smooth muscle in calcium-free solution. Am J Physiol. 1982 Jan;242(1):C36–C40. doi: 10.1152/ajpcell.1982.242.1.C36. [DOI] [PubMed] [Google Scholar]
- Marx J. L. A new view of receptor action. Science. 1984 Apr 20;224(4646):271–274. doi: 10.1126/science.6143399. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Jones L. M., Downes C. P., Creba J. A. The stimulation of inositol lipid metabolism that accompanies calcium mobilization in stimulated cells: defined characteristics and unanswered questions. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):123–138. doi: 10.1098/rstb.1981.0177. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Rosenberger L. B., Triggle D. J. The mechanism of action of ionophore A 23187 on guinea pig intestinal smooth muscle. Can J Physiol Pharmacol. 1979 Apr;57(4):348–358. doi: 10.1139/y79-053. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Warenycia M. W., Vohra M. M. The effects of the ionophore A-23187 on the rat vas deferens. Can J Physiol Pharmacol. 1983 Jan;61(1):97–101. doi: 10.1139/y83-012. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., McKinney J. S., Putney J. W., Jr Receptor-mediated net breakdown of phosphatidylinositol 4,5-bisphosphate in parotid acinar cells. Biochem J. 1982 Sep 15;206(3):555–560. doi: 10.1042/bj2060555. [DOI] [PMC free article] [PubMed] [Google Scholar]


