Abstract
Constant exposure to harmful substances from both inside and outside the body can mess up the body’s natural ways of keeping itself in balance. This can cause severe skin damage, including basal cell carcinoma (BCC), squamous cell carcinoma (SCC), and melanoma. However, plant-derived compounds found in fruits and vegetables have been shown to protect against skin cancer-causing free radicals and other harmful substances. It has been determined that these dietary phytochemicals are effective in preventing skin cancer and are widely available, inexpensive, and well-tolerated. Studies have shown that these phytochemicals possess anti-inflammatory, antioxidant, and antiangiogenic properties that can aid in the prevention of skin cancers. In addition, they influence crucial cellular processes such as angiogenesis and cell cycle control, which can halt the progression of skin cancer. The present paper discusses the benefits of specific dietary phytochemicals found in fruits and vegetables, as well as the signaling pathways they regulate, the molecular mechanisms involved in the prevention of skin cancer, and their drawbacks.
Keywords: antioxidant, basal cell carcinoma, dietary phytochemicals, melanoma skin cancer, squamous cell carcinoma
Introduction
Highlights
Skin cancer is a type of cancer that arises from the uncontrolled growth of cells in the skin. There are three main types of skin cancer.
This mutation also leads to the activation of the MAPK/ERK signaling pathway and abnormally promotes cell growth and division.
The BRAF and NRAS genes are members of the RAS family of genes, which regulate cell growth and division.
The CDKN2A gene encodes two proteins, p16INK4a and p14ARF, which act as tumor suppressors by regulating cell growth and division.
Signaling pathways (MAPK pathway, PI3K/Akt pathway, Wnt pathway, Hedgehog pathway, Notch pathway, etc.) are involved in cell growth and proliferation.
Phytochemicals found in fruits and vegetables, as well as the signaling pathways they regulate, the molecular mechanisms involved in the prevention of skin cancer.
Skin structure
The skin is the largest organ in the human body and serves as a barrier between the internal and external environment. It plays a crucial role in protecting the body from physical, chemical, and biological damage, as well as in regulating body temperature, maintaining hydration, and providing sensory input1. The skin is composed of three main layers: the epidermis, dermis, and hypodermis (subcutaneous tissue) (Fig. 1)2. Each layer has its own unique structure and function, which work together to provide a protective and functional barrier. The epidermis is the outermost layer of the skin and is composed of several layers of keratinized epithelial cells. The thickness of the epidermis varies depending on the location on the body, with the thickest epidermis found on the soles of the feet and the thinnest on the eyelids. The epidermis is responsible for protecting the body from physical damage, UV radiation, and infection3. The dermis is the second layer of the skin and is located beneath the epidermis. The dermis is composed of connective tissue and is responsible for providing support and elasticity to the skin. The dermis also contains blood vessels, nerves, and sensory receptors. The hypodermis, also known as the subcutaneous tissue, is the deepest layer of the skin. The hypodermis is composed of adipose tissue and connective tissue and serves as a cushion for the underlying muscles and bones4.
Figure 1.
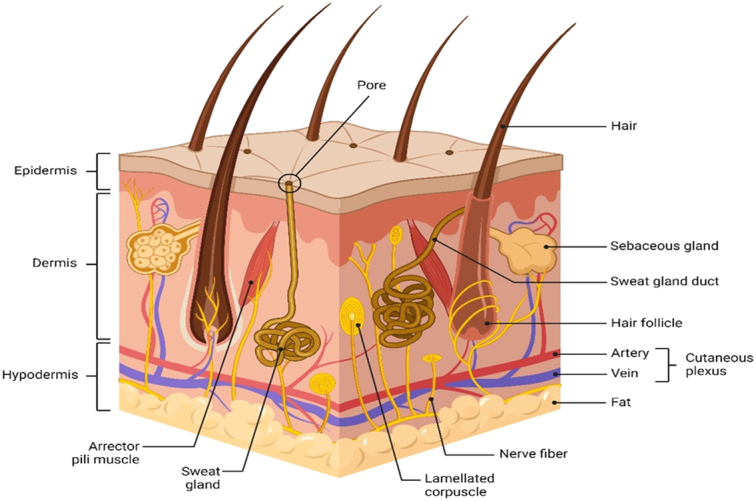
Skin anatomy (created with BioRender).
There are several types of cells present in the skin, each with its own unique function. Some of the main cell types in the skin include Keratinocytes, Melanocytes, Langerhans cells, Merkel cells, and Fibroblasts5. Skin cancer is the most common type of cancer in the United States and is caused by the uncontrolled growth of abnormal cells in the skin. The different cell types in the skin play a critical role in protecting against skin cancer. For example, melanocytes produce melanin, which helps to protect against UV radiation and reduces the risk of skin cancer. Langerhans cells are involved in the immune response and can help to identify and destroy abnormal cells before they can develop into skin cancer. Additionally, fibroblasts produce the extracellular matrix, which helps to prevent the uncontrolled growth and spread of abnormal cells. Overall, the different types of cells in the skin work together to maintain the integrity of the skin and protect against the development of skin cancer. Regular skin checks and sun protection can also help to reduce the risk of skin cancer6,7.
Molecular mechanism of skin cancer
Skin cancer is a type of cancer that arises from the uncontrolled growth of cells in the skin. There are three main types of skin cancer: basal cell carcinoma (BCC), squamous cell carcinoma (SCC), and melanoma8–10. The molecular mechanisms underlying skin cancer development involve mutations in several genes and pathways that regulate cell growth, division, and apoptosis11,12. BCC is the most common type of skin cancer, accounting for ~80% of all cases. It typically develops in the basal cells, which are located in the deepest layer of the epidermis. The molecular mechanisms underlying BCC development involve mutations in the sonic hedgehog (SHH) signaling pathway. The SHH signaling pathway plays an important role in embryonic development, and its activation is necessary for the development of the skin, hair, and nails. In adults, the SHH pathway is normally inactive in the skin. However, mutations in genes that regulate the SHH pathway can lead to its activation and the development of BCC13,14. The most common mutation in BCC is in the Patched-1 (PTCH1) gene, which normally acts as a tumor suppressor by inhibiting the SHH pathway. Mutations in PTCH1 lead to the activation of the SHH pathway, which results in the uncontrolled growth of basal cells and the development of tumors15,16. Other genetic and environmental factors can also contribute to the development of BCC. Mutations in the Tumor Protein 53 (TP53) gene, which is another tumor suppressor gene, can also lead to BCC development. Environmental factors, such as exposure to arsenic and ionizing radiation, can also increase the risk of developing BCC17,18. SCC is the second most common type of skin cancer, accounting for ~16% of all cases. It typically develops in the squamous cells, which are located in the upper layers of the epidermis19. The molecular mechanisms underlying SCC development involve mutations in several genes, including TP53, Cyclin-dependent kinase inhibitor 2A (CDKN2A), and Harvey rat sarcoma viral oncogene homolog (HRAS). Like BCC, mutations in TP53 and CDKN2A are commonly found in SCC. TP53 mutations can lead to the uncontrolled growth of squamous cells and the development of tumors20,21. CDKN2A mutations can also contribute to SCC development by preventing cells from undergoing apoptosis, which is a process that helps to eliminate damaged cells from the body22,23. Mutations in the HRAS gene, which is a member of the Rat Sarcoma (RAS) family of genes, are also found in SCC. The RAS genes normally regulate cell growth and division, but mutations in these genes can lead to the development of tumors. In SCC, mutations in HRAS lead to the uncontrolled growth of squamous cells and the development of tumors24,25. Melanoma is a type of skin cancer that arises from the uncontrolled growth of melanocytes, which are the cells that produce the pigment melanin. Melanoma is the most dangerous type of skin cancer, as it can metastasize and spread to other parts of the body. The molecular mechanisms underlying melanoma development also involve mutations in several genes and pathways that regulate cell growth, division, and apoptosis26–29.
B-Raf proto-oncogene, serine/threonine kinase (BRAF), and neuroblastoma RAS viral oncogene homolog (NRAS) mutations
The BRAF and NRAS genes are members of the RAS family of genes, which regulate cell growth and division. Mutations in BRAF and NRAS are commonly found in melanoma and can lead to the uncontrolled growth of melanocytes and the development of tumors27. The BRAF gene is mutated in ~50% of all melanomas. The most common mutation is a substitution of valine for glutamic acid at position 600 (BRAFV600E). This mutation leads to the activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) signaling pathway, which promotes cell growth and division. The NRAS gene is mutated in ~15% of all melanomas. The most common mutation is a substitution of glutamine for arginine at position 61 (NRASQ61R). This mutation also leads to the activation of the MAPK/ERK signaling pathway and promotes cell growth and division30,31.
CDKN2A mutations
The CDKN2A gene encodes two proteins, p16INK4a and p14ARF, which act as tumor suppressors by regulating cell growth and division. Mutations in CDKN2A are commonly found in melanoma and can lead to the uncontrolled growth of melanocytes and the development of tumors. Loss of p16INK4a function is found in ~50% of all melanomas. This loss can occur through mutations in the CDKN2A gene or through epigenetic silencing of the gene. Loss of p16INK4a function leads to the activation of the cyclin-dependent kinase (CDK) 4/6 pathway, which promotes cell growth and division. Loss of p14ARF function is found in ~20% of all melanomas. This loss can occur through mutations in the CDKN2A gene or through epigenetic silencing of the gene. Loss of p14ARF function leads to the stabilization of the oncoprotein mouse double minute 2 (MDM2) homolog, which promotes the degradation of the tumor suppressor protein p5332,33.
TP53 mutations
The TP53 gene encodes the tumor suppressor protein p53, which regulates cell growth, division, and apoptosis. Mutations in TP53 are found in ~25% of all melanomas and can lead to the uncontrolled growth of melanocytes and the development of tumors34–36. Loss of p53 function can occur through mutations in the TP53 gene or through the stabilization of the oncoprotein MDM2, which promotes the degradation of p53. Loss of p53 function leads to the inhibition of apoptosis and the promotion of cell growth and division37,38.
Skin cancer therapy via diet-based phytochemicals
Dietary phytochemicals are naturally occurring compounds found in plant-based foods, including fruits, vegetables, whole grains, nuts, seeds, and herbs. Phytochemicals are not considered essential nutrients, but they have been shown to have a wide range of health benefits, including antioxidant, anti-inflammatory, anticancer, and neuroprotective properties39–42. There are thousands of different phytochemicals, each with its unique chemical structure and biological activity. Some of the most well-known and studied phytochemicals include flavonoids, carotenoids, phenolic acids, and lignans43–46. Flavonoids, such as quercetin and kaempferol, are potent antioxidants that have been shown to have anti-inflammatory and anticancer effects47,48. Carotenoids, such as β-carotene, are responsible for the red, orange, and yellow colors in fruits and vegetables and have been shown to have potent antioxidant and anticancer properties49,50. Phenolic acids, such as caffeic acid and ferulic acid, are found in a wide range of plant-based foods and have been shown to have antioxidant and anti-inflammatory effects43. Lignans are phytoestrogens found in flaxseeds, sesame seeds, and whole grains and have been shown to have anticancer effects51,52. The health benefits of dietary phytochemicals are believed to come from their ability to interact with cellular signaling pathways, enzymes, and other molecules in the body, thereby modulating various biological processes (Table 1A–N)127–129.
Table 1.
Some important dietary phytochemicals effective in preventing skin cancer.
| S. no. | Dietary phytochemical | Structure | IUPAC name | Signaling pathway | References |
|---|---|---|---|---|---|
| A | |||||
| 1. | Rosmarinic acid |
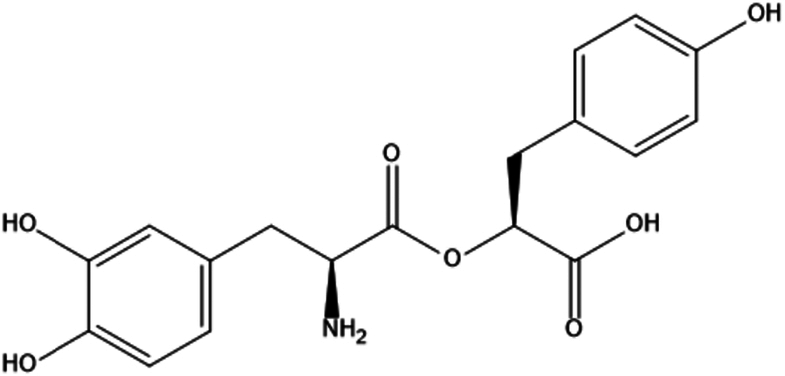
|
(2S)-2-[(3-(3,4-dihydroxyphenyl)-l-alanyl)oxy]-3-(4-hydroxyphenl)propanoic acid | (1) Inhibition of PI3K/AKT/mTOR pathway. | 53 |
| (2) Inhibition of MAPK/ERK pathway. | 54 | ||||
| (3) Inhibit the activation of NF-κB. | 55 | ||||
| (4) Inhibit the Wnt/β-catenin pathway by decreasing the expression of β-catenin and its downstream target genes, leading to decreased cell proliferation and invasion. | 56,57 | ||||
| B | |||||
| 2. | Allicin |
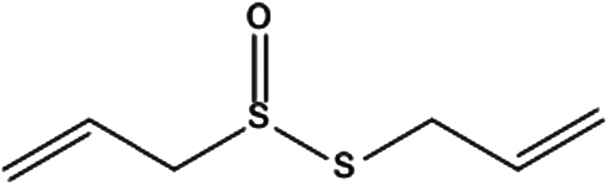
|
2-propene-1-sulfinothioic acid S-2-propen-1-yl ester | (1) Inhibit the activation of NF-κB, potentially reducing the risk of various types of cancer development, including skin cancer. | 58 |
| (2) Inhibit the activation of the Wnt/β-catenin pathway, potentially reducing the risk of various types of cancer development, including skin cancer. | 59 | ||||
| C | |||||
| 3. | Sulforaphane |
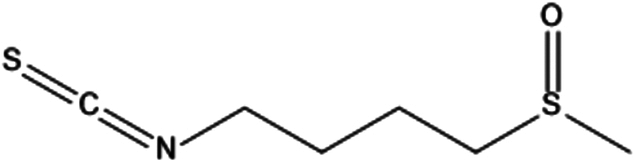
|
(RS)-1-isothiocyanato-4-(methylsulfinyl)butane | (1) Activate the Nrf2–Keap1 pathway, leading to increased production of antioxidant and detoxification enzymes that help protect against the damaging effects of environmental toxins and UV radiation. | 60 |
| (2) Inhibit the activation of these PI3K/Akt and MAPK pathways potentially reducing the risk of cancer development, including skin cancer. | 61,62 | ||||
| D | |||||
| 4. | Ellagic acid |
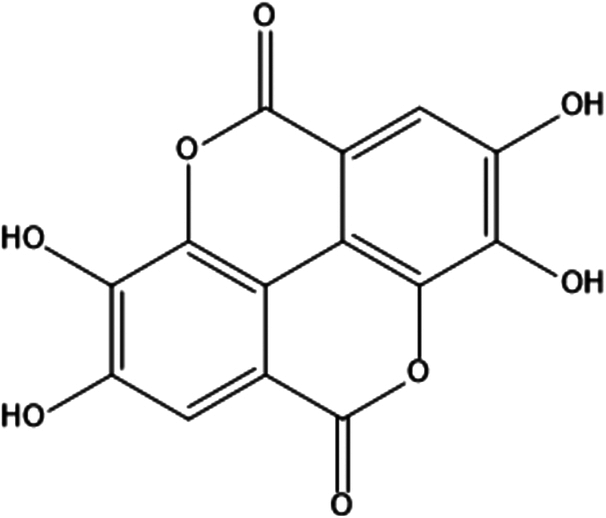
|
2,3,7,8-tetrahydroxychromeno[5,4,3-cde]chromene-5,10-dione | (1) Inhibit the activation of NF-κB signaling pathway to prevent the proliferation of skin cancer cells and induce apoptosis. | 63–65 |
| (2) Activate the p53 signaling pathway, which can induce cell cycle arrest and apoptosis in skin cancer cells. | 66 | ||||
| (3) Modulation of MAPK signaling pathway. | 63 | ||||
| (4) Inhibition of PI3K/Akt signaling pathway. | 67 | ||||
| E | |||||
| 5. | Betulinic acid |
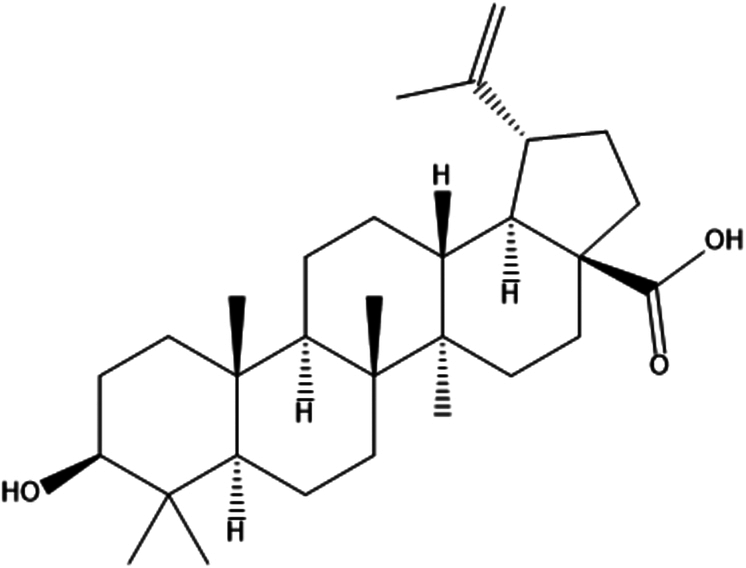
|
(1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-9-hydroxy-5a,5b,8,8,11a-pentamethyl-1-prop-1-en-2-yl-1,2,3,4,5,6,7,7a,9,10,11,11b,12,13,13a,13b-hexadecahydrocyclopenta[a]chrysene-3a-carboxylic acid | (1) Inhibit the activation of NF-κB signaling pathway. | 68 |
| (2) Activation of p53 signaling pathway. | 69,70 | ||||
| (3) Modulation of MAPK signaling pathway. | 71 | ||||
| (4) Inhibition of PI3K/Akt signaling pathway. | 72 | ||||
| (5) Inhibition of Notch signaling pathway. | 73 | ||||
| F | |||||
| 6. | Apigenin |
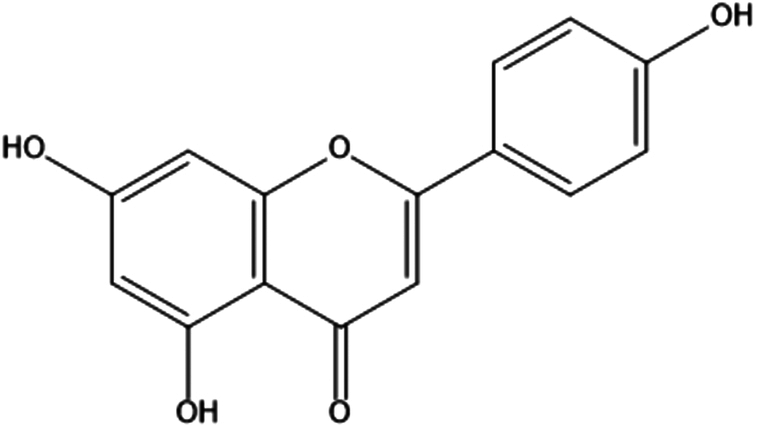
|
4′,5,7-trihydroxyflavone | (1) Inhibition of PI3K/Akt signaling pathway. | 74 |
| (2) Inhibition of MAPK signaling pathway. | 75 | ||||
| (3) By inhibiting the activation of signal transducer and activator of transcription 3 (STAT3). | 76 | ||||
| (4) Apigenin can activate the p53 signaling pathway, which can induce cell cycle arrest and apoptosis in skin cancer cells. | 77 | ||||
| (5) By inhibiting NF-κB signaling pathway. | 78,79 | ||||
| G | |||||
| 7. | Gingerol |
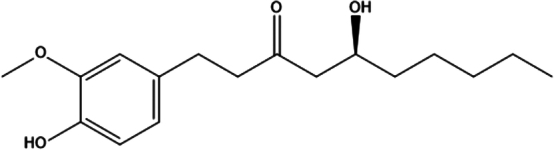
|
(S)-5-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-3-decanone | (1) Inhibition of PI3K/Akt/mTOR signaling pathway. | 80 |
| (2) Inhibition of NF-κB signaling pathway. | 81 | ||||
| (3) Activation of nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway. | 82 | ||||
| (4) Inhibition of MAPK signaling pathway. | 83 | ||||
| (5) Induction of apoptosis. | 80 | ||||
| H | |||||
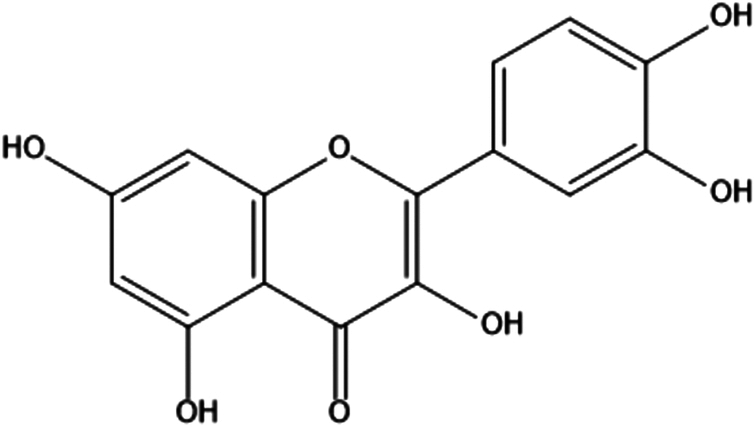
| 8. | Quercetin |
|
3,3′,4′,5,7-pentahydroxyflavone | (1) Inhibition of oxidative stress. | 84 |
| (2) Inhibit the production of pro-inflammatory cytokines such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α). | 85,86 | ||||
| (3) Inhibition of cell proliferation. | 58 | ||||
| (4) Inhibition of angiogenesis. | 87 | ||||
| I | |||||
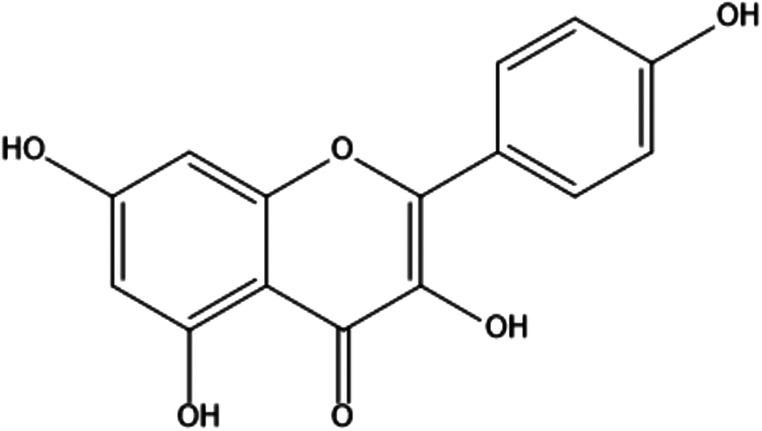
| 9. | Kaempferol |
|
3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one | (1) Inhibition of oxidative stress. | 88,89 |
| (2) Inhibition of inflammation. | 90 | ||||
| (3) Inhibition of cell proliferation. | 88 | ||||
| (4) Inhibition of angiogenesis. | 91,92 | ||||
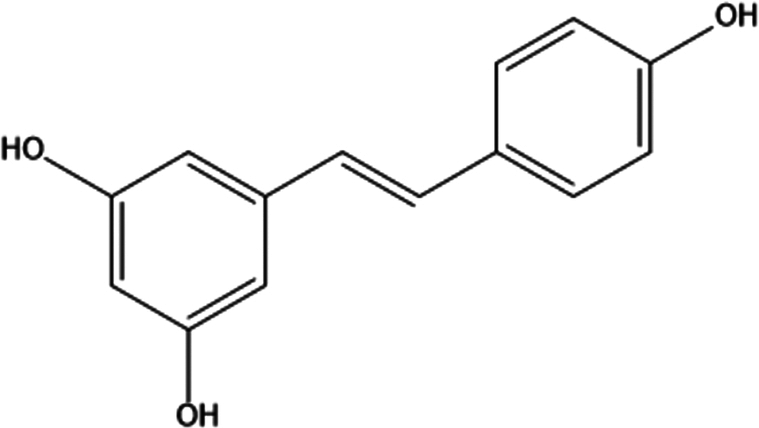
| 10. | Resveratrol |
|
3,5,4′-trihydroxy-trans-stilbene | (1) Inhibition of oxidative stress. | 93 |
| (2) Inhibition of inflammation. | 94,95 | ||||
| (3) Inhibition of cell proliferation. | 96 | ||||
| (4) Inhibition of angiogenesis. | 97,98 | ||||
| J | |||||
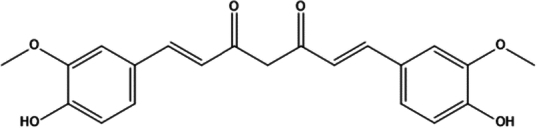
| 11. | Curcumin |
|
(1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione | (1) Inhibition of oxidative stress. | 99,100 |
| (2) Inhibition of inflammation. | 101 | ||||
| (3) Inhibition of cell proliferation. | 102,103 | ||||
| (4) Inhibition of angiogenesis. | 104,105 | ||||
| K | |||||
| 12. | Epigallocatechin gallate |
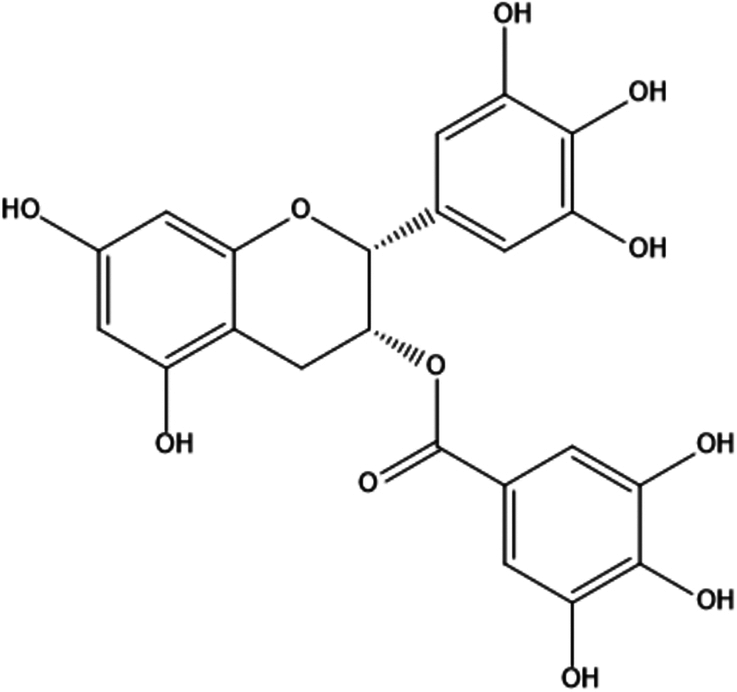
|
[(2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-3-yl]3,4,5-trihydroxybenzoate | (1) Inhibition of oxidative stress. | 106,107 |
| (2) Inhibition of inflammation. | 108,109 | ||||
| (3) Inhibition of cell proliferation. | 110,111 | ||||
| (4) Inhibition of angiogenesis. | 112,113 | ||||
| L | |||||
| 13. | β-Carotene |
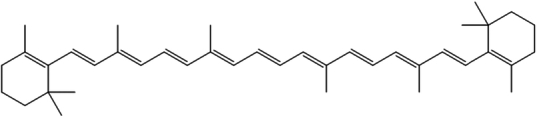
|
1,3,3-trimethyl-2-[3,7,12,16-tetramethyl-18-(2,6,6-trimethyl-1-cyclohexenyl)octadeca-1,3,5,7,9,11,13,15,17-nonaenyl]cyclohexene | (1) Inhibition of oxidative stress. | 114,115 |
| (2) Modulation of immune function. | 116,117 | ||||
| (3) Inhibition of inflammation. | 118,119 | ||||
| (4) Regulation of cell cycle and apoptosis. | 115 | ||||
| (5) Inhibition of angiogenesis. | 120 | ||||
| M | |||||
| 14. | Caffeic acid |
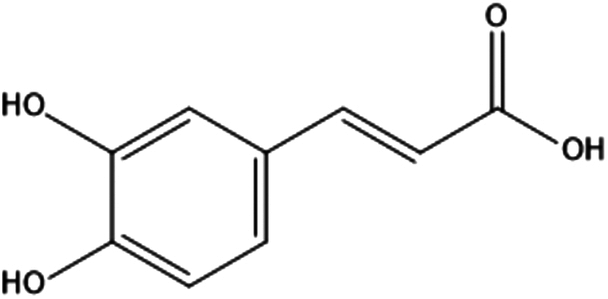
|
3-(3,4-dihydroxyphenyl)prop-2-enoic acid | (1) Inhibit the activation of NF-κB by preventing the degradation of its inhibitor protein, IκBα. | 121,122 |
| (2) Regulates the expression of mitogen-activated protein kinases (MAPKs), which regulate cell proliferation and survival, and cyclooxygenase-2 (COX-2), which promotes inflammation and cell proliferation. | 123,124 | ||||
| N | |||||
| 15. | Ferulic acid |
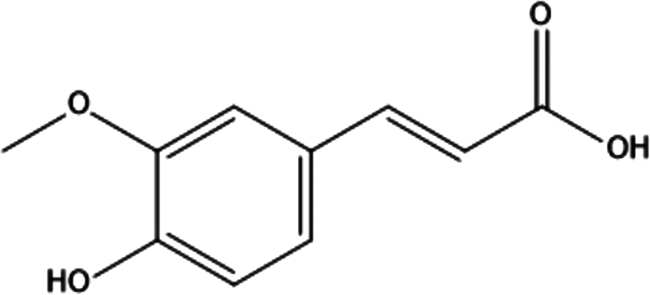
|
(E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoic acid | (1) Inhibit the activation of NF-κB by preventing the degradation of its inhibitor protein, IκBα. | 125 |
| (2) Regulates the expression of MAPKs, which regulate cell proliferation and survival, and COX-2, which promotes inflammation and cell proliferation. | 126 | ||||
Overall, skin cancer therapy via diet-based phytochemicals is a promising approach for the prevention and treatment of skin cancer. Further research is needed to identify the optimal dosage, duration, and combination of phytochemicals for maximum therapeutic efficacy. However, the evidence to date supports the inclusion of phytochemical-rich foods in the diet as an important strategy for skin cancer prevention and therapy. The goal of this review article is to give a basic understanding of phytochemicals in the context of diet by describing their most important sources, chemical classes, and ability to prevent skin cancer.
Rosmarinic acid
Rosmarinic acid is a naturally occurring polyphenol found in various plant species, particularly in the Lamiaceae family130,131. Some of the most common botanical sources of rosmarinic acid include Rosemary (Rosmarinus officinalis), Sage (Salvia officinalis), Lemon balm (Melissa officinalis), Oregano (Origanum vulgare), Thyme (Thymus vulgaris), Mint (Mentha spp.), Basil (Ocimum basilicum), Perilla (Perilla frutescens), Lavender (Lavandula angustifolia), and Peppermint (Mentha piperita)132–134.
Rosmarinic acid has been shown to have potential chemopreventive effects against skin cancer through multiple molecular mechanisms135. Rosmarinic acid has potent antioxidant properties, which can protect skin cells from oxidative stress caused by environmental factors such as UV radiation. This can prevent DNA damage and mutations that can lead to the development of skin cancer136–141. One mechanism through which rosmarinic acid may prevent skin cancer is by inhibiting the activity of enzymes called matrix metalloproteinases (MMPs). MMPs play a crucial role in the breakdown of collagen and other extracellular matrix components. Overactive MMPs can lead to the destruction of the extracellular matrix, which can promote the development of skin cancer. Rosmarinic acid has been shown to inhibit the activity of MMPs, which may help prevent skin cancer142,143. Chronic inflammation is a key contributor to skin cancer development. Rosmarinic acid has been shown to have anti-inflammatory effects by inhibiting the production of pro-inflammatory cytokines and enzymes such as cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS). By reducing inflammation, rosmarinic acid can prevent the progression of precancerous lesions to skin cancer144,145. Rosmarinic acid has been shown to induce apoptosis (programmed cell death) in skin cancer cells, which can inhibit their growth and proliferation146–150. Rosmarinic acid has been shown to arrest the cell cycle of skin cancer cells, preventing them from dividing and proliferating151,152. Angiogenesis, the formation of new blood vessels, is necessary for tumor growth and progression. Rosmarinic acid has been shown to inhibit angiogenesis in skin cancer cells, preventing their growth and spread153.
In recent years, researchers have investigated the molecular mechanisms underlying the effects of rosmarinic acid on skin cancer cells, including its ability to induce apoptosis, inhibit inflammation, and regulate cell cycle progression. For example, Gupta et al. investigated the toxic effects of UVB radiation on the skin and the potential therapeutic effects of plant-based natural agents. The study found that UVB exposure induced endoplasmic reticulum (ER) stress and inhibited mitophagy, leading to intracellular damage and apoptosis. Treatment with the natural agent rosmarinic acid prevented intracellular damage by alleviating ER stress and promoting mitophagy. The study highlights the potential of rosmarinic acid as a therapeutic agent for photodamage and provides mechanistic insights into the toxic effects of UVB radiation on skin154. Another study, conducted by Huang et al., investigated the anticancer effects of rosmarinic acid in melanoma cells by downregulating (a disintegrin and metalloproteinase 17) ADAM17. Results showed that rosmarinic acid treatment reduced cell viability, proliferation, migration, and invasion abilities while increasing apoptosis and reducing melanin content. Rosmarinic acid also inhibited the expression of ADAM17/epidermal growth factor receptor (EGFR)/protein kinase B (AKT)/glycogen synthase kinase 3 beta (GSK3β), which was further suppressed by TPD, an ADAM17 inhibitor. The study concludes that rosmarinic acid exerts an inhibitory effect on melanoma cell growth and promotes apoptosis, potentially through the inhibition of the ADAM17/EGFR/AKT/GSK3β axis155. A study by Sharmila et al. investigated the mechanisms by which rosmarinic acid affects the expression of MAPK signaling proteins and their downstream targets in mice with dermal cancer, as well as to analyze the docking interaction of rosmarinic acid with the extracellular signal-regulated kinase 2 (ERK2) protein. Dermal cancer was induced in mice by applying 7,12-dimethylbenz[a]anthracene (DMBA), and various analyses were performed to observe the expression of proteins related to MAPK signaling, as well as histopathological changes. The study found that rosmarinic acid significantly reduced the expression of various proteins related to MAPK signaling in dermal tissues and inhibited the activation of ERK2 protein, which may contribute to the inhibitory effect of rosmarinic acid on dermal cancer in mice156. The study conducted by Lukmanul Hakkim et al. aimed to investigate whether natural antioxidants including caffeic acid, rosmarinic acid, trans-cinnamic acid, p-coumaric acid, and hydroxyphenyllactic acid could offer radiation protection for skin cells. Non-toxic concentrations of these compounds were tested for radiation protection in human keratinocytes. Results showed that pretreatment with caffeic acid, rosmarinic acid, and trans-cinnamic acid could protect skin cells by scavenging γ-radiation-induced reactive oxygen species and decreasing the number of post-irradiation DNA double-strand break foci. The inclusion of these compounds in chemo-radiotherapy could potentially facilitate achieving multiple target protection, including anticancer and skin radio protection157.
Rosmarinic acid has been shown to regulate several major signaling pathways in the human body including:
The phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway
The PI3K/AKT/mTOR pathway is frequently activated in various types of cancer, including skin cancer. Rosmarinic acid has been shown to inhibit this pathway by decreasing the phosphorylation of AKT and mTOR, leading to decreased cell proliferation and survival53.
The MAPK/ERK pathway
The MAPK/ERK pathway is another important signaling pathway involved in skin cancer development. Rosmarinic acid has been shown to inhibit this pathway by decreasing the phosphorylation of ERK, leading to decreased cell proliferation and invasion54.
The nuclear factor-κB (NF-κB) pathway
The NF-κB pathway is a transcription factor that plays a critical role in inflammation and cancer development. Rosmarinic acid has been shown to inhibit the activation of NF-κB by preventing the degradation of the inhibitor of kappa B alpha (IκBα), leading to decreased inflammation and cell proliferation55.
The Wnt/β-catenin pathway
The Wnt/β-catenin pathway is involved in the regulation of cell proliferation, differentiation, and apoptosis. Dysregulation of this pathway has been linked to various types of cancer, including skin cancer. Rosmarinic acid has been shown to inhibit this pathway by decreasing the expression of β-catenin and its downstream target genes, leading to decreased cell proliferation and invasion56,57.
The ability of rosmarinic acid to regulate major signaling pathways in the human body suggests that it may have therapeutic potential for the prevention and treatment of various diseases, including cancer158. While rosmarinic acid has shown promising anticancer properties in vitro and in animal studies, there are several potential drawbacks and limitations to its use in treating skin cancer131,159. While rosmarinic acid is generally considered safe, there are some potential side effects, including nausea, vomiting, and allergic reactions160. Rosmarinic acid has poor bioavailability, which means that it may not be absorbed well by the body when taken orally or applied topically161. The amount of rosmarinic acid in herbal supplements or preparations can vary widely, which makes it difficult to establish a standardized dose or formulation for treating skin cancer. This may limit its effectiveness in treating skin cancer162,163. More research is needed to determine its safety and efficacy and to optimize the dosage and delivery of rosmarinic acid for maximum efficacy.
Allicin
Allicin is a natural compound found in garlic (Allium sativum)164. Allicin belongs to the class of secondary metabolites called organosulfur compounds. These compounds are characterized by the presence of sulfur atoms in their chemical structure and are often produced by plants as a defense mechanism against pests and pathogens165–167. When garlic is crushed or chopped, an enzyme called alliinase is activated, which converts alliin, a sulfur-containing amino acid derivative, into allicin168. Allicin is responsible for the characteristic odor and flavor of fresh garlic, and it is also believed to be responsible for many of the health benefits associated with garlic consumption169,170. The molecular mechanism of allicin’s action against skin cancer is not completely understood, but several studies have suggested that it targets multiple pathways involved in cancer development and progression171. A study investigated the effect of allicin on the migration and invasion of human melanoma cells (A375 and SK-MEL-28). Allicin was found to inhibit the migration and invasion of melanoma cells by downregulating the expression of genes involved in epithelial–mesenchymal transition (EMT), a process that allows cancer cells to acquire invasive and metastatic properties. Allicin was also found to downregulate the expression of the COX-2 gene, which is involved in inflammation and cell proliferation. Furthermore, allicin was found to inhibit the activation of the NF-κB pathway and the expression of its downstream target genes, which are involved in cell survival, inflammation, and tumor progression58. In a study conducted by Wang et al., it was observed that several compounds, including allicin, allyl sulfides, ajoene, diallyl trisulfide (DATS), and S-allyl cysteine (SAC), have shown anticancer activity against various types of cancer, including skin cancer. DATS is more potent than mono- and disulfides against skin cancer. DATS inhibits cell growth of human melanoma A375 cells and basal cell carcinoma (BCC) cells by increasing the levels of intracellular reactive oxygen species (ROS) and DNA damage, inducing G2/M arrest, endoplasmic reticulum (ER) stress, and mitochondria-mediated apoptosis, including the caspase-dependent and caspase-independent pathways171. In one of the studies, Jobani et al. aimed to investigate the potential of allicin to sensitize malignant melanoma cells to all-trans retinoic acid (ATRA) therapy. The CD44+ and CD117+ melanoma cell subpopulations were sorted, and the effects of ATRA, allicin, and allicin/ATRA on cell proliferation and cell cycle arrest were examined. The results showed that CD44+ melanoma cells were more resistant to ATRA and allicin than CD117+ cells. However, allicin was found to sensitize melanoma cells to ATRA-induced cell death, and the combination treatment significantly reduced the IC50 value obtained for ATRA alone in CD44+ melanoma cells. Furthermore, allicin and ATRA combination treatment showed inhibitory effects on CD44+ and CD117+ melanoma cells, and allicin alone reduced matrix metalloproteinase-9 (MMP-9) mRNA expression in both cell subpopulations. The findings suggest that allicin may reinforce the ATRA-mediated inhibitory effects on melanoma cells, providing a new approach for the treatment of malignant melanoma172.
In another study conducted by Omar and Al-Wabel, it was observed that garlic contains chemical compounds that have been shown to protect against several diseases, including cancer, particularly in the stomach, colorectal, breast, and skin. The protective effects are related to the presence of organosulfur compounds (like allicin, etc.), which inhibit carcinogenesis in various experimental animals. The compounds modulate the activity of several metabolizing enzymes and inhibit the formation of DNA adducts. Antiproliferative activity has been observed in tumor cell lines, possibly mediated by induction of apoptosis and alterations of the cell cycle. Garlic’s organosulfur compounds are potential cancer-preventive agents, but clinical trials are necessary to define the effective dose with no toxicity in humans173.
Allicin has been shown to scavenge free radicals and protect against oxidative damage induced by UV radiation, which can contribute to the development of skin cancer174. Allicin has been shown to suppress the production of inflammatory cytokines, such as interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α), which can promote the development and progression of skin cancer175. Allicin has been shown to induce apoptosis (programmed cell death) in cancer cells, including skin cancer cells, by activating caspases and other apoptosis-related proteins176. Allicin has been shown to arrest the cell cycle of cancer cells, preventing them from dividing and multiplying, which can help to prevent the growth and spread of skin cancer177. Allicin has been shown to inhibit the formation of new blood vessels, which is a critical step in the growth and spread of cancer178.
The major signaling pathways that have been reported to be regulated by allicin are as follows:
NF-κB pathway
Allicin has been shown to inhibit the activation of NF-κB, a transcription factor that plays a key role in inflammation and cancer development58.
PI3K)/Akt/mTOR pathway
Allicin has been shown to inhibit the activation of this pathway, which is frequently dysregulated in cancer cells and promotes cell survival, growth, and proliferation179.
Wnt/β-catenin pathway
Allicin has been shown to inhibit the activation of this pathway, which is frequently dysregulated in cancer cells and promotes cell proliferation and survival59.
MAPK pathway
Allicin has been shown to inhibit the activation of the MAPK pathway, which plays a critical role in cell proliferation, differentiation, and survival180.
STAT3 pathway
Allicin has been shown to inhibit the activation of STAT3, a transcription factor that plays a critical role in inflammation and cancer development181.
Overall, these pathways play important roles in regulating cell growth, survival, and inflammation and dysregulation of these pathways can contribute to the development and progression of skin cancer. By regulating these pathways, allicin has the potential to prevent or slow down the progression of skin cancer178–180. While allicin may have some benefits, there are also potential drawbacks when it comes to using it against skin cancer. Allicin can be a skin irritant and may cause redness, itching, or burning when applied topically. This can be especially problematic for people with sensitive skin or those who are already experiencing skin irritation due to cancer treatments182. Allicin is not widely available in a standardized form for medical use, which can make it difficult to obtain and ensure quality183. It can be concluded that while allicin may have potential benefits for skin cancer, there are also potential drawbacks and limitations to its use.
Sulforaphane
Sulforaphane is a naturally occurring compound found in cruciferous vegetables, such as broccoli, cauliflower, Brussels sprouts, and kale184. Sulforaphane belongs to the family of isothiocyanates. It is formed when the enzyme myrosinase comes into contact with glucoraphanin, a glucosinolate compound present in these vegetables185. Broccoli is the richest dietary source of sulforaphane, with broccoli sprouts containing even higher concentrations of the compound186. Sulforaphane is also available as a dietary supplement, which is typically derived from broccoli sprouts or broccoli seed extract187. It is important to note that the content of sulforaphane in cruciferous vegetables can vary widely depending on factors such as plant variety, growing conditions, and preparation methods. For example, chopping, chewing, or blending cruciferous vegetables can activate myrosinase and increase the formation of sulforaphane, whereas cooking or boiling can reduce the amount of the compound188,189.
Sulforaphane has been shown to exhibit chemopreventive and therapeutic effects against various types of cancer, including skin cancer190,191. Its mechanism of action against skin cancer involves several pathways and cellular processes:
Induction of phase II detoxification enzymes
Sulforaphane activates the nuclear factor erythroid 2-related factor 2–antioxidant response element (Nrf2–ARE) signaling pathway, leading to the induction of phase II detoxification enzymes, such as glutathione S-transferases (GSTs), which are involved in the elimination of carcinogens and other toxic compounds from the body192.
Inhibition of inflammation
Sulforaphane can modulate the expression of genes involved in inflammation and immune responses, such as NF-κB and COX-2, leading to a reduction in pro-inflammatory cytokines and chemokines193. Chronic inflammation is known to contribute to the development and progression of skin cancer.
Induction of apoptosis
Sulforaphane can induce apoptosis (programmed cell death) in cancer cells by activating caspases and other apoptotic pathways194.
Inhibition of angiogenesis
Sulforaphane can suppress the formation of new blood vessels (angiogenesis) that supply nutrients to cancer cells, thereby inhibiting their growth and proliferation195.
Modulation of epigenetic mechanisms
Sulforaphane can modulate epigenetic mechanisms, such as DNA methylation and histone acetylation, leading to changes in gene expression that can affect various cellular processes, including those involved in the development and progression of cancer196–198.
Several studies have been conducted by researchers to investigate the molecular mechanism of action of sulforaphane against skin cancer. For instance, Eom et al. investigated the effects of sulforaphane treatment on B16F10 melanoma cells and zebrafish models. The results showed that sulforaphane treatment reduced cell proliferation, increased tyrosinase production, and induced cytoskeletal reorganization, leading to an elongated appearance of melanoma cells. Sulforaphane treatment also regulated the protein expression of microphthalmia-associated transcription factor (MITF), protein kinase C beta 1 (PKCβ1), and tyrosinase. The study further demonstrated that sulforaphane-induced biosynthesis of melanin in melanoma cells occurs through changes in actin, as shown by co-treatment of sulforaphane with cytochalasin D (CD) and jasplakinolide (JAS). The same results were obtained in zebrafish models, where sulforaphane upregulated melanin levels despite the presence of the melanin inhibitor phenylthiourea (PTU)199. A study performed by Balasubramanian et al. investigated the impact of sulforaphane (SFN), a potential cancer-preventative agent found in cruciferous vegetables, on the expression and function of PcG proteins, which are known to promote cell survival and suppress gene expression in cancer cells. The study found that SFN treatment resulted in a concentration-dependent reduction of PcG protein expression in skin cancer cells, leading to a decrease in histone H3 trimethylation and an accumulation of cells in the G2/M phase. The treatment also increased apoptosis, as evidenced by enhanced cleavage of caspase and PARP proteins. The results suggest that SFN may inhibit PcG-dependent pro-survival epigenetic events via proteasome-dependent degradation, thus suppressing cancer progression196. In another study, Dickinson et al. investigated the chemopreventive properties of sulforaphane, an isothiocyanate found in cruciferous vegetables, against UVB-induced squamous cell carcinoma in mice. The study found that sulforaphane treatment reduced the multiplicity and tumor burden of squamous cell carcinoma in mice co-treated with the carcinogen and sulforaphane. The study also showed that sulforaphane was able to reduce the activity of the transcription factor activator protein-1 (AP-1) in the skin of transgenic mice after UVB. Chromatin immunoprecipitation analysis revealed that sulforaphane inhibited c-Fos, a constituent of the AP-1 dimer, from binding to the AP-1 DNA-binding site. The study also found that sulforaphane and diamide, both known to react with cysteine amino acids, effectively inhibited AP-1 from binding to its response element. Mutation of critical cysteines in the DNA-binding domain of c-Fos and c-Jun resulted in loss of sensitivity to sulforaphane and diamide. These findings suggest that inhibition of AP-1 activity by sulforaphane may be an important mechanism for the chemoprevention of squamous cell carcinoma200.
While sulforaphane may have some benefits, there are also potential drawbacks when it comes to using it against skin cancer. The potency of sulforaphane can vary depending on how it is prepared and stored. This can make it difficult to determine the appropriate dosage and ensure consistent results201. Sulforaphane may interact with certain medications, including blood thinners and medications used to treat HIV. Overall, the multiple mechanisms of action of sulforaphane suggest its potential as a promising chemopreventive and therapeutic agent against skin cancer. However, more research is needed to fully understand its effects, safer dose, drug interaction studies and to develop effective treatment strategies.
Ellagic acid
Ellagic acid is a naturally occurring polyphenolic compound found in several fruits and vegetables, including strawberries, raspberries, blackberries, pomegranates, and walnuts202. It is formed from the hydrolysis of ellagitannins, which are water-soluble compounds present in these foods203. Ellagic acid has been shown to exhibit chemopreventive and therapeutic effects against various types of cancer204, including skin cancer. Its mechanism of action against skin cancer involves several pathways and cellular processes:
Antioxidant activity
Ellagic acid is a potent antioxidant that can scavenge free radicals and protect cells from oxidative stress-induced damage, which is implicated in the development of skin cancer205.
Inhibition of inflammation
Ellagic acid can modulate the expression of genes involved in inflammation and immune responses, such as NF-κB and COX-2, leading to a reduction in pro-inflammatory cytokines and chemokines206. Chronic inflammation is known to contribute to the development and progression of skin cancer.
Induction of apoptosis
Ellagic acid can induce apoptosis (programmed cell death) in cancer cells by activating caspases and other apoptotic pathways. This mechanism can help eliminate cancer cells and prevent their proliferation207–209.
Inhibition of angiogenesis
Ellagic acid can suppress the formation of new blood vessels (angiogenesis) that supply nutrients to cancer cells, thereby inhibiting their growth and proliferation123.
Modulation of cellular signaling pathways
Ellagic acid can modulate various signaling pathways involved in the regulation of cell growth, proliferation, and survival, including the MAPK/ERK, PI3K/AKT, and Wnt/β-catenin pathways. Dysregulation of these pathways is implicated in the development of skin cancer67,104.
Researchers have extensively studied the molecular mechanism of action of ellagic acid against skin cancer. Hseu et al. investigated the protective effects of ellagic acid against UVA-induced oxidative stress and apoptosis in human keratinocyte cells. Ellagic acid was found to increase cell viability, suppress ROS generation, prevent DNA damage, and inhibit UVA-induced apoptosis. The antioxidant potential of ellagic acid was linked to the increased expression of HO-1 and SOD, downregulation of Keap1, and the activation of Nrf2. Nrf2 knockdown diminished the protective effects of ellagic acid, indicating its potential use for the treatment of UVA-induced skin damage and skin cancer prevention204. In addition, the effects of ellagic acid, a polyphenolic compound from pomegranate fruit extracts, on melanoma cells were investigated. The results showed that ellagic acid significantly inhibited the proliferation, migration, and invasion of WM115 and A375 melanoma cells. Ellagic acid treatment decreased the expression of p-EGFR and vimentin, while it increased the expression of E-cadherin in both cell lines. EGFR activation abolished the effect of ellagic acid on melanoma cells. Additionally, ellagic acid treatment impaired in vivo tumorigenesis of A375 cells, and elevated phosphorylated epidermal growth factor receptor (p-EGFR) expression was an independent detrimental factor for melanoma patients. The study suggests that ellagic acid may be useful for the development of new therapeutic strategies for melanoma via the EGFR signaling pathway by Wang et al.210. Moreover, Bia et al. investigated the stress-resistant action of ellagic acid in Caenorhabditis elegans (C. elegans) and found that 50 μM ellagic acid significantly prolonged the lifespan of C. elegans under ultraviolet radiation stress, heat stress, oxidative stress, and Pseudomonas aeruginosa infection stress. Ellagic acid was also found to reduce damage caused by ultraviolet radiation by inducing the nucleus translocation of Dauer formation 16 (DAF-16) and activating a series of target genes to resist ultraviolet radiation stress. Ellagic acid increased the expression of superoxide dismutase 3 (SOD3) to clean out harmful reactive oxygen species in C. elegans exposed to ultraviolet radiation stress. The results suggest that ellagic acid plays an important role in resisting ultraviolet radiation stress in C. elegans, probably in an insulin/insulin-like growth factor-1(IGF-1) signaling pathway-dependent way, and its effects are dependent on the DAF-16 gene, thus helps in preventing skin cancer and other diseases including diabetes, arteriosclerosis, neurodegenerative diseases, stroke, and cataracts211.
While ellagic acid has shown promising health benefits, there are also some potential drawbacks that should be considered. Ellagic acid is poorly absorbed in the body and has low bioavailability. It is rapidly metabolized and eliminated, limiting its potential therapeutic efficacy. Strategies to improve its bioavailability, such as combining it with other compounds or using nanoformulations, are being explored212. Ellagic acid may interact with certain drugs and supplements, including anticoagulants and antiplatelet agents, and may increase the risk of bleeding or bruising. It may also interact with certain chemotherapy drugs and affect their efficacy213. Ellagic acid may cause allergic reactions in some individuals, particularly those who are allergic to berries or other foods that contain the compound214. There is no standardized dosage for ellagic acid, and the optimal dose may vary depending on the specific health condition being targeted215. Additionally, the amount of ellagic acid in foods and supplements can vary widely, making it difficult to determine the actual dose being consumed216. While ellagic acid has shown promising results in preclinical studies, there is limited clinical evidence to support its use in humans. Overall, the multiple mechanisms of action of ellagic acid suggest its potential as a promising chemopreventive and therapeutic agent against skin cancer. However, more research is needed to fully understand its effects, safer dose, drug interaction studies, allergic reaction studies and to develop effective treatment strategies.
Betulinic acid
Betulinic acid is a naturally occurring triterpenoid compound that is found in the bark of several tree species, including white birch (Betula pubescens), which is its primary botanical source217. Betulinic acid can also be found in other plants, such as the Chinese herb Zizyphus jujuba Mill var. spinosa 218, and in some fruits and vegetables, such as apples219 and strawberries220, although in lower concentrations. The compound can be extracted from the bark of the white birch tree221 using various methods, including maceration and solvent extraction. Betulinic acid has shown a range of biological activities, including anti-inflammatory, antiviral, and anticancer effects222,223.
Betulinic acid has been shown to regulate several signaling pathways involved in the development and progression of skin cancer. These pathways include:
PI3K/Akt/mTOR pathway
Betulinic acid can inhibit the PI3K/Akt/mTOR pathway, which is involved in cell growth and survival. This pathway is commonly activated in cancer cells, including skin cancer cells, and contributes to their proliferation and survival224.
MAPK/ERK pathway
Betulinic acid can also modulate the MAPK/ERK pathway, which regulates cell proliferation and differentiation. This pathway is often dysregulated in skin cancer and contributes to its development and progression225,226.
Wnt/β-catenin pathway
Betulinic acid has been shown to inhibit the Wnt/β-catenin pathway, which plays a key role in cell proliferation and stem cell self-renewal227. Aberrant activation of this pathway has been implicated in the development of various types of cancer, including skin cancer.
NF-κB pathway
Betulinic acid can also inhibit the NF-κB pathway, which is involved in inflammation, cell survival, and proliferation. This pathway is commonly activated in cancer cells and contributes to their survival and resistance to chemotherapy68.
Several studies have been conducted to investigate the molecular mechanism of action of betulinic acid against skin cancer. For instance, Wróblewska-Łuczka et al. investigated the effects of betulinic acid, alone and in combination with taxanes, on the growth of melanoma cell lines. Betulinic acid had no cytotoxic effect on normal cells but significantly inhibited the growth of melanoma cells in vitro, with IC50 values ranging from 2.21 to 15.94 µM. Co-treatment with betulinic acid and taxanes showed desirable drug interactions, with additive and additive with a tendency to synergy interactions observed. These findings suggest that betulinic acid may be a potential therapeutic agent for melanoma, either alone or in combination with taxanes228. One of the studies performed by Liao et al. examined the biological effects of betulinic acid (BA)-functionalized GNP in human keratinocytes and melanoma cells. Betulinic acid was grafted onto citrate-capped GNP (BA-GNP) using cysteamine as a linker. The results showed that the BA-GNP formulation had selective cytotoxic and antiproliferative effects on melanoma cells compared to free betulinic acid. Further analysis revealed a pro-apoptotic effect, as evidenced by morphological changes and western blot data showing downregulation of anti-apoptotic Bcl-2 expression and upregulation of pro-apoptotic Bax. GNP also significantly inhibited mitochondrial respiration, demonstrating its mitochondrial-targeted activity. These findings suggest that BA-functionalized GNP could be a potential therapeutic option for melanoma229.
The study performed by Kallimanis et al. aimed to identify natural compounds that could inhibit aryl hydrocarbon receptor (AhR) activation by these ligands. The methanolic Rosmarinus officinalis L. extracts (ROE) were assayed for their activities as antagonists of AhR ligand binding with guinea pig cytosol. The isolated metabolites (viz. carnosic acid, carnosol, 7-O-methyl-epi-rosmanol, 4′,7-O-dimethylapigenin, and betulinic acid) were assayed for their agonist and antagonist activity in the presence and absence of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) using the gel retardation assay. All assayed extracts showed almost complete inhibition of AhR activation by TCDD at 100 ppm. The methanol ROE at 10 ppm showed significant inhibition against TCDD, 6-formylindolo[3,2-b]carbazole (FICZ), indirubin (IND), and pyrazolo[1,5-a]pyrimidine (PZ), respectively, in human keratinocytes. Most assayed metabolites exhibited dose-dependent antagonist activity. The results suggest that ROE could be useful for the prevention or treatment of skin diseases mediated by the activation of AhR230.
Betulinic acid is generally considered safe and non-toxic at therapeutic doses, and it has shown promising anticancer activity in preclinical studies. However, there are some potential drawbacks associated with its use. Betulinic acid has poor water solubility and low bioavailability, which can limit its effectiveness in vivo. Various delivery systems and formulations have been developed to enhance its bioavailability and effectiveness231–235. Although betulinic acid has shown promising activity against skin cancer in preclinical studies, there is limited clinical data on its safety and efficacy in humans. Further clinical studies are needed to determine its potential as a therapeutic agent for skin cancer236.
Overall, while betulinic acid has shown promise as a potential therapeutic agent against skin cancer, more research is needed to fully understand its bioavailability, safety, efficacy, and optimal use in clinical settings.
Apigenin
Apigenin is a naturally occurring flavone that can be found in various plants, including parsley, chamomile, celery, thyme, and red pepper237. It is also present in many fruits and vegetables, such as oranges, grapefruit, onions, and broccoli. Apigenin can be extracted from these sources using various methods, including microwave-assisted extraction238, ultrasound-assisted extraction239, supercritical carbon dioxide extraction, enzyme-assisted extraction240, high-speed counter-current chromatography241, etc. The mechanism of action of apigenin against skin cancer involves multiple pathways, including:
Induction of apoptosis
Apigenin can induce programmed cell death or apoptosis in cancer cells. It does so by activating caspase enzymes, which are responsible for cleaving and degrading proteins that are essential for cell survival. By inducing apoptosis, apigenin can reduce the number of cancer cells in the skin and prevent the formation of tumors242.
Inhibition of cell proliferation
Apigenin can inhibit the proliferation of cancer cells by regulating cell cycle progression. It does so by suppressing the expression of cyclin-dependent kinases and upregulating the expression of cell cycle inhibitors. This results in the inhibition of cell division and reduced growth of skin cancer cells243.
Regulation of signaling pathways
Apigenin can regulate several signaling pathways that are involved in the development and progression of skin cancer. It can inhibit the activation of the PI3K/Akt75,243, MAPK112,243, and Wnt/β-catenin pathways244, which promote cell survival, proliferation, and migration. It can also activate the Nrf2/ARE pathway, which is involved in cellular antioxidant and detoxification responses245. By regulating these pathways, apigenin can prevent the growth and spread of skin cancer cells.
Anti-inflammatory effects
Apigenin has been shown to have anti-inflammatory effects that can protect the skin from UV radiation-induced damage. It can reduce the production of pro-inflammatory cytokines and inhibit the activity of enzymes that promote inflammation. By reducing inflammation, apigenin can prevent the formation of skin cancer cells246.
Several studies have investigated the molecular mechanism of action of apigenin against skin cancer. In one study conducted by Bridgeman et al., it was found that apigenin inhibited UVB-induced mTOR activation, cell proliferation, and cell cycle progression in mouse skin and keratinocytes. The inhibition of UVB-induced mTOR signaling by apigenin was not Akt-dependent but instead was driven by adenosine monophosphate-activated protein kinase (AMPK) activation. Additionally, mTOR inhibition by apigenin enhanced autophagy and decreased proliferation in keratinocytes, providing a new target and strategy for better prevention of UV-induced skin cancer247. Another study by Das et al. aimed to evaluate the antiproliferative effects of apigenin-loaded poly (lactic-co-glycolide) nanoparticles (NAp) on A375 skin cancer cells in vitro. NAp was characterized for particle size, morphology, zeta potential, drug release, and encapsulation. The cellular entry and intracellular localization of NAp were evaluated, along with the stability of dsDNA and relevant markers of mitochondrial functioning, such as ATPase activity, cytochrome-c release, and caspase-3 activity. NAp showed better efficacy due to their smaller size and faster mobility with site-specific action. The study revealed that NAp could intercalate with dsDNA, leading to ROS accumulation and depletion of antioxidant enzyme activities, resulting in DNA damage and apoptosis through mitochondrial dysfunction. The study suggests that NAp could be a potential therapeutic option for combating skin melanoma248. A study was performed by Jangdey et al. to optimize transfersomes, which are vesicular carriers for drug delivery, using a modified rotary evaporation sonication technique and surfactant Tween 80. The Box-Behnken design with three factors and three levels was applied using response surface methodology. The formulations were characterized for size, shape, entrapment efficiency, stability, and in-vitro permeation. The optimized formulation had an entrapment efficiency of 84.24%, a vesicle size of 35.41 nm, and a drug loading of 8.042%, with good stability. This approach shows promise for the sustained release of apigenin for an extended period of time249. Waheed et al. performed a study to develop and optimize lyotropic liquid crystalline nanoparticles (LLC NPs) loaded with apigenin (API) for effective dermal delivery using a quality-by-design (QbD) approach. The optimized API-LLC NPs showed particle size, polydispersity index (PDI), and entrapment efficiency of 287.7±9.53 nm, 0.152±0.051 and 80±2.2 %, respectively. In-vitro and ex-vivo studies showed sustained release and a better permeation profile. The developed API-LLC NPs exhibited better penetration of deeper skin layers, with cytotoxic efficacy assessed on B16F10 cell lines showing a dose-dependent efficacy of API-LLC NPs with an IC50 of 45.74±0.05, making it a promising topical drug delivery nanocarrier for the treatment and management of skin cancer250.
Overall, the mechanism of action of apigenin against skin cancer is multifaceted and involves the modulation of several cellular processes. Apigenin is generally considered safe and well-tolerated, and side effects are rare. However, high doses of apigenin supplements or extracts may cause some adverse effects. High doses of apigenin may cause digestive issues such as diarrhea, nausea, and stomach upset251. In some individuals, apigenin may cause an allergic reaction, especially if they have an allergy to other flavonoids or plants in the same family as apigenin252. Apigenin may interact with certain medications, including blood thinners, chemotherapy drugs, and medications that are metabolized by the liver253,254. Although preclinical studies have shown promising results, there is currently a lack of clinical evidence to support the efficacy and safety of apigenin as a treatment for skin cancer. More research is needed to determine its potential benefits and risks.
Gingerol
Gingerol is a bioactive compound found in ginger (Zingiber officinale), a spice and medicinal plant that has been used for centuries for its health benefits255. Gingerol belongs to the class of secondary metabolites known as phenolic compounds256. Gingerol is a member of the gingerols, a group of compounds that are responsible for the pungent flavor and aroma of ginger257. Gingerol is known for its anti-inflammatory, antioxidant, and anticancer properties258–260. It has been studied for its potential to help manage a variety of health conditions, including nausea, vomiting, pain, and inflammation261,262.
Gingerol has been shown to regulate several signaling pathways involved in the development and progression of skin cancer, including the MAPK/ERK83 and PI3K/Akt80 pathways. The MAPK/ERK pathway is a signaling pathway that regulates cell growth, division, and survival. Dysregulation of this pathway can contribute to the development of skin cancer. Gingerol has been shown to inhibit the activation of the MAPK/ERK pathway in skin cancer cells, which can help prevent the growth and proliferation of cancer cells83. The PI3K/Akt pathway is another signaling pathway that plays an important role in regulating cell growth, division, and survival. Dysregulation of this pathway has also been implicated in the development of skin cancer. Gingerol has been shown to inhibit the activation of the PI3K/Akt pathway in cancer cells, which can help prevent the growth and proliferation of cancer cells80. In addition to regulating these signaling pathways, gingerol has also been shown to modulate the expression of several genes involved in skin cancer development and progression. For example, gingerol has been shown to upregulate the expression of tumor suppressor genes such as p53 and phosphatase and tensin (PTEN) homolog, which can help prevent the development of skin cancer263.
Several studies have been conducted to investigate the molecular mechanism of action of gingerol against skin cancer. A study performed by Praveena et al. aimed to investigate the anticancer activity of [6]-gingerol, a bioactive compound found in the rhizome of Zingiber officinale and its structural analogs against skin cancer. The ethanolic crude extract of the plant was subjected to phytochemical and gas chromatography–mass spectrometry (GC–MS) analysis to confirm the presence of [6]-gingerol. The anticancer activity was evaluated using the A431 human skin adenocarcinoma cell line, and the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay showed promising cytotoxicity with an IC50 of 81.46 μg/ml. In silico studies were conducted using [6]-gingerol and 21 structural analogs to investigate their anticancer potential and drug-likeliness properties. The study targeted the skin cancer protein, DEAD-box helicase 3 X-linked (DDX3X), which regulates all stages of ribonucleic acid (RNA) metabolism, and the compounds were docked to identify the most potent lead molecule based on the lowest binding energy value. The study suggests that [6]-gingerol and its structural analogs could be used as lead molecules for future drug development against skin cancer264. One of the studies conducted by Nigam et al. investigated the chemopreventive potential of [6]-gingerol, a pungent ingredient found in ginger rhizome, against benzo[a]pyrene (B[a]P)-induced mouse skin tumorigenesis. Topical treatment of [6]-gingerol was given to animals prior to and after B[a]P treatment. Results showed a delay in tumorigenesis onset, reduced tumor numbers and volume, and elevated apoptotic propensity in tumor tissues. Western blot analysis showed [6]-gingerol treatment increased p53 levels, Bcl-2-associated X protein (Bax), and apoptotic protease-activating factor-1 (Apaf-1) while decreasing B-cell lymphoma 2 (Bcl-2) and Survivin expression. The study suggests that [6]-gingerol has apoptotic potential as a mechanism of chemoprevention and warrants further investigation265. Another study by Park et al. reported the potential cancer chemopreventive properties of [6]-gingerol, a phenolic compound found in ginger, using a two-stage mouse skin carcinogenesis model. The results showed that topical application of [6]-gingerol prior to each dose of 12-O-tetradecanoylphorbol-13-acetate (TPA) significantly inhibited the development of skin papillomagenesis induced by 7,12-dimethylbenz[a]anthracene. The compound also suppressed TPA-induced epidermal inflammation and ornithine decarboxylase activity. These findings suggest that [6]-gingerol may have potential as an antitumor promotional agent266.
Gingerol has been associated with several potential health benefits. However, there are also some drawbacks and potential side effects of gingerol. Gingerol may have blood-thinning effects, which can increase the risk of bleeding or interfere with the effectiveness of blood-thinning medications267. Gingerol may interact with certain medications, including anticoagulants, antiplatelets267, and blood pressure medications268. Gingerol may not be suitable for pregnant women269. Overall, gingerol appears to regulate multiple signaling pathways involved in the development and progression of skin cancer, which may contribute to its anticancer effects270. However, more research is needed to fully understand the drug interaction studies and safer doses for use in humans.
Quercetin
Quercetin is a natural flavonoid compound found in many fruits, vegetables, and grains271. Some of the food sources of quercetin include onions (Allium cepa), apples (Malus spp.), berries such as blueberries (Vaccinium spp.) and cranberries (Vaccinium macrocarpon), citrus fruits such as grapefruit (Citrus paradisi), oranges (Citrus sinensis), leafy green vegetables such as kale (Brassica oleracea) and spinach (Spinacia oleracea), and grains such as buckwheat (Fagopyrum esculentum)272–277. Flavonoids are responsible for giving plants their vibrant colors47. Quercetin is known for its antioxidant and anti-inflammatory properties and has been studied for its potential health benefits278. The molecular mechanism of action of quercetin against skin cancer involves several pathways and cellular processes. Here are some of the molecular mechanisms by which quercetin acts against skin cancer:
Inhibiting cell proliferation
Quercetin can inhibit cell proliferation by inducing cell cycle arrest at the G1/S phase, which prevents cancer cells from dividing and growing58.
Inducing apoptosis
Quercetin can induce apoptosis, or programmed cell death, in cancer cells. This can help eliminate cancer cells and prevent the spread of cancer279.
Inhibiting angiogenesis
Quercetin can inhibit the formation of new blood vessels, which is necessary for cancer cells to grow and spread87.
Reducing oxidative stress
Quercetin has antioxidant properties and can reduce oxidative stress and damage to skin cells, which can lead to the development of skin cancer84.
Regulating signaling pathways
Quercetin can regulate several signaling pathways involved in skin cancer development and progression, including the PI3K/Akt279, MAPK280, Wnt/β-catenin281, NF-κB282 and JAK/STAT pathways270.
Enhancing the immune system
Quercetin can enhance the immune system’s ability to recognize and destroy cancer cells, reducing the risk of skin cancer84.
In recent years, several studies have investigated the molecular mechanism of action of quercetin against skin cancer. Imran et al. developed a nanostructured lipid carrier (NLC) gel loaded with two drugs, quercetin and resveratrol, to enhance their delivery to the dermal and epidermal layers of the skin. The NLC formulation was optimized using a central composite rotatable design (CCRD) and contained a lipid binary mixture, Cremophor RH40 as a surfactant, and had good particle size, polydispersity index, zeta potential, and entrapment efficiency. Dermatokinetic studies showed that the NLC gel significantly increased the disposition of the drugs in the skin compared to a conventional gel, and this was confirmed by confocal microscopic studies. The cytotoxic effect of the NLC gel was assessed in a human epidermoid carcinoma cell line and found to be lower than that of the conventional gel. These results suggest that the NLC gel could be a promising carrier for the delivery of quercetin and resveratrol into deeper layers of the skin for the treatment of skin cancer283.
Another study by Caddeo et al., focuses on developing liposomes for delivering two natural polyphenols, quercetin and resveratrol. The liposomes were found to be small, spherical, and uni/bilamellar in nature. The incorporation of polyphenols did not affect their antioxidant activity. Liposomal delivery of polyphenols showed higher cellular uptake and better scavenging ability of ROS in fibroblasts. The in-vivo study in a mouse model of skin lesions demonstrated that topical administration of liposomes reduced tissue damage, edema, and leukocyte infiltration. The study suggests that liposomal delivery of polyphenols may be a promising approach for treating inflammation/oxidative stress associated with precancerous/cancerous skin lesions284.
Paliwal et al. used ultrasound to enhance the potency of quercetin as a chemotherapeutic drug for the treatment of prostate and skin cancer. The short application of low-frequency ultrasound selectively induced cytotoxicity in cancer cells, while having minimal effect on normal cell lines. The treatment resulted in a significant reduction of viable skin cancer cell population within 48 h. Ultrasound reduced the LC50 of quercetin for skin cancer cells by almost 80-fold, while showing no effect on LC50 for nonmalignant skin cells. The study suggests that ultrasound can be used as a selective sensitizing agent to enhance the efficacy of bioflavonoids for cancer treatment285. Furthermore, Jung et al. focus on identifying the molecular targets of quercetin and its effect on the inhibition of IGF-1 signaling in skin carcinogenesis. The results show that a quercetin diet remarkably delayed the incidence of skin tumor and reduced tumor multiplicity in a mouse skin carcinogenesis protocol. Moreover, skin hyperplasia was significantly inhibited by quercetin supplementation. Further analysis of the skin papilloma cell line showed that quercetin treatment suppressed IGF-1-induced phosphorylation of insulin-like growth factor 1 receptor (IGF-1R), insulin receptor substrate 1 (IRS-1), Akt and ribosomal protein S6 kinase (S6K) and inhibited IGF-1 stimulated cell proliferation. The study suggests that quercetin has potent anticancer activity through the inhibition of IGF-1 signaling and can be considered as a potential therapeutic agent for cancer treatment286.
While quercetin has many potential benefits for preventing and treating skin cancer, there are some drawbacks to its use. Quercetin has low bioavailability, which means that much of the compound may be broken down and excreted before it can exert its therapeutic effects287. This can limit its effectiveness against skin cancer. Quercetin may interact with certain medications, such as blood thinners288, chemotherapy drugs289, and antibiotics290. This can affect their effectiveness and increase the risk of side effects. While preclinical studies have shown promising results for the use of quercetin against skin cancer, there is limited clinical evidence to support its use in humans291.
Overall, the molecular mechanisms of action of quercetin against skin cancer are complex and involve multiple cellular processes and pathways. These mechanisms work together to prevent the growth and spread of cancer cells, making quercetin a promising natural compound for skin cancer prevention and treatment. More research is needed to determine its optimal dosage, effectiveness, and safety for preventing and treating skin cancer.
Kaempferol
Kaempferol is a natural flavonoid compound that is found in a variety of plant-based foods such as fruits, vegetables, and herbs91. It is a yellow crystalline solid that belongs to the flavonol subclass of flavonoids292. Kaempferol can be found in plant species like Ginkgo Folium 293. The molecular mechanism of kaempferol against skin cancer involves several pathways. One of the primary mechanisms is its ability to induce cell cycle arrest and apoptosis in cancer cells. Kaempferol activates the tumor suppressor protein p53, which leads to cell cycle arrest and apoptosis294. Additionally, kaempferol can inhibit the activity of anti-apoptotic proteins such as Bcl-2, which further promotes cell death in cancer cells295. Kaempferol can suppress the production of inflammatory cytokines and chemokines, which can lead to chronic inflammation that promotes the growth of cancer cells296. It can also reduce the generation of reactive oxygen species and enhance the activity of antioxidant enzymes, which helps to protect cells from oxidative damage that can contribute to the development of cancer297. Moreover, kaempferol has been shown to inhibit the activity of several enzymes that are involved in the progression of skin cancer, including tyrosinase298, matrix metalloproteinases299, and cyclooxygenase-2300. By inhibiting these enzymes, kaempferol can prevent the proliferation and invasion of cancer cells.
Several studies have been performed to elucidate the molecular mechanism of action of kaempferol against skin cancer. In one of the studies, Yang et al. evaluated the anticancer activity of kaempferol against the human malignant melanoma A375 cell line and its effects on apoptosis, cell cycle, cell migration, and mTOR/PI3K/AKT pathway. The results showed that kaempferol exhibited significant anticancer activity against A375 cells with an IC50 of 20 µM. It reduced colony formation in a dose-dependent manner and initiated apoptosis in human malignant melanoma A375 cells. Additionally, kaempferol triggered G2/M cell cycle arrest, inhibited cell migration, and downregulated mTOR, pm-TOR, PI3K, p-PI3K, and Akt protein levels in A375 cells. The findings suggest that kaempferol exerts potent anticancer effects by targeting multiple pathways in melanoma cells301. Yao et al. performed a study that investigates the role of the 90 kDa ribosomal S6 kinase (RSK) and mitogen and stress-activated protein kinase (MSK) in solar ultraviolet (SUV) irradiation-induced skin carcinogenesis. The study shows that phosphorylation of RSK and MSK1 is upregulated in human squamous cell carcinoma (SCC) and SUV-treated mouse skin. The study also examines the potential of kaempferol, a natural flavonol found in tea, broccoli, grapes, apples, and other plant sources, as a chemopreventive agent against SUV-induced skin carcinogenesis. The study reveals that kaempferol inhibits RSK2 and MSK1 kinase activities and, by doing so, attenuates solar UV-induced phosphorylation of cAMP response element-binding protein (CREB) and histone H3 in mouse skin cells. The study further shows that kaempferol is a potent inhibitor of SUV-induced mouse skin carcinogenesis and acts by targeting RSK2 and MSK1. Overall, the study identifies kaempferol as a safe and novel chemopreventive agent against solar UV-induced skin carcinogenesis302. Furthermore, a study by Lee et al. investigates the effects of kaempferol, a flavonoid with anti-inflammatory and anti-oxidative properties, on UVB-induced skin inflammation and photocarcinogenesis. The study shows that kaempferol suppresses UVB-induced COX-2 protein expression in mouse skin epidermal JB6 P+ cells and attenuates the UVB-induced transcriptional activities of COX-2 and AP-1. The study further demonstrates that kaempferol attenuates the UVB-induced phosphorylation of several MAPKs, including ERKs, p38, and c-Jun N-terminal kinases (JNKs), by blocking Src kinase activity. The study also shows that kaempferol competes with adenosine triphosphate (ATP) for direct binding to Src and docks easily into the ATP-binding site of Src. The study suggests that kaempferol is a potent chemopreventive agent against skin cancer through its inhibitory interaction with Src. Overall, the study provides insights into the potential of kaempferol as a chemopreventive agent against UVB-induced skin inflammation and photocarcinogenesis303.
Overall, the molecular mechanism of kaempferol against skin cancer involves inducing cell cycle arrest and apoptosis, modulating the signaling pathways involved in inflammation and oxidative stress, and inhibiting the activity of enzymes that promote the growth and invasion of cancer cells304.
While kaempferol has shown promise in preventing and treating skin cancer, there are some potential drawbacks to its use. Kaempferol has relatively low bioavailability305, which means that the body may not absorb it efficiently. Like many natural compounds, kaempferol can have toxic effects at high doses. While it is generally considered safe, some studies have suggested that it can be toxic to certain cells and tissues at high concentrations306. Kaempferol can interact with some medications, particularly those that are metabolized by the liver307. While there have been some promising studies on the use of kaempferol in skin cancer, more research is needed to determine its safety and effectiveness. It is important to note that most of the research on kaempferol has been done in cell cultures and animal models, and more clinical studies are needed to confirm its effects in humans.
Resveratrol
Resveratrol is a naturally occurring polyphenolic compound found in many plant-based foods, including grapes and red wine308. The botanical name for the grapevine is Vitis vinifera, and the most commonly cultivated variety of grapes used for wine production is Vitis vinifera subsp. vinifera 309. Resveratrol is found in the skin of grapes, and its concentration is highest in red grapes compared to white grapes310. Resveratrol is also found in other plants, including peanuts311, berries312, and knotweed313. The botanical name for the peanut plant is Arachis hypogaea, while the most commonly consumed berries that contain resveratrol are blueberries (Vaccinium spp.), cranberries (Vaccinium macrocarpon), and bilberries (Vaccinium myrtillus)314. The Japanese knotweed (Fallopia japonica), a plant native to Asia, is also a rich source of resveratrol313. It is classified as a phytoalexin, which means it is produced by plants in response to stress, injury, or infection315. Resveratrol has been extensively studied for its potential health benefits, including its antioxidant316, anti-inflammatory317, and anticancer318 properties. Resveratrol has been shown to have potential anticancer effects against skin cancer through several molecular mechanisms. Here are some of the key ways in which resveratrol may act against skin cancer:
Inhibition of inflammation
Chronic inflammation is a risk factor for the development of many cancers, including skin cancer. Resveratrol has been shown to inhibit the expression of pro-inflammatory cytokines, such as interleukin-1β and tumor necrosis factor-α, which may help to reduce inflammation and the risk of cancer development319,320.
Induction of apoptosis
Resveratrol has been shown to induce apoptosis, or programmed cell death, in skin cancer cells. This is thought to be due in part to its ability to activate certain signaling pathways, such as the p53 pathway, which can trigger cell death321,322.
Inhibition of cell proliferation
Resveratrol can also inhibit the proliferation of skin cancer cells, which may help to slow the growth and spread of cancer cells96.
Protection against DNA damage
Resveratrol has been shown to have DNA-protective effects, which may help to prevent mutations and other DNA damage that can lead to cancer323.
Regulation of cell cycle
Resveratrol can regulate the cell cycle, which is the process by which cells divide and grow. By modulating the activity of certain proteins involved in cell cycle regulation, resveratrol may help to prevent uncontrolled cell growth that can lead to cancer development324.
Several studies have investigated the molecular mechanisms underlying the anticancer effects of resveratrol against skin cancer. One study conducted by Osmond et al. evaluated the potential of resveratrol as an adjunct to chemotherapy in melanoma treatment. In vitro, resveratrol significantly decreased melanoma cell viability in all lines tested and selectively spared nonmalignant fibroblast cell lines. Treatment of malignant cells with resveratrol and temozolomide (TMZ) enhanced cytotoxicity compared to TMZ alone. However, in vivo, there was no significant difference. The study suggests that resveratrol has selective antitumor activity in vitro, but barriers to translating these results to in vivo models need to be overcome325. Another study by Aziz et al. discusses the protective role of resveratrol against skin cancer induced by UVR. Resveratrol modulates various molecular mechanisms involved in cell cycle regulation, ROS production, apoptosis, autophagy, cell proliferation, tumor promotion, and cancer-related gene expression. The evidence suggests that resveratrol has effective chemopreventive activity and could potentially be used as a preventive and therapeutic agent in managing UVR-induced skin carcinogenesis326. In addition, resveratrol has been found to modulate the expression of several genes and proteins involved in cell cycle regulation, such as cyclins, cyclin-dependent kinases (CDKs), and checkpoint proteins327. Kundu et al. investigate the effects of resveratrol, a compound found in grapes, on COX-2 expression induced by the tumor promoter (TPA) in mouse skin. Results showed that resveratrol significantly inhibited COX-2 expression by suppressing activation of NF-κB, ERK, and p38 mitogen-activated protein (MAP) kinase. Resveratrol blunted TPA-induced phosphorylation of p65 and its interaction with CBP/p300, rendering NF-κB transcriptionally inactive. The study also found that resveratrol targets IκB kinase (IKK) in blocking TPA-induced NF-κB activation and COX-2 expression in mouse skin in vivo 328. Furthermore, a study performed by Reagan-Shaw et al. investigated the role of cell cycle regulatory molecules in resveratrol-mediated protection against multiple exposures to UVB radiation in hairless mice skin. Resveratrol was topically applied before each UVB exposure, resulting in a decrease in skin thickness, hyperplasia, and leukocyte infiltration. Resveratrol downregulated the upregulation of critical cell cycle regulatory proteins induced by UVB, including proliferating cell nuclear antigen (PCNA), cyclin-dependent kinases, and cyclins. Resveratrol also upregulated cyclin kinase inhibitor WAF1/p21 and tumor suppressor p53, and decreased the upregulation of MAPK, suggesting that the antiproliferative effects of resveratrol may be mediated via modulation of the expression and function of cell cycle regulatory proteins and inhibition of the MAPK pathway. Resveratrol may be useful for preventing UVB-mediated cutaneous damage, including skin cancer329. A study performed by Wu et al., aimed to investigate the effects of resveratrol, a natural polyphenol, on the proliferation and expression of aquaporin 3 (AQP3) in normal human epidermal keratinocytes (NHEKs), which are involved in hyperplastic skin disorders. The results showed that resveratrol inhibited NHEK proliferation in a concentration-dependent manner, and this was associated with downregulation of AQP3 expression and ERK phosphorylation, as well as upregulation of aryl hydrocarbon receptor nuclear translocator (ARNT) expression. The study suggests that the inhibitory effects of resveratrol on AQP3 expression were mediated by Sirtuin 1 (SIRT1)/ARNT/ERK signaling pathway. This finding may provide insights into the development of new drugs for hyperplastic skin disorders330.
Resveratrol’s anticancer effects are likely due to its ability to modulate multiple cellular pathways and processes involved in cancer development and progression. While resveratrol has many potential health benefits, including anticancer effects318 against skin cancer, there are also some drawbacks and potential risks associated with its use. Resveratrol can interact with certain medications, such as blood thinners331, nonsteroidal anti-inflammatory drugs (NSAIDs)332, and some antidepressants333. It may also interfere with the effectiveness of chemotherapy drugs334. Resveratrol can cause side effects, including gastrointestinal symptoms (such as nausea, vomiting, and diarrhea)335, headaches335 and allergic reactions336. Resveratrol supplements are not regulated by the U.S. Food and Drug Administration (FDA), and there is no standardized dosage or quality control. This can make it difficult to ensure the safety and effectiveness of resveratrol supplements337. Overall, while resveratrol has many potential health benefits, it is important to use caution when using it as a supplement, particularly in high doses or in combination with other medications.
Curcumin
Curcumin is a natural compound found in the root of the turmeric plant (Curcuma longa), which is a member of the ginger family338. It is a bright yellow pigment and has been used for thousands of years in traditional medicine systems, such as Ayurveda and traditional Chinese medicine, to treat a wide range of conditions339. Curcumin is a polyphenol and is considered to be the most active constituent in turmeric340. It has antioxidant341, anti-inflammatory342, and anticancer properties343 and has been studied extensively for its potential health benefits. It is also a common ingredient in many foods and supplements, including curry powder and turmeric supplements338. In terms of its molecular mechanism against skin cancer, curcumin has been found to inhibit various signaling pathways involved in cancer development and progression. One of the key pathways that curcumin targets is the nuclear factor-kappa B (NF-κB) signaling pathway101. NF-κB is a transcription factor that regulates genes involved in inflammation, cell proliferation, and apoptosis, and its activation is commonly found in various types of cancer, including skin cancer. Curcumin has been shown to inhibit NF-κB activation by suppressing the activity of IKK, which is the kinase that phosphorylates IκBα and leads to its degradation and subsequent release of NF-κB from the cytoplasm to the nucleus. Curcumin has also been shown to inhibit the expression of various NF-κB-regulated genes involved in cell survival, proliferation, and inflammation344. Additionally, curcumin has been found to induce apoptosis (programmed cell death) in skin cancer cells by activating the caspase pathway and inhibiting the anti-apoptotic protein Bcl-2345. Curcumin has also been shown to inhibit angiogenesis (the formation of new blood vessels), which is critical for tumor growth and metastasis105.
Multiple investigations have been conducted to explore the molecular mechanisms responsible for the anticancer properties of curcumin against skin cancer. In one of the studies, Zhao et al. investigated the effects of curcumin on melanoma cells and found that it effectively inhibited cell proliferation and invasion potential and induced autophagy. Curcumin also arrested the cells at the G2/M phase of the cell cycle and suppressed the activation of AKT, mTOR, and P70S6K proteins, which are part of the AKT/mTOR signaling pathway. These findings suggest that curcumin could be a potential therapeutic candidate for managing melanoma, which is a highly malignant and resistant cancer346. Another study by Wu et al. aimed to investigate the effects of curcumin on the expression of signal transducer and activator of transcription 3 (STAT3) in skin squamous cell carcinoma tissues and the possible mechanisms of curcumin in preventing and treating skin squamous cell carcinoma. Results showed that curcumin treatment inhibited the growth of A431 cells in a time-dependent and dose-dependent manner, with doses above 15 μmol/l for more than 24 h showing significant cytotoxic effects. Curcumin treatment also decreased the invasion and adhesive abilities of A431 cells and inhibited the transcription level of STAT3 mRNA in a dose-dependent manner. The study suggests that curcumin may reduce the invasive ability of A431 cells by inhibiting the activation of the STAT3 signaling pathway and expression of STAT3 as a target gene in the pathway, which may provide insights into the development of new therapies for skin squamous cell carcinoma347. In a study by Kunnumakkara et al., the authors investigated the role of curcumin in the regulation of signaling pathways involved in skin cancer development and progression. The authors demonstrated that curcumin inhibited the expression and activity of various pro-inflammatory cytokines, growth factors, and enzymes involved in skin carcinogenesis, including COX-2, MMP-9, and EGFR. They also showed that curcumin inhibited the activation of the PI3K/Akt/mTOR signaling pathway and the upregulation of its downstream target genes involved in cell survival and proliferation. Furthermore, the authors showed that curcumin enhanced the expression and activity of various tumor suppressor genes, including p53 and p21, which are involved in the regulation of cell cycle progression and apoptosis348.
While curcumin has shown promising results in preclinical studies as a potential therapeutic agent against skin cancer, its clinical application has some limitations and challenges. One of the major challenges is its low bioavailability, which refers to the fraction of the administered dose that reaches the systemic circulation in an unchanged form and is available to exert its pharmacological effects. Curcumin is rapidly metabolized in the liver and intestines and has poor solubility in water, which leads to limited absorption and rapid elimination from the body349. Furthermore, curcumin’s potential interactions with other drugs and its effects on drug metabolism enzymes have also raised concerns about its safety and efficacy. For instance, curcumin has been reported to inhibit the activity of cytochrome P450 (CYP) enzymes, which play a crucial role in the metabolism of many drugs. This inhibition may result in altered drug efficacy or toxicity when administered in combination with other medications350.
Overall, the molecular mechanisms of curcumin against skin cancer are complex and involve multiple pathways and targets. Its ability to modulate inflammation, apoptosis, and angiogenesis makes it a promising natural compound for the prevention and treatment of skin cancer. However, more research is needed to fully understand its bioavailability studies, safer dose, and to optimize its use in clinical settings.
Epigallocatechin gallate
Epigallocatechin gallate is a type of flavonoid, a class of plant-derived compounds that are known for their antioxidant351 and anti-inflammatory properties352. Epigallocatechin gallate is found primarily in green tea, which is derived from the leaves of the Camellia sinensis plant353. However, it can also be found in smaller amounts in black tea354, white tea355, and oolong tea356. Epigallocatechin gallate has been studied for its potential health benefits, including its anticancer properties357. In addition to its anticancer properties, Epigallocatechin gallate has also been studied for its potential role in reducing the risk of cardiovascular disease358, improving cognitive function359, and promoting weight loss358. It has also been found to have anti-inflammatory effects, which may make it useful in the prevention and treatment of chronic inflammatory diseases360 and inflammatory bowel disease361. Epigallocatechin gallate has been shown to have potential chemopreventive and therapeutic effects against skin cancer through several molecular mechanisms of action. These mechanisms include:
Antioxidant activity
Epigallocatechin gallate has strong antioxidant activity, which can protect skin cells from oxidative stress-induced DNA damage, a key contributor to skin cancer development106,107.
Anti-inflammatory activity
Epigallocatechin gallate has been found to inhibit the production of pro-inflammatory cytokines and chemokines, which can promote tumor growth and progression. In particular, Epigallocatechin gallate has been shown to inhibit the NF-κB signaling pathway, which is involved in inflammation and cell survival362.
Inhibition of cell proliferation
Epigallocatechin gallate has been found to inhibit the proliferation of skin cancer cells by blocking the cell cycle and inducing apoptosis (programmed cell death). Epigallocatechin gallate has also been found to inhibit the expression of genes involved in cell cycle regulation and DNA repair110,111.
Inhibition of angiogenesis
Epigallocatechin gallate has been shown to inhibit angiogenesis, the process by which new blood vessels are formed, which is critical for tumor growth and metastasis149,150.
Inhibition of MMPs
Epigallocatechin gallate has been found to inhibit the activity of MMPs, which are enzymes that degrade the extracellular matrix and promote tumor invasion and metastasis363.
Numerous studies have been carried out to investigate the molecular mechanisms underlying the anticancer effects of Epigallocatechin gallate in the context of skin cancer. In one of the studies, El-Kayal et al. investigated the potential use of ultra-deformable colloidal vesicular systems, including penetration enhancer-containing vesicles, ethosomes, and transethosomes, for the topical delivery of (–)-epigallocatechin-3-gallate as a nutraceutical for skin cancer treatment. The prepared vesicles showed good physical stability and preservation of the antioxidant properties of (–)-epigallocatechin-3-gallate. (–)-epigallocatechin-3-gallate-loaded PEVs and TEs demonstrated an inhibitory effect on epidermoid carcinoma cells and reduced tumor sizes in mice, with decreased skin oxidative stress biomarkers and lipid peroxidation. These findings suggest that (-)-epigallocatechin-3-gallate PEVs could be an effective topical delivery system for skin cancer treatment364. Zhang et al. investigated the interaction between Epigallocatechin-3-gallate and TRAF6 and its effects on the E3 ubiquitin ligase activity of TRAF6, as well as its role in the NF-κB pathway and melanoma cell growth, migration, and invasion. The results demonstrated that Epigallocatechin-3-gallate binds to TRAF6 and inhibits its E3 ubiquitin ligase activity, leading to the inactivation of the NF-κB pathway and suppression of melanoma cell growth, migration, and invasion. These findings suggest that Epigallocatechin-3-gallate may be a promising agent for the prevention and treatment of melanoma by targeting TRAF6365. Moreover, Ellis et al. describe a study that investigated the mechanism of action of Epigallocatechin-3-gallate, a polyphenolic component of green tea, in inhibiting melanoma cell growth. The study found that Epigallocatechin-3-gallate inhibited melanoma cell growth at physiological doses and that this inhibition was associated with reduced activity of the NF-κB pathway and decreased secretion of the inflammatory cytokine IL-1β. The study further demonstrated that Epigallocatechin-3-gallate downregulated the inflammasome component NOD-like receptor (NLR) family pyrin domain-containing 1 (NLRP1). NLRP1 and reduced caspase-1 activation, and silencing NLRP1 expression abolished Epigallocatechin-3-gallate induced inhibition of tumor cell proliferation both in vitro and in vivo. These findings suggest that inflammasomes and Interleukin-1 beta (IL-1β) could be potential targets for future melanoma therapeutics366.
While Epigallocatechin gallate has shown promise as a potential treatment for skin cancer, there are some drawbacks and limitations to its use. The optimal dose of Epigallocatechin gallate for skin cancer treatment is unclear, and the effective dose may vary depending on the type and stage of the cancer. High doses of Epigallocatechin gallate may also cause adverse effects, such as liver toxicity367. The content of Epigallocatechin gallate in green tea and other natural sources can vary widely, depending on factors such as the plant species, growing conditions, and processing methods. This makes it difficult to standardize the dose and quality of Epigallocatechin gallate in natural sources368. Epigallocatechin gallate has low bioavailability, meaning that it is not well absorbed by the body and has a short half-life. This can limit its effectiveness in vivo and make it difficult to achieve therapeutic concentrations in target tissues369.
Overall, Epigallocatechin gallate’s molecular mechanisms of action against skin cancer involve multiple pathways, including antioxidant351, anti-inflammatory352, antiproliferative370, antiangiogenic371, and antimetastatic effects371. These mechanisms suggest that Epigallocatechin gallate may be a promising natural compound for the prevention and treatment of skin cancer, although more research is needed to fully understand its safer dose and potential clinical applications.
β-Carotene
β-Carotene is a red-orange pigment and a type of carotenoid, which is a group of plant pigments responsible for the bright colors of fruits and vegetables372. It is a precursor of vitamin A, meaning that the body can convert it into vitamin A when needed373. β-Carotene is found in high amounts in fruits and vegetables such as carrots, sweet potatoes, pumpkin, spinach, and apricots374. β-Carotene has antioxidant properties and is believed to have various health benefits, such as reducing the risk of certain types of cancer375 and improving immune function376.
β-Carotene is obtained from a variety of plants, like carrots (Daucus carota), sweet potatoes (Ipomoea batatas), pumpkins (Cucurbita spp.), etc.377. The molecular mechanism of action of β-carotene against skin cancer involves its ability to neutralize reactive oxygen species (ROS) and reduce oxidative stress, which is a key factor in the development of cancer. ROS are highly reactive molecules that are produced in cells as a result of various physiological and environmental stressors, including exposure to ultraviolet radiation. When ROS levels exceed the capacity of the cell’s antioxidant defense system, they can cause oxidative damage to DNA, lipids, and proteins, which can ultimately lead to cancer. β-Carotene can scavenge ROS and neutralize them, thereby reducing the amount of oxidative stress in the cell114,115. Additionally, β-carotene can stimulate the body’s natural antioxidant defense system by upregulating the expression of antioxidant enzymes such as superoxide dismutase and catalase. This further helps to reduce oxidative stress and prevent DNA damage378. Moreover, β-carotene also exhibits anti-inflammatory properties that can help reduce chronic inflammation, which is also a risk factor for the development of skin cancer. Chronic inflammation is associated with the production of ROS and DNA damage, which can ultimately lead to the development of cancer379.
β-Carotene’s ability to scavenge ROS, stimulate antioxidant enzyme expression, and reduce chronic inflammation makes it a potent antioxidant and a promising candidate for the prevention and treatment of skin cancer379.
Several researchers have conducted studies on the effect of β-carotene against skin cancer. One such study was conducted by Greenberg et al. aimed to investigate whether β-carotene could prevent the occurrence of new nonmelanoma skin cancer in patients who had a recent history of the condition. The study randomly assigned 1805 patients to receive either 50 mg of β-carotene or placebo daily and conducted annual skin examinations. After 5 years, there was no significant difference in the rate of occurrence of new nonmelanoma skin cancer between the groups. The active treatment also showed no efficacy in patients with the lowest initial plasma β-carotene level or in those who currently smoked. The study concludes that β-carotene does not reduce the occurrence of new skin cancers in patients with a previous nonmelanoma skin cancer over a 5-year period of treatment and observation380.
In a similar study, Kune et al. investigated the relationship between dietary and serum levels of β-carotene, vitamin A, and the risk of nonmelanocytic skin cancer in males. The study found that a high intake of vegetables, including cruciferous vegetables, β-carotene, and vitamin C-containing foods and fish, was inversely related to the risk of skin cancer. The study also found that cases had lower serum levels of β-carotene and vitamin A than controls, and the incidence of skin cancer was inversely related to serum β-carotene levels. Smoking and alcohol consumption did not show a significant association with the risk of skin cancer. The study concludes that further investigation is required to determine the etiologic relevance of low serum levels of β-carotene and vitamin A and the protective effects of a high intake of vegetables and fish on nonmelanocytic skin cancer381.
While β-carotene has potential benefits in preventing skin cancer, there are also some drawbacks associated with its use.
Pro-oxidant effects
High doses of β-carotene may have pro-oxidant effects, meaning that they can increase oxidative stress and damage cells. This is particularly true in smokers, as studies have shown that β-carotene supplements can increase the risk of lung cancer in smokers382.
Lack of efficacy in some studies
While some studies have suggested that β-carotene can reduce the risk of skin cancer, other studies have shown no significant effect. More research is needed to determine the optimal dose, duration, and form of β-carotene supplementation for skin cancer prevention383.
Interactions with other nutrients
β-carotene can interact with other nutrients, such as vitamin E and selenium, which can affect its efficacy384. It is important to ensure adequate intake of these nutrients when supplementing with β-carotene.
Not a substitute for sun protection
β-carotene supplements should not be seen as a substitute for sun protection. While β-carotene may help reduce the risk of skin cancer, it cannot replace the importance of wearing protective clothing and using sunscreen when exposed to ultraviolet radiation385.
It can be concluded that while β-carotene may have potential benefits in preventing skin cancer, more research is needed to understand its interaction with other nutrients, which can affect its efficacy.
Caffeic acid
Caffeic acid is a naturally occurring polyphenolic compound and one of the most common hydroxycinnamic acid derivatives386. Caffeic acid is found in a wide variety of plants, including coffee (Coffea arabica, Coffea robusta), apples (Malus domestica), pears (Pyrus communis), grapes (Vitis vinifera), blueberries (Vaccinium spp.), kiwi fruits (Actinidia deliciosa), thyme (Thymus vulgaris), basil (Ocimum basilicum), rosemary (Rosmarinus officinalis), sage (Salvia officinalis), etc.387. Caffeic acid has been studied for its antioxidant388, anti-inflammatory389, and anticancer properties390. Caffeic acid has been shown to have potential chemopreventive effects against skin cancer390.
The molecular mechanism of action of caffeic acid against skin cancer involves its ability to modulate multiple cellular pathways involved in cell growth, differentiation, and apoptosis. One of the key mechanisms by which caffeic acid exerts its anticancer effects is through the regulation of signaling pathways that control cell proliferation and survival126. Caffeic acid has been shown to inhibit the activity of various enzymes and transcription factors, such as Akt, ERK, NF-κB121,122, and AP-1, which are involved in cell survival and proliferation. By inhibiting these pathways, caffeic acid can induce cell cycle arrest and promote apoptosis, leading to the death of cancer cells391–393. Caffeic acid has also been shown to possess potent antioxidant and anti-inflammatory properties, which can help to protect cells against oxidative stress and reduce chronic inflammation. Chronic inflammation is a major risk factor for the development of skin cancer, as it can cause DNA damage and impair the immune system’s ability to detect and eliminate cancer cells394–396. Additionally, caffeic acid has been shown to enhance the activity of the body’s natural defense mechanisms against cancer, such as the activation of the Nrf2 pathway. This pathway is involved in the regulation of antioxidant enzymes and can help to protect cells against oxidative damage397–399.
Studies have investigated the molecular mechanisms of action of caffeic acid against skin cancer. One such study was conducted by Yang et al., which investigated the molecular mechanism underlying the anticancer activity of caffeic acid, a phenolic phytochemical found in coffee. The study found that caffeic acid inhibited the colony formation of human skin cancer cells and the neoplastic transformation of HaCaT cells. Topical application of caffeic acid to mouse skin also significantly suppressed tumor incidence and volume in a solar UV-induced skin carcinogenesis model. The study further demonstrated that caffeic acid directly interacted with and inhibited the activity of ERK1 and 2, two proteins involved in mitogen-activated protein kinase signaling. The co-crystal structure of ERK2 complexed with caffeic acid was also resolved, revealing the amino acid residues at which caffeic acid interacts with ERK2. Finally, the study showed that the knockdown of ERK2 in skin cancer cells reduced their sensitivity to caffeic acid in a xenograft mouse model. These findings suggest that caffeic acid exerts its chemopreventive activity against skin carcinogenesis by targeting ERK1 and 2400. Another study by Kudugunti et al. investigated the anti-melanoma effects of phenethyl ester of caffeic acid in vitro and in vivo. The phenethyl ester of caffeic acid was found to inhibit the growth of five different melanoma cell lines, cause intracellular GSH depletion, increase ROS formation, and induce apoptosis in B16-F0 cells. In an in vivo study using a B16-F0 melanoma tumor model in mice, it was found to inhibit tumor growth at doses of 10–30 mg/kg/day with minimal toxicity. However, higher doses of phenethyl ester of caffeic acid were associated with increased plasma ALT levels and lipid peroxidation levels in liver and kidney homogenates. The study suggests that phenethyl ester of caffeic acid has potential as an anti-melanoma agent at lower doses401. Furthermore, a study by Balupillai et al. investigated the effect of caffeic acid on acute and chronic UVB-irradiation-induced inflammation and photocarcinogenesis in Swiss albino mice. The results showed that caffeic acid administration before UVB exposure decreased inflammation and oxidative stress, enhanced antioxidant status, and activated peroxisome proliferator-activated receptors (PPARγ) in the mice’s skin. PPARγ is considered a potential target for photo chemoprevention because it inhibits UVB-mediated inflammatory responses. Moreover, both topical and intraperitoneal caffeic acid treatment before each UVB exposure reduced tumor incidence and multiplicity in the mice’s skin. Therefore, caffeic acid offers protection against UVB-induced photocarcinogenesis through the activation of the anti-inflammatory transcription factor PPARγ in mice402. Another study by Balupillai et al. investigated the mechanisms by which caffeic acid prevents UVB-induced photocarcinogenesis in human dermal fibroblasts (HDFa) and mouse skin. The results showed that caffeic acid inhibited the formation of cyclobutane pyrimidine dimers (CPDs), oxidative DNA damage, ROS generation, and apoptotic cell death in HDFa. Furthermore, CA prevented UVB-induced expression of PI3K and AKT kinases through activation of PTEN and promoted XPC-dependent nucleotide excision repair (NER) proteins such as XPC, XPE, transcription factor IIH (TFIIH-p44), and excision repair cross-complementation group 1 (ERCC1) in human dermal fibroblasts (HDFa) cells and mouse skin tissue. Caffeic acid directly activated PTEN through hydrogen bonds and hydrophobic interactions. These findings suggest that caffeic acid prevents UVB-induced photodamage through the activation of PTEN expression in human dermal fibroblasts and mouse skin403. Another study by Agilan et al. investigated the role of JAK-STAT3 signaling in UVB-induced skin carcinogenesis and the protective effect of caffeic acid against it in mouse skin. Chronic UVB irradiation increased the expression of IL-10 and JAK1, which activated STAT3 and led to the transcription of proliferative and anti-apoptotic markers. Caffeic acid inhibited JAK-STAT3 signaling, induced apoptotic cell death, and upregulated the expression of pro-apoptotic markers. Additionally, chronic UVB exposure decreased the expression of thrombospondin-1 (TSP-1), an antiangiogenic protein, and pretreatment with caffeic acid prevented this loss in UVB-irradiated mouse skin. Thus, caffeic acid offers protection against UVB-induced photocarcinogenesis by modulating the JAK-STAT3 signaling pathway in the mouse skin404.
While caffeic acid has shown promising anticancer properties against skin cancer, there are also some potential drawbacks to its use. Some of the possible drawbacks of caffeic acid include:
Dose-dependent toxicity
At high doses, caffeic acid can be toxic to cells and tissues. This can lead to oxidative stress and inflammation, which can actually promote the growth and spread of cancer cells405. Therefore, it is important to use caffeic acid at appropriate doses and under medical supervision.
Bioavailability
Caffeic acid has relatively low bioavailability, which means that a significant amount of it may be metabolized or excreted before it can exert its anticancer effects. To overcome this limitation, researchers are exploring ways to enhance the bioavailability of caffeic acid, such as by using nanoparticles or liposomes406.
Lack of clinical trials
While many preclinical studies have shown that caffeic acid has potential anticancer properties, there is a lack of clinical trials to determine its efficacy and safety in humans. More research is needed to establish the optimal dose, route of administration, and duration of treatment405.
Interactions with other drugs
Caffeic acid may interact with other drugs or supplements, which could lead to adverse effects or reduce its efficacy. Therefore, it is important to consult a healthcare provider before using caffeic acid as a complementary or alternative therapy for skin cancer408.
Overall, the molecular mechanism of action of caffeic acid against skin cancer involves its ability to modulate multiple cellular pathways involved in cell growth, differentiation, and apoptosis. Its potent antioxidant388 and anti-inflammatory properties389, along with its ability to enhance the body’s natural defense mechanisms, may be used for the prevention and treatment of skin cancer390. However, further research is needed to fully understand its benefits and risks in the prevention and treatment of skin cancer.
Ferulic acid
Ferulic acid is a type of phenolic acid, which is a group of compounds that are widely distributed in plants409. It is a natural antioxidant and has been found in many different plant sources, including fruits, vegetables, grains, and herbs410–412. Ferulic acid is found in many different plant sources, including rice bran (Oryza sativa), wheat (Triticum aestivum), barley (Hordeum vulgare), oats (Avena sativa), corn (Zea mays), coffee (Coffea arabica), pineapple (Ananas comosus), apples (Malus pumila), oranges (Citrus sinensis), and artichokes (Cynara scolymus)413–415.
Ferulic acid has been shown to have various molecular mechanisms of action against skin cancer. Some of the key mechanisms are:
Antioxidant properties
Ferulic acid has strong antioxidant properties, which means that it can scavenge free radicals and protect skin cells from oxidative stress. This can help to prevent DNA damage and reduce the risk of skin cancer development416.
Anti-inflammatory properties
Chronic inflammation is known to contribute to the development of skin cancer. Ferulic acid has been shown to have anti-inflammatory properties, which can help reduce inflammation and prevent the progression of precancerous cells to cancerous cells126.
Inhibition of UV-induced damage
Ferulic acid has been shown to inhibit the DNA damage caused by UV radiation, which is a major risk factor for skin cancer. It can also help to repair damaged DNA and prevent the formation of cancerous cells417.
Induction of apoptosis
Ferulic acid has been shown to induce apoptosis (programmed cell death) in skin cancer cells. This can help to prevent the growth and spread of cancer cells418.
Inhibition of angiogenesis
Ferulic acid has also been shown to inhibit angiogenesis, which is the process by which new blood vessels form to supply nutrients to tumors. By inhibiting angiogenesis, ferulic acid can help to prevent the growth and spread of skin cancer cells419.
A study performed by Alias et al. aimed to compare the chemopreventive potential of orally administered and topically applied ferulic acid in 7,12-dimethylbenz[a]anthracene (DMBA)-induced skin carcinogenesis. The status of phase I and phase II detoxication agents, lipid peroxidation byproducts, and antioxidants were assessed to determine the mechanistic pathway of their chemopreventive efficacy. Skin squamous cell carcinoma was induced in mice by applying DMBA twice weekly for 8 weeks. Oral administration of ferulic acid completely prevented the formation of skin tumors, whereas topically applied ferulic acid did not show significant chemopreventive activity. Ferulic acid had a modulating effect on the status of lipid peroxidation, antioxidants, and detoxication agents during DMBA-induced skin carcinogenesis. The study concludes that orally administered ferulic acid has a potent suppressing effect on cell proliferation during DMBA-induced skin carcinogenesis420. One study conducted by Choi et al. examined the anticancer activity of ferulic acid on National Institutes of Health 3T3 (NIH3T3) fibroblasts and human skin melanoma cells (SK-MEL-3) by measuring the cytotoxicity of ferulic acid on these cells. Ferulic acid decreased cell viability in a dose-dependent manner after human skin melanoma cells were treated with various concentrations of ferulic acid for 48 h. At a concentration of 120 μM, ferulic acid significantly decreased cell viability in human skin melanoma cells, while it did not show a significant decrease in cell viability at concentrations of 30–120 μM in NIH3T3 fibroblasts. These results suggest that ferulic acid has anticancer activity in cancer cells, such as human skin melanoma cells, by significantly decreasing cell viability421. Another study by Murakami et al. reports the synthesis of a novel ferulic acid analog called FA15, which was found to suppress phorbol ester-induced Epstein–Barr virus activation and superoxide anion generation in vitro. The study also demonstrated that FA15 suppressed inducible nitric oxide synthase and cyclooxygenase-2 protein expressions, inhibited the release of tumor necrosis factor-alpha, and suppressed I-kappaB degradation in RAW264.7, a murine macrophage cell line. In mouse skin, topical application of FA15 attenuated hydrogen peroxide production and edema formation, as well as papilloma development, while FA did not. The study concludes that FA15, derived from natural sources, is a novel chemopreventive agent, both structurally and functionally422.
While ferulic acid has many potential benefits for the prevention and treatment of skin cancer, there are also some drawbacks and potential side effects. Ferulic acid can cause skin irritation, especially in individuals with sensitive skin. This can manifest as redness, itching, or burning sensations. Ferulic acid can increase the skin’s sensitivity to UV radiation, which can increase the risk of sunburn and skin damage. It is important to use sunscreen and limit sun exposure when using ferulic acid-containing skincare products. Ferulic acid can interact with certain medications, including blood thinners and chemotherapy drugs.
Overall, the molecular mechanisms of action of ferulic acid against skin cancer involve its antioxidant416 and anti-inflammatory properties126, as well as its ability to inhibit UV-induced damage417, induce apoptosis418, and inhibit angiogenesis419. These properties make ferulic acid a promising natural agent for the prevention and treatment of skin cancer. While ferulic acid is generally considered safe when used in appropriate amounts, it is important to be aware of the potential side effects and to use caution when using it to prevent or treat skin cancer. It is also important to use high-quality, pure products to minimize the risk of skin irritation and other adverse effects.
Importance of a diet rich in fruits and vegetables for preventing skin cancer
A diet rich in fruits and vegetables has been associated with many health benefits, including the prevention of skin cancer423. The high nutrient content of fruits and vegetables provides the body with the necessary vitamins, minerals, and antioxidants to maintain healthy skin and prevent damage from the sun’s harmful UV rays424. One of the most important benefits of a diet rich in fruits and vegetables is its high antioxidant content. Antioxidants are molecules that neutralize free radicals, which are unstable molecules that damage cells and increase the risk of cancer425. Fruits and vegetables contain antioxidants such as vitamin C, vitamin E, and β-carotene, which can help protect the skin from UV radiation and reduce the risk of skin cancer426.
Vitamin C is necessary for the production of collagen, a protein that helps to maintain the elasticity of the skin427. β-carotene, which is found in orange and yellow fruits and vegetables, is converted into vitamin A in the body and is important for skin cell growth and repair428. Other vitamins and minerals found in fruits and vegetables, such as potassium and folate, are also essential for maintaining healthy skin429. A diet rich in fruits and vegetables is also high in fiber, which can reduce inflammation in the body430. Chronic inflammation has been linked to an increased risk of many types of cancer, including skin cancer. By reducing inflammation in the body, a diet rich in fruits and vegetables can help prevent the development of skin cancer431. A diet rich in fruits and vegetables can help with weight management432. Being overweight or obese has been linked to an increased risk of certain types of skin cancer, including melanoma. By maintaining a healthy weight, individuals can reduce their risk of developing skin cancer433.
To maximize the benefits, individuals should aim to consume a variety of fruits and vegetables in their diet and limit their exposure to UV radiation by wearing protective clothing, seeking shade during peak hours, and using sunscreen434.
Fruits and vegetables are rich sources of dietary phytochemicals that play a crucial role in the regulation of cell signaling pathways, which are important in the prevention of skin cancer435. Cell signaling pathways are complex networks of molecular interactions that govern cellular processes such as growth, differentiation, and survival. Abnormalities in these pathways have been linked to the development of skin cancer436,437.
Many dietary phytochemicals found in fruits and vegetables that belong to the class of secondary metabolites, like flavonoids, carotenoids, polyphenols, etc., have been shown to regulate cell signaling pathways and prevent the development of skin cancer438. For example, polyphenols found in blueberries have been shown to inhibit the growth of skin cancer cells by regulating the Akt/mTOR signaling pathway439. Flavonoids found in citrus fruits, such as hesperidin and naringenin, have been shown to inhibit the growth of melanoma cells by regulating the MAPK signaling pathway440,441.
Fruits and vegetables also have anti-inflammatory properties that can prevent the development of skin cancer by regulating the immune response442. Inflammation is a key component of the skin’s response to UV radiation, and chronic inflammation has been linked to an increased risk of skin cancer443. Bioactive compounds found in fruits and vegetables, such as quercetin found in apples and onions, have been shown to inhibit the production of inflammatory cytokines and prevent inflammation in the skin444.
It is important to complement dietary changes with other sun safety measures, such as wearing protective clothing, using sunscreen, and limiting sun exposure during peak hours445. This section includes the dietary sources of some important dietary phytochemicals effective against skin cancer (Table 2A, B).
Table 2.
Dietary sources of some important dietary phytochemicals.
| S. no. | Dietary phytochemical | Group of plant secondary metabolite | Dietary sources | Reference |
|---|---|---|---|---|
| A | ||||
| 1. | Rosmarinic acid | Phenolic acids | Rosemary, sage, thyme, mint, basil, oregano, broccoli, spinach, lemon balm tea, green tea, and some types of honey, particularly those made from plants such as rosemary or thyme. | 446–455 |
| 2. | Allicin | Organosulfur compounds | Raw garlic, onions, leeks, shallots, and chives. | 456–461 |
| 3. | Sulforaphane | Organosulfur compounds | Broccoli, cauliflower, brussels sprouts, kale, cabbage, bok choy, arugula, radishes, and turnips. | 462–470 |
| 4. | Ellagic acid | Polyphenolic compounds | Strawberries, raspberries, blackberries, pomegranates, cranberries, walnuts, pecans, green tea, and black tea. | 471–478 |
| 5. | Betulinic acid | Pentacyclic triterpenoid compounds | Chaga mushrooms, white asparagus, and wild celery. | 479–482 |
| 6. | Apigenin | Flavonoids | Parsley, chamomile, cilantro, thyme, oregano, peppermint, rosemary, celery, onions, artichokes, broccoli, bell peppers, grapefruit, oranges, cherries, chamomile tea, and peppermint tea. | 254,483–495 |
| 7. | Gingerol | Gingerols | Ginger. | 496,497 |
| 8. | Quercetin | Flavonoids | Apples, berries (such as blueberries, cranberries, and elderberries), cherries, grapes, oranges, pomegranates, onions, shallots, kale, broccoli, red leaf lettuce, tomatoes, green peppers, spinach, quinoa, buckwheat, thyme, green tea, and red wine. | 444,498–516 |
| B | ||||
| 9. | Kaempferol | Flavonoids | Kale, spinach, broccoli, brussels sprouts, cabbage, grapes, apples, thyme, green tea, black tea, chickpeas and kidney beans. | 517–528 |
| 10. | Resveratrol | Polyphenolic compounds (stilbenes) | Grapes, red wine, blueberries, raspberries, mulberries, peanuts, pistachios, cocoa, dark chocolate, and tomatoes. | 529–533 |
| 11. | Curcumin | Polyphenolic compounds (curcuminoids) | Turmeric. | 534 |
| 12. | Epigallocatechin gallate | Polyphenolic compounds (catechins) | Black tea, green tea (particularly Japanese green tea varieties such as matcha), white tea, strawberries, raspberries, blueberries, grapes, hazelnuts, and pecans. | 354,535–541 |
| 13. | β-Carotene | Terpenoid compounds (carotenoids) | Carrots, sweet potatoes, butternut squash, acorn squash, pumpkin, spinach, kale, honeydew melon, mangoes, and apricots. | 542–550 |
| 14. | Caffeic acid | Polyphenolic compounds (hydroxycinnamic acids) | Coffee, apples, pears, blueberries, cherries, tomatoes, carrots, thyme, oregano, rosemary, sage, almonds, green tea, sunflower seeds, and sesame seeds. | 486,551–562 |
| 15. | Ferulic acid | Polyphenolic compounds (hydroxycinnamic acids) | White bran, germ portions of wheat, rice, oats, oranges, apples, pineapples, cherries, spinach, broccoli, carrots, sweet potatoes, cinnamon, flaxseeds, and sesame seeds. | 386,563–575 |
Blueberries (Vaccinium corymbosum)
Blueberries belong to the class of secondary metabolites known as flavonoids576. Specifically, blueberries contain high levels of a type of flavonoid called anthocyanins, which give them their characteristic deep blue color577. Anthocyanins are known for their antioxidant properties578, which may have a number of health benefits, including reducing inflammation479, improving cardiovascular health480, and protecting against certain types of cancer, including skin cancer423. Another phytochemical present in blueberries that may help to prevent skin cancer is pterostilbene581, which has been shown to have antioxidant and anti-inflammatory properties and may help to inhibit the growth of skin cancer cells582. Additionally, blueberries are a good source of vitamin C583, which has been found to have protective effects against skin damage caused by UV radiation and may also help to prevent skin cancer584. Various research groups have examined its impact on skin cancer. A recent study performed by Alsadi et al. examined the effects of a polyphenol-enriched blueberry preparation (PEBP) and non-fermented blueberry juice (NBJ) on the expression of miRNAs and target proteins associated with skin cancer. The study found that PEBP was able to inhibit the proliferation of skin cancer stem cells, reduce the formation of melanophores, and decrease the expression of the CD133+ stem cell marker. The study also found that the expression of tumor suppressor miR-200s was upregulated, and a protein target of the tumor suppressor miR200b, ZEB1, was significantly modulated. These findings suggest that PEBP has potent anticancer and antimetastatic potentials and may represent a novel chemopreventative agent against skin cancer. Qi et al. investigated the effects of blueberries on skin cancer prevention in mice. The researchers found that blueberries inhibited skin tumor growth via the regulation of multiple signaling pathways, including the PI3K/AKT, MAPK, and NF-κB pathways. In one of the studies, Afaq et al. investigated the effects of green tea and black tea extracts on skin cancer in mice. The researchers found that both green tea and black tea extracts inhibited skin cancer via the regulation of multiple signaling pathways, including the MAPK and PI3K/AKT pathways. Overall, these studies demonstrate that blueberries protect against skin cancer.
Raspberries (Rubus idaeus)
Raspberries contain a class of secondary metabolites known as flavonoids, specifically anthocyanins. These are responsible for the fruit’s characteristic red color and are also known for their antioxidant properties585. Some studies have suggested that anthocyanins may have protective effects against skin cancer by reducing oxidative stress and inflammation in the skin423. Raspberries are a rich source of dietary phytochemicals, many of which have been found to have potential anticancer properties586. Specifically, several of the compounds found in raspberries have been studied for their ability to prevent or treat skin cancer587. One such compound is ellagic acid, a polyphenol found in raspberries that has been shown to have anti-inflammatory and antioxidant effects214. Studies have found that ellagic acid can inhibit the growth of skin cancer cells and prevent the formation of skin tumors in animal models210. Another phytochemical found in raspberries with potential anticancer properties is quercetin, a flavonoid498 with antioxidant499 and anti-inflammatory effects122. Quercetin has been shown to induce cell death in skin cancer cells and inhibit the growth of tumors in animal models283.
Numerous research teams have looked at its effect on skin cancer. A study performed by Duncan et al. investigates the potential of a black raspberry extract (BRE) in reducing cutaneous UVB-induced inflammation and carcinogenesis. Female hairless mice were exposed to UVB radiation and treated topically with either BRE or vehicle control. Results showed that mice treated with BRE had a significant reduction in tumor number and size, which correlated with a decrease in tumor-infiltrating regulatory T-cells. In addition, topical BRE treatment significantly reduced acute UVB-induced inflammation, as evidenced by decreased edema, p53 protein levels, oxidative DNA damage, and neutrophil activation. These findings suggest that BRE may have clinical efficacy in preventing human skin cancers588. Oberyszyn describes the increased incidence of skin cancer due to exposure to sunlight and changes in tanning practices. Skin tumors are the most common form of cancer in humans, and current treatments for nonmelanoma skin cancers can have severe side effects and limited clinical efficacy. The study proposes natural compounds derived from functional foods as an alternative strategy for the prevention and treatment of skin cancer. Specifically, the study discusses the potential of black raspberry extracts as a chemopreventive/chemotherapeutic agent against nonmelanoma skin cancers589. Mace et al. examine the potential of black raspberry extracts (BRB) and their phytochemical metabolites in modulating immune processes relevant to carcinogenesis and immunotherapy. The study found that BRB extracts and their metabolites inhibited the proliferation and viability of CD4+ and CD8+ T lymphocytes, as well as the expansion and suppressive capacity of myeloid-derived suppressor cells (MDSC). Additionally, pretreatment of immune cells with BRB extracts and metabolites attenuated IL-6-mediated phosphorylation of STAT3 and IL-2-induced STAT5 phosphorylation. The study concludes that BRB extracts and their metabolites contain phytochemicals that affect immune processes relevant to carcinogenesis and immunotherapy and could be a potential source of lead compounds for drug development590. Overall, raspberries exhibit promising natural anticancer properties.
Kiwifruit (Actinidia deliciosa)
Kiwifruit contains a variety of dietary phytochemicals, including flavonoids, carotenoids, and phenolic acids, that have been studied for their potential benefits in preventing or treating skin cancer591. Flavonoids, such as quercetin and kaempferol, are particularly abundant in kiwifruit592 and have been shown to have antioxidant, anti-inflammatory, and anticancer properties593,594. Studies have suggested that quercetin and kaempferol may be particularly effective against skin cancer due to their ability to inhibit the growth and proliferation of cancer cells, as well as their ability to protect against DNA damage caused by UV radiation283,303. Additionally, the carotenoids present in kiwifruit, such as β-carotene595,596, lutein596,597, and zeaxanthin597, have been studied for their potential to protect against UV-induced skin damage and reduce the risk of skin cancer598,599. Phenolic acids, such as chlorogenic acid, are also present in kiwifruit and have been shown to have antioxidant and anti-inflammatory effects600. Numerous research groups have examined its impact on skin cancer. One of the studies performed by Kou et al. investigated the effects of kiwifruit extract on CRL-11147 melanoma cancer cells and the possible mechanisms behind the results. The study found that kiwifruit extract decreased the percentage of cancer cell colonies and increased apoptosis, indicating antitumor effects. The antiproliferative effect of kiwifruit extract was attributed to the downregulation of Cyclin E and CDK4, while the pro-apoptotic effect was attributed to the upregulation of TRAILR1. Overall, the study suggests that kiwifruit extract may have potential for use in the treatment of melanoma601. Another study carried out by Kou et al. investigated the potential of kiwifruit extract as a radiosensitizer for Cell Repository Line (CRL-11147) melanoma cancer cells and the underlying mechanisms. Melanoma is a deadly form of skin cancer that is often resistant to radiation therapy. The study used various assays to evaluate the effects of kiwifruit extract on cell proliferation and apoptosis in combination with radiation therapy. The results showed that the combination of kiwifruit extract and radiation therapy decreased the percentage of colonies, Proliferating Cell Nuclear Antigen (PCNA) staining intensity, and optical density value of cancer cells. The study also found that kiwifruit extract increased relative caspase-3 activity, indicating increased apoptosis of cancer cells. The antitumor effect of kiwifruit extract was correlated with increased expression of the antiproliferative molecule p27 and the pro-apoptotic molecule Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand Receptor 1 (TRAILR1). The study suggests that kiwifruit extract could be used as a potential radiosensitizer for melanoma treatment by upregulating both p27 and TRAILR1 to inhibit proliferation and increase apoptosis, respectively602. Overall, these studies show that kiwifruit can help prevent skin cancer.
Watermelon (Citrullus lanatus)
Watermelon is a rich source of several dietary phytochemicals that have been found to be effective against skin cancer607. One such phytochemical is lycopene, a carotenoid that gives watermelon its red color. Lycopene is a potent antioxidant that has been shown to protect against UV-induced skin damage, which can lead to skin cancer604. Studies have also found that lycopene can induce cell death in cancer cells and inhibit their growth605. Another phytochemical found in watermelon is citrulline, an amino acid that has been found to have anti-inflammatory properties. Inflammation is a key factor in the development and progression of many types of cancer, including skin cancer. Citrulline has been shown to reduce inflammation and oxidative stress in the skin, potentially reducing the risk of skin cancer606. Watermelon also contains β-carotene, another carotenoid with antioxidant properties. β-Carotene has been shown to protect against UV-induced skin damage and may help reduce the risk of skin cancer607. Its effect on skin cancer has been studied by a plethora of research teams. Ascenso et al. focus on the photoprotective properties of lycopene, one of the most potent antioxidants, and its role in anticarcinogenic action at different levels. However, the photoprotective properties of lycopene remain contradictory as some studies show a positive effect while others show a negative effect in both in vitro and in vivo models. The study highlights the need for a better understanding of the molecular mechanisms of UV damage on skin cells and the role of carotenoids as effective modulators of apoptosis and cell cycle dynamics. The use of lycopene and other effective phytocompounds as preventive and/or corrective agents may be a potential approach for reducing UVB-induced damage and developing novel therapeutic strategies for skin disorders608. Another study by Fazekas et al. investigated the protective effects of topical lycopene, a carotenoid found in tomatoes and watermelon, against acute ultraviolet B (UVB)-induced photodamage. The results showed that the application of lycopene inhibited UVB-induced ornithine decarboxylase and myeloperoxidase and reduced skin thickness. Lycopene also prevented cleavage of caspase-3, restored normal PCNA staining, and maintained normal cell proliferation, suggesting that it may act as a preventative agent by reducing inflammation, maintaining normal cell proliferation, and preventing DNA damage. Thus, topical lycopene showed protective effects against acute UVB-induced photodamage609. Overall, these studies indicate that watermelon can aid in the prevention of skin cancer.
Mangoes (Mangifera indica)
Mangoes contain several dietary phytochemicals that have been found to be effective against skin cancer610. One of the primary phytochemicals found in mangoes is quercetin, which has been shown to possess strong antioxidant properties and inhibit the growth of cancer cells82,284. Another important phytochemical found in mangoes is mangiferin, which has been found to possess anti-inflammatory, antioxidant, and anticancer properties611. Studies have shown that mangiferin can inhibit the proliferation of cancer cells and induce apoptosis, which is programmed cell death612. Additionally, β-carotene and lutein, two other important dietary phytochemicals found in mangoes, have been shown to possess photoprotective properties and protect the skin against UV-induced damage, which is a major risk factor for skin cancer613,614. Norathyriol is a flavonoid compound that has been identified in mangoes. It is a naturally occurring phytochemical that belongs to the class of flavanones. Norathyriol has been reported to have various biological activities, including antioxidant, anti-inflammatory, and anticancer properties615. The combination of these dietary phytochemicals found in mangoes makes it a potentially effective dietary intervention for preventing and treating skin cancer. A multitude of research groups have investigated its impact on skin cancer. Li et al. describe a study where norathyriol, a compound derived from plants and known to have anticancer activity, was identified through virtual screening of the Chinese Medicine Library as a potential chemopreventive agent for skin cancer. Norathyriol was found to inhibit extracellular signal-regulated kinase (ERK)1/2 activity and attenuate UVB-induced phosphorylation in mitogen-activated protein kinases signaling cascades. It was confirmed to bind specifically with ERK2 through co-crystal structural analysis. Norathyriol was shown to inhibit in vitro cell growth and significantly suppress solar UV-induced skin carcinogenesis in mouse skin tumorigenesis assays by inhibiting ERK-dependent activity of transcriptional factors AP-1 and NF-κB during UV-induced skin carcinogenesis. Overall, norathyriol was identified as a safe and highly effective chemopreventive agent against the development of UV-induced skin cancer615. In one of the studies, Saleem et al. investigated the antitumor-promoting effects of lupeol, a triterpene found in common fruit plants like mangoes and medicinal herbs, in a mouse skin tumorigenesis model. Topical application of lupeol inhibited conventional and novel biomarkers of tumor promotion in a time-dependent and dose-dependent manner. Lupeol treatment also inhibited the activation of signaling pathways involved in tumor promotion, resulting in reduced tumor incidence, lower tumor burden, and delayed tumor appearance. The results suggest that lupeol is an effective agent for cancer chemoprevention and should be evaluated in tumor models, including skin carcinogenesis616. Overall, the results of these studies suggest that eating mangoes may help lower the risk of getting skin cancer.
Carrots (Daucus carota)
Carrots are a rich source of dietary phytochemicals that have been shown to have potential health benefits, including the prevention of skin cancer617. The most notable phytochemicals present in carrots are carotenoids, which include β-carotene, α-carotene, and lutein. These compounds have been shown to have antioxidant properties that protect skin cells from oxidative stress caused by ultraviolet radiation, the most important factor contributing to skin cancer development618. Carotenoids can also enhance immune function and stimulate repair mechanisms in the skin619,620. In addition, falcarinol, a polyacetylene compound found in carrots, has been found to have chemopreventive properties against skin cancer by inducing apoptosis (cell death) in cancer cells and inhibiting their proliferation621. Studies have shown that consuming a diet rich in carrots and other carotenoid-containing vegetables can reduce the risk of developing skin cancer, particularly squamous cell carcinoma628. Therefore, incorporating carrots into a balanced and healthy diet may be an effective strategy for reducing the risk of skin cancer. Numerous research groups have studied its effect on skin cancer. Shebaby et al. performed a study that investigates the effects of wild carrot oil fractions on skin cancer. Four fractions were tested for their cytotoxic effects on human epidermal keratinocytes, with one fraction showing a significant antitumor effect on DMBA/TPA-induced skin carcinogenesis in mice. The study also identified a major compound in the fraction, which caused an accumulation of cells in the sub-G1 apoptotic phase and decreased the population of cells in the S and G2/M phases. Additionally, the fraction caused an upregulation of the expression of pro-apoptotic proteins and a downregulation of the expression of anti-apoptotic proteins. The data suggest that the antitumor activity of the fraction may be mediated through inhibition of the MAPK/ERK and PI3K/AKT pathways. Overall, the study suggests that wild carrot oil fractions, particularly the pentane/diethyl ether fraction, may have the potential as a chemopreventive agent against skin cancer623. Natarajmurthy and Dharmesh determined the contribution of phenolics and β-carotene to the antioxidant, tyrosinase inhibitory, and antiproliferative properties of carrots and evaluated their potential in preventing skin cancer in mice induced by DMBA-UV. Phenolic fractions of carrots were extracted and quantified using high-performance liquid chromatography (HPLC), and their capacities were determined. The results showed that phenolics in CRFP and β-carotene, rather than those in CRBP, exhibited a higher reduction in tumor formation, tyrosinase, and galectin-3 levels and increased antioxidant levels, indicating their potential to prevent skin cancer. Thus, carrots enriched with phenolics and β-carotene may be an efficient natural source in preventing skin cancer, as evidenced by the in vitro and in vivo studies624. In another study, Zeinab et al. investigated the chemopreventive effects of oil extract of Daucus carota (DCOE) umbels on 7,12-dimethyl benz(a)anthracene (DMBA)-induced skin cancer in mice. The oil extract was administered to animals via gavage, intraperitoneal, and topical routes for 20 weeks, and tumor appearance, incidence, yield, and volume were compared with those of a non-treated control group. The results showed that DCOE has remarkable antitumor activity against DMBA-induced skin cancer compared with non-treated animals, with the topical route being the most effective623. Overall, these studies suggest that carrot consumption may reduce the risk of developing skin cancer.
Future prospects of dietary phytochemicals to prevent skin cancer
The use of dietary phytochemicals as chemopreventive agents in topical skin cancer formulations has gained significant attention in recent years435. While there is promising evidence for the use of phytochemicals in preventing skin cancer, further studies are needed to meet the challenges of topical skin cancer formulations. These challenges include skin penetration, optimum drug concentration, stability, dosing strategy, and sustained drug release following topical application625. Skin penetration is a crucial factor that affects the efficacy of topical formulations625. Dietary phytochemicals, such as flavonoids and polyphenols, have been shown to have low skin penetration due to their large molecular size and low lipophilicity627. Various strategies have been explored to improve the skin penetration of phytochemicals, including the use of nanoemulsions, liposomes, and penetration enhancers628. Optimum drug concentration is another critical factor that needs to be considered in the development of topical skin cancer formulations containing dietary phytochemicals625. The concentration of phytochemicals needs to be optimized to ensure that they are effective in preventing or treating skin cancer while minimizing toxicity. This concentration may vary depending on the phytochemical, the skin type, and the intended use of the formulation629. Stability is also an important factor that needs to be addressed in the development of topical skin cancer formulations containing dietary phytochemicals. The formulation must remain stable during storage and use to ensure that the phytochemicals remain effective435. Stability can be affected by factors such as pH, temperature, and light exposure. Dosing strategy is another factor that needs to be considered in the development of topical skin cancer formulations containing dietary phytochemicals435. The dosing strategy must be optimized to ensure that the phytochemicals are delivered in the most effective manner. Factors such as the frequency of application, the amount of phytochemicals applied, and the duration of treatment must be carefully considered629. Finally, sustained drug release following topical application is a crucial factor that needs to be addressed in the development of topical skin cancer formulations containing dietary phytochemicals. The phytochemicals must be released in a controlled manner to ensure that they remain effective for the intended duration of treatment630. Various strategies have been explored to achieve sustained drug release, including the use of polymers, liposomes, and nanoparticles631. Overall, while there is promising evidence for the use of dietary phytochemicals in preventing skin cancer, further studies are needed to meet the challenges of topical skin cancer formulations. Addressing these challenges is critical to the development of effective and safe topical formulations containing dietary phytochemicals for the prevention and treatment of skin cancer. The development of such formulations has the potential to revolutionize the management of skin cancer and improve patient outcomes.
Conclusion
Dietary phytochemicals are biologically active compounds that are naturally present in fruits and vegetables. These compounds have been shown to possess numerous health benefits, including the prevention of skin cancer by protecting cells from DNA damage, inhibiting the production of pro-inflammatory cytokines and chemokines, reducing inflammation and the risk of cancer development, inhibiting the activation of signaling pathways (MAPK pathway, PI3K/Akt pathway, Wnt pathway, Hedgehog pathway, Notch pathway, etc.) involved in cell growth and proliferation, which are dysregulated in skin cancer, and inducing apoptosis in cancer cells through various mechanisms like activation of caspases, regulation of Bcl-2 family proteins, modulation of p53, inhibition of NF-κB, induction of oxidative stress, etc. Assessing the applicability of the preclinical data to humans may require additional research, such as short-term human studies. In addition, studies on skin cancer prevention involving fruits and vegetables seem to be necessary for high-risk humans, such as those with weakened immune systems. In models of high-risk skin carcinogenesis, preclinical studies may reveal positive effects. In addition, fruits and vegetables can be combined with current treatment methods to enhance the treatment of skin cancer.
Ethical approval
Ethics approval was not required for this review.
Consent
Informed consent was not required for this review.
Source of funding
No funding.
Author contribution
H.S. and A.K.M.: conceptualization, data curation, writing – original draft preparation, and writing – reviewing and editing; S.M., A.K., A.M., and R.A.: data curation, writing – original draft preparation, and writing – reviewing and editing; C.R.D.: writing – reviewing and editing and visualization; T.B.E.: writing – reviewing and editing, visualization, and supervision.
Conflicts of interest disclosure
All authors report no conflicts of interest relevant to this article.
Research registration unique identifying number (UIN)
Name of the registry: not applicable.
Unique identifying number or registration ID: not applicable.
Hyperlink to your specific registration (must be publicly accessible and will be checked): not applicable.
Guarantor
Talha Bin Emran, PhD, Associate Professor, Department of Pharmacy, BGC Trust University Bangladesh, Chittagong 4381, Bangladesh; Tel.: +88 030 3356193; fax: +88 031 2550224; cell: +88 01819 942214; https://orcid.org/0000-0003-3188-2272; e-mail: talhabmb@bgctub.ac.bd.
Data availability statement
Not applicable to this article.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 7 August 2024
Contributor Information
Harpreet Singh, Email: harpreetproctor@rediffmail.com.
Arun Kumar Mishra, Email: arun_azam@rediffmail.com.
Sourav Mohanto, Email: mohanto111@gmail.com.
Arvind Kumar, Email: akarvindsr01@gmail.com.
Amrita Mishra, Email: amrita_azam@rediffmail.com.
Ruhul Amin, Email: ruhulglp18@gmail.com.
Chellappan Ronald Darwin, Email: ronaldpharma@gmail.com.
Talha Bin Emran, Email: talhabmb@bgctub.ac.bd;talhabmb@gmail.com.
References
- 1.Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol 2008;17:1063–1072. [DOI] [PubMed] [Google Scholar]
- 2.Elias PM; Williams ML. Overview of the epidermis and dermis. In: Goldsmith S, Wolff K, Katz S, Gilchrest B, Paller A, Leffell D, eds. Fitzpatrick’s Dermatology In General Medicine, 9th ed. McGraw-Hill Education; 2019. pp. 61–72.
- 3.Madison KC. Barrier function of the skin: “La raison d’être” of the epidermis. J Invest Dermatol 2003;121:231–241. [DOI] [PubMed] [Google Scholar]
- 4.Losquadro WD. Anatomy of the skin and the pathogenesis of nonmelanoma skin cancer. Facial Plast Surg Clin North Am 2017;25:283–289. [DOI] [PubMed] [Google Scholar]
- 5.Driskell RR, Watt FM. Understanding fibroblast heterogeneity in the skin. Trends Cell Biol 2015;25:92–99. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen AV, Soulika AM. The dynamics of the Ssin’s immune system. Int J Mol Sci 2019;20:1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Senechal B, Elain G, Jeziorski E, et al. Expansion of regulatory T cells in patients with Langerhans cell histiocytosis. PLoS Med 2007;4:e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol 2015;151:1081–1086. [DOI] [PubMed] [Google Scholar]
- 9.Leiter U, Eigentler T, Garbe C. Epidemiology of skin cancer. Adv Exp Med Biol 2014;810:120–140. [DOI] [PubMed] [Google Scholar]
- 10.Stratigos AJ, Garbe C, Dessinioti C, et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 2. Treatment. Eur J Cancer 2020;128:83–102. [DOI] [PubMed] [Google Scholar]
- 11.Kwong LN, Davies MA. Navigating the therapeutic complexity of PI3K pathway inhibition in melanoma. Clin Cancer Res 2013;19:5310–5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol 2012;166:1069–1080. [DOI] [PubMed] [Google Scholar]
- 13.Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer 2008;8:743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong SY, Reiter JF. The primary cilium at the crossroads of mammalian hedgehog signalling. Curr Top Dev Biol 2008;85:225–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang JY, Aszterbaum M, Athar M, et al. Basal cell carcinoma chemoprevention with nonsteroidal anti-inflammatory drugs in genetically predisposed PTCH1+/− humans and mice. Cancer Prev Res (Phila) 2010;3:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin VT, Esmaeli B. Targeting the Hedgehog pathway for locally advanced and metastatic basal cell carcinoma. Curr Pharm Des 2017;23:655–659. [DOI] [PubMed] [Google Scholar]
- 17.Rivlin N, Brosh R, Oren M, et al. Mutations in the p53 tumor suppressor gene: important milestones at the various steps of tumorigenesis. Genes Cancer 2011;2:466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohan SV, Chang AL. Advanced basal cell carcinoma: epidemiology and therapeutic innovations. Curr Dermatol Rep 2014;3:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanofsky VR, Mercer SE, Phelps RG. Histopathological variants of cutaneous squamous cell carcinoma: a review. J Skin Cancer 2011;2011:210813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol 2010;2:a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weissmueller S, Manchado E, Saborowski M, et al. Mutant p53 drives pancreatic cancer metastasis through cell-autonomous PDGF receptor beta signalling. Cell 2014;157:382–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harismendy O, Notani D, Song X, et al. 9p21 DNA variants associated with coronary artery disease impair interferon-gamma signalling response. Nature 2011;470:264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stiewe T, Zimmermann S, Frilling A, et al. Transactivation-deficient DeltaTA-p73 acts as an oncogene. Cancer Res 2002;62:3598–3602. [PubMed] [Google Scholar]
- 24.Basu TN, Gutmann DH, Fletcher JA, et al. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature 1992;356:713–715. [DOI] [PubMed] [Google Scholar]
- 25.Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer 2007;7:295–308. [DOI] [PubMed] [Google Scholar]
- 26.Hodis E, Watson IR, Kryukov GV, et al. A landscape of driver mutations in melanoma. Cell 2012;150:251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dankort D, Curley DP, Cartlidge RA, et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet 2009;41:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chin L, Garraway LA, Fisher DE. Malignant melanoma: genetics and therapeutics in the genomic era. Genes Dev 2006;20:2149–2182. [DOI] [PubMed] [Google Scholar]
- 29.Lin WM, Baker AC, Beroukhim R, et al. Modeling genomic diversity and tumor dependency in malignant melanoma. Cancer Res 2008;68:664–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viros A, Fridlyand J, Bauer J, et al. Improving melanoma classification by integrating genetic and morphologic features. PLoS Med 2008;5:e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hocker T, Tsao H. Ultraviolet radiation and melanoma: a systematic review and analysis of reported sequence variants. Hum Mut 2007;28:578–588. [DOI] [PubMed] [Google Scholar]
- 32.Hayward NK, Wilmott JS, Waddell N, et al. Whole-genome landscapes of major melanoma subtypes. Nature 2017;545:175–180. [DOI] [PubMed] [Google Scholar]
- 33.Shain AH, Yeh I, Kovalyshyn I, et al. The genetic evolution of melanoma from precursor lesions. N Engl J Med 2015;373:1926–1936. [DOI] [PubMed] [Google Scholar]
- 34.Oren M, Levine AJ. Molecular cloning of a cDNA specific for the murine p53 cellular tumor antigen. Proc Natl Acad Sci U S A 1983;80:56–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soussi T, Wiman KG. Shaping genetic alterations in human cancer: the p53 mutation paradigm. Cancer Cell 2007;12:303–312. [DOI] [PubMed] [Google Scholar]
- 36.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature 2000;408:307–310. [DOI] [PubMed] [Google Scholar]
- 37.Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev 2012;26:1268–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toledo F, Wahl GM. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer 2006;6:909–923. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Zhang T, Korkaya H, et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. sulforaphane inhibits breast cancer stem cells. Clin Cancer Res 2010;16:2580–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patil JR, Jayaprakasha GK, Murthy KC, et al. Apoptosis-mediated proliferation inhibition of human colon cancer cells by volatile principles of Citrus aurantifolia. Food Chem 2009;114:1351–1358. [Google Scholar]
- 41.Grosso G, Bei R, Mistretta A, et al. Effects of vitamin C on health: a review of evidence. Front Biosci (Landmark Ed) 2013;18:1017–1029. [DOI] [PubMed] [Google Scholar]
- 42.Spencer JP, Vafeiadou K, Williams RJ, et al. Neuroinflammation: modulation by flavonoids and mechanisms of action. Mol Aspects Med 2012;33:83–97. [DOI] [PubMed] [Google Scholar]
- 43.Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2009;2:270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson IT. Phytochemicals and cancer. Proc Nutr Soc 2007;66:207–215. [DOI] [PubMed] [Google Scholar]
- 45.Manach C, Scalbert A, Morand C, et al. Polyphenols: food sources and bioavailability. Am J Clin Nut 2004;79:727–747. [DOI] [PubMed] [Google Scholar]
- 46.Liu RH. Health-promoting components of fruits and vegetables in the diet. Adv Nutr 2013;4:384S–392S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kesarkar S, Bhandage A, Deshmukh S, et al. Flavonoids: an overview. J Pharm Res 2009;2:1148–1154. [Google Scholar]
- 48.Grosso G, Godos J, Lamuela‐Raventos R, et al. A comprehensive meta‐analysis on dietary flavonoid and lignan intake and cancer risk: level of evidence and limitations. Mol Nutr Food Res 2017;61:1600930. [DOI] [PubMed] [Google Scholar]
- 49.Stahl W, Sies H. Antioxidant activity of carotenoids. Mol Aspects Med 2003;24:345–351. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka T, Shnimizu M, Moriwaki H. Cancer chemoprevention by caroteno. Molecules 2012;17:3202–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coulman KD, Liu Z, Hum WQ, et al. Whole sesame seed is as rich a source of mammalian lignan precursors as whole flaxseed. Nutr Cancer 2005;52:156–165. [DOI] [PubMed] [Google Scholar]
- 52.Adlercreutz H. Lignans and human health. Crit Rev Clin Lab Sci 2007;44:483–525. [DOI] [PubMed] [Google Scholar]
- 53.Shiau JP, Chuang YT, Tang JY, et al. The impact of oxidative stress and AKT pathway on cancer cell functions and its application to natural products. Antioxidants (Basel) 2022;11:1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sana SS, Pasha A, Kumbhakar DV, et al. SnO2-NPs (synthesized from crude rosmarinic acid) inhibit HeLa cells progression in vitro for anti-tumor investigation and act as an angiogenic inhibitor in ovo CAM xenograft. Nano-Struct Nano-Objects 2023;33:100918. [Google Scholar]
- 55.Eskandani R, Kazempour M, Farahzadi R, et al. Engineered nanoparticles as emerging gene/drug delivery systems targeting the nuclear factor-κB protein and related signalling pathways in cancer. Biomed Pharmacother 2022;156:113932. [DOI] [PubMed] [Google Scholar]
- 56.Yuan Y, Guo M, Gu C, et al. The role of Wnt/β-catenin signalling pathway in the pathogenesis and treatment of multiple myeloma (review). Am J Transl Res 2021;13:9932–9949. [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang X, Xie K, Zhou H, et al. Role of non-coding RNAs and RNA modifiers in cancer therapy resistance. Mol Cancer 2020;19:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gupta SC, Kim JH, Prasad S, et al. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev 2010;29:405–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mondal A, Banerjee S, Bose S, et al. Garlic constituents for cancer prevention and therapy: From phytochemistry to novel formulations. Pharmacolog Res 2022;175:105837. [DOI] [PubMed] [Google Scholar]
- 60.Huang Y, Li W, Su ZY, et al. The complexity of the Nrf2 pathway: beyond the antioxidant response. J Nutr Biochem 2015;26:1401–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roy SK, Srivastava RK, Shankar S. Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of FOXO transcription factor, leading to cell cycle arrest and apoptosis in pancreatic cancer. J Mol Signal 2010;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang L, Tian Z, Yang Q, et al. Sulforaphane inhibits thyroid cancer cell growth and invasiveness through the reactive oxygen species-dependent pathway. Oncotarget 2015;6:25917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Olędzka AJ, Czerwińska ME. Role of plant-derived compounds in the molecular pathways related to inflammation. Int J Mol Sci 2023;24:4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edderkaoui M, Odinokova I, Ohno I, et al. Ellagic acid induces apoptosis through inhibition of nuclear factor κB in pancreatic cancer cells. World J Gastroenterol 2008;14:3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang HM, Zhao L, Li H, et al. Research progress on the anticarcinogenic actions and mechanisms of ellagic acid. Cancer Biol Med 2014;11:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qiu S, Zhong C, Zhao B, et al. Transcriptome analysis of signalling pathways targeted by Ellagic acid in hepatocellular carcinoma cells. Biochim Biophys Acta Gen Subj 2021;1865:129911. [DOI] [PubMed] [Google Scholar]
- 67.Ceci C, Lacal PM, Tentori L, et al. Experimental evidence of the antitumor, antimetastatic and antiangiogenic activity of ellagic acid. Nutrients 2018;10:1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takada Y, Aggarwal BB. Betulinic acid suppresses carcinogen-induced NF-κB activation through inhibition of IκBα kinase and p65 phosphorylation: abrogation of cyclooxygenase-2 and matrix metalloprotease-9. J Immunol 2003;171:3278–3286. [DOI] [PubMed] [Google Scholar]
- 69.Mullauer FB, Kessler JH, Medema JP. Betulinic acid, a natural compound with potent anticancer effects. Anticancer Drugs 2010;21:215–227. [DOI] [PubMed] [Google Scholar]
- 70.Fulda S, Friesen C, Los M, et al. Betulinic acid triggers CD95 (APO-1/Fas)-and p53-independent apoptosis via activation of caspases in neuroectodermal tumors. Cancer Res 1997;57:4956–4964. [PubMed] [Google Scholar]
- 71.Jiang W, Li X, Dong S, et al. Betulinic acid in the treatment of tumour diseases: application and research progress. Biomed Pharmacother 2021;142:111990. [DOI] [PubMed] [Google Scholar]
- 72.Liu W, Li S, Qu Z, et al. Betulinic acid induces autophagy-mediated apoptosis through suppression of the PI3K/AKT/mTOR signalling pathway and inhibits hepatocellular carcinoma. Am J Transl Res 2019;11:6952. [PMC free article] [PubMed] [Google Scholar]
- 73.Jiang N, Hu Y, Wang M, et al. The Notch signalling pathway contributes to angiogenesis and tumor immunity in breast cancer. Breast Cancer (Dove Med Press) 2022;14:291–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tong X, Pelling JC. Targeting the PI3K/Akt/mTOR axis by apigenin for cancer prevention. Anticancer Agents Med Chem 2013;13:971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Javed Z, Sadia H, Iqbal MJ, et al. Apigenin role as cell-signalling pathways modulator: implications in cancer prevention and treatment. Cancer Cell Int 2021;21:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao G, Han X, Cheng W, et al. Apigenin inhibits proliferation and invasion, and induces apoptosis and cell cycle arrest in human melanoma cells. Oncol Rep 2017;37:2277–2285. [DOI] [PubMed] [Google Scholar]
- 77.Jia M, Mateoiu C, Souchelnytskyi S. Protein tyrosine nitration in the cell cycle. Biochem Biophys Res Commun 2011;413:270–276. [DOI] [PubMed] [Google Scholar]
- 78.Shukla S, Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res 2010;27:962–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Imran M, Aslam Gondal T, Atif M, et al. Apigenin as an anticancer agent. Phytother Res 2020;34:1812–1828. [DOI] [PubMed] [Google Scholar]
- 80.Deng J, Bai X, Feng X, et al. Inhibition of PI3K/Akt/mTOR signalling pathway alleviates ovarian cancer chemoresistance through reversing epithelial–mesenchymal transition and decreasing cancer stem cell marker expression. BMC Cancer 2019;19:618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim SO, Kundu JK, Shin YK, et al. [6]-Gingerol inhibits COX-2 expression by blocking the activation of p38 MAP kinase and NF-κB in phorbol ester-stimulated mouse skin. Oncogene 2005;24:2558–2567. [DOI] [PubMed] [Google Scholar]
- 82.Surh YJ, Na HK. NF-κB and Nrf2 as prime molecular targets for chemoprevention and cytoprotection with anti-inflammatory and antioxidant phytochemicals. Genes Nutr 2008;2:313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang HC, Chou YC, Wu CY, et al. [8]-Gingerol inhibits melanogenesis in murine melanoma cells through down-regulation of the MAPK and PKA signal pathways. Biochem Biophys Res Commun 2013;438:375–381. [DOI] [PubMed] [Google Scholar]
- 84.Rather RA, Bhagat M. Quercetin as an innovative therapeutic tool for cancer chemoprevention: molecular mechanisms and implications in human health. Cancer Med 2020;9:9181–9192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiong G, Ji W, Wang F, et al. Quercetin inhibits inflammatory response induced by LPS from Porphyromonas gingivalis in human gingival fibroblasts via suppressing NF-κB signalling pathway. Biomed Res Int 2019;2019:6282635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zehsaz F, Farhangi N, Mirheidari L. The effect of Zingiber officinale R. rhizomes (ginger) on plasma pro-inflammatory cytokine levels in well-trained male endurance runners. Cent Eur J Immunol 2014;39:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zalpoor H, Nabi-Afjadi M, Forghaniesfidvajani R, et al. Quercetin as a JAK–STAT inhibitor: a potential role in solid tumors and neurodegenerative diseases. Cell Mol Biol Lett 2022;27:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sekiguchi A, Motegi SI, Fujiwara C, et al. Inhibitory effect of kaempferol on skin fibrosis in systemic sclerosis by the suppression of oxidative stress. J Dermatolog Sci 2019;96:8–17. [DOI] [PubMed] [Google Scholar]
- 89.Sharma V, Joseph C, Ghosh S, et al. Kaempferol induces apoptosis in glioblastoma cells through oxidative stress. Mol Cancer Ther 2007;6:2544–2553. [DOI] [PubMed] [Google Scholar]
- 90.Ashrafizadeh M, Gholami MH, Mirzaei S, et al. Dual relationship between long non-coding RNAs and STAT3 signalling in different cancers: new insight to proliferation and metastasis. Life Sci 2021;270:119006. [DOI] [PubMed] [Google Scholar]
- 91.Chen AY, Chen YC. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem 2013;138:2099–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim GD. Kaempferol inhibits angiogenesis by suppressing HIF-1α and VEGFR2 activation via ERK/p38 MAPK and PI3K/Akt/mTOR signalling pathways in endothelial cells. Prevent Nutr Food Sci 2017;22:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chikara S, Nagaprashantha LD, Singhal J, et al. Oxidative stress and dietary phytochemicals: role in cancer chemoprevention and treatment. Cancer Lett 2018;413:122–134. [DOI] [PubMed] [Google Scholar]
- 94.Kundu JK, Shin YK, Surh YJ. Resveratrol modulates phorbol ester-induced pro-inflammatory signal transduction pathways in mouse skin in vivo: NF-κB and AP-1 as prime targets. Biochem Pharmacol 2006;72:1506–1515. [DOI] [PubMed] [Google Scholar]
- 95.Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatolog Res 2010;302:71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu Z, Liu B, E C, et al. Resveratrol inhibits the proliferation of human melanoma cells by inducing G1/S cell cycle arrest and apoptosis. Mol Med Rep 2015;11:400–404. [DOI] [PubMed] [Google Scholar]
- 97.Aziz MH, Reagan‐Shaw S, Wu J, et al. Chemoprevention of skin cancer by grape constituent resveratrol: relevance to human disease? FASEB J 2005;19:1193–1195. [DOI] [PubMed] [Google Scholar]
- 98.Elshaer M, Chen Y, Wang XJ, et al. Resveratrol: an overview of its anti-cancer mechanisms. Life Sci 2018;207:340–349. [DOI] [PubMed] [Google Scholar]
- 99.Nakamura Y, Ohto Y, Murakami A, et al. Inhibitory effects of curcumin and tetrahydrocurcuminoids on the tumor promoter‐induced reactive oxygen species generation in leukocytes in vitro and in vivo. Jpn J Cancer Res 1998;89:361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.He Y, Yue Y, Zheng X, et al. Curcumin, inflammation, and chronic diseases: how are they linked? Molecules 2015;20:9183–9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chao SC, Hu DN, Roberts J, et al. Inhibition effect of curcumin on UVB-induced secretion of pro-inflammatory cytokines from corneal limbus epithelial cells. Int J Ophthalmol 2017;10:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu D, An F, He X, et al. Curcumin inhibits the proliferation and invasion of human osteosarcoma cell line MG-63 by regulating miR-138. Int J Clin Exp Pathol 2015;8:14946. [PMC free article] [PubMed] [Google Scholar]
- 103.Arbiser JL, Klauber N, Rohan R, et al. Curcumin is an in vivo inhibitor of angiogenesis. Mol Med 1998;4:376–383. [PMC free article] [PubMed] [Google Scholar]
- 104.Panahi Y, Fazlolahzadeh O, Atkin SL, et al. Evidence of curcumin and curcumin analogue effects in skin diseases: a narrative review. J Cell Physiol 2019;234:1165–1178. [DOI] [PubMed] [Google Scholar]
- 105.Bhandarkar SS, Arbiser JL. Curcumin as an inhibitor of angiogenesis. Adv Exp Med Biol 2007;595:185–195. [DOI] [PubMed] [Google Scholar]
- 106.Huang CC, Wu WB, Fang JY, et al. (-)-Epicatechin-3-gallate, a green tea polyphenol is a potent agent against UVB-induced damage in HaCaT keratinocytes. Molecules 2007;12:1845–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Salaheldin TA, Adhami VM, Fujioka K, et al. Photochemoprevention of ultraviolet beam radiation-induced DNA damage in keratinocytes by topical delivery of nanoformulated epigallocatechin-3-gallate. Nanomedicine 2022;44:102580. [DOI] [PubMed] [Google Scholar]
- 108.Kim I, He YY. Ultraviolet radiation-induced non-melanoma skin cancer: regulation of DNA damage repair and inflammation. Genes Dis 2014;1:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sharifi-Rad M, Pezzani R, Redaelli M, et al. Preclinical activities of epigallocatechin gallate in signalling pathways in cancer. Molecules 2020;25:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aggarwal V, Tuli HS, Tania M, et al. Molecular mechanisms of action of epigallocatechin gallate in cancer: recent trends and advancement. Semin Cancer Biol 2022;80:256–275. [DOI] [PubMed] [Google Scholar]
- 111.Choudhury SR, Balasubramanian S, Chew YC, et al. (-)-Epigallocatechin-3-gallate and DZNep reduce polycomb protein level via a proteasome-dependent mechanism in skin cancer cells. Carcinogenesis 2011;32:1525–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Katiyar S, Elmets CA, Katiyar SK. Green tea and skin cancer: photoimmunology, angiogenesis and DNA repair. J Nutr Biochem 2007;18:287–296. [DOI] [PubMed] [Google Scholar]
- 113.Jung YD, Ellis LM. Inhibition of tumour invasion and angiogenesis by epigallocatechin gallate (EGCG), a major component of green tea. Int J Exp Pathol 2001;82:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.O’Connor I, O’Brien N. Modulation of UVA light-induced oxidative stress by β-carotene, lutein and astaxanthin in cultured fibroblasts. J Dermatolog Sci 1998;16:226–230. [DOI] [PubMed] [Google Scholar]
- 115.Fuchs J. Potentials and limitations of the natural antioxidants RRR-alpha-tocopherol, L-ascorbic acid and β-carotene in cutaneous photoprotection. Free Radic Biol Med 1998;25:848–873. [DOI] [PubMed] [Google Scholar]
- 116.Anjani G, Ayustaningwarno F, Eviana R. Critical review on the immunomodulatory activities of carrot’s β-carotene and other bioactive compounds. J Funct Foods 2022;99:105303. [Google Scholar]
- 117.Hughes DA. Effects of carotenoids on human immune function. Proc Nutr Soc 1999;58:713–718. [DOI] [PubMed] [Google Scholar]
- 118.Hadad N, Levy R. The synergistic anti-inflammatory effects of lycopene, lutein, β-carotene, and carnosic acid combinations via redox-based inhibition of NF-κB signalling. Free Radic Biol Med 2012;53:1381–1391. [DOI] [PubMed] [Google Scholar]
- 119.Wertz K, Hunziker PB, Seifert N, et al. β-carotene interferes with ultraviolet light A-induced gene expression by multiple pathways. J Investigat Dermatol 2005;124:428–434. [DOI] [PubMed] [Google Scholar]
- 120.Furman-Toczek D, Zagórska-Dziok M, Dudra-Jastrzębska M, et al. A review of selected natural phytochemicals in preventing and treating malignant skin neoplasms. J Pre Clin Clin Res 2016;10:127–130. [Google Scholar]
- 121.Búfalo MC, Ferreira I, Costa G, et al. Propolis and its constituent caffeic acid suppress LPS-stimulated pro-inflammatory response by blocking NF-κB and MAPK activation in macrophages. J Ethnopharmacol 2013;149:84–92. [DOI] [PubMed] [Google Scholar]
- 122.Bharti AC, Aggarwal BB. Nuclear factor-kappa B and cancer: its role in prevention and therapy. Biochem Pharmacol 2002;64:883–888. [DOI] [PubMed] [Google Scholar]
- 123.Dorai T, Aggarwal BB. Role of chemopreventive agents in cancer therapy. Cancer Lett 2004;215:129–140. [DOI] [PubMed] [Google Scholar]
- 124.Desai SJ, Prickril B, Rasooly A. Mechanisms of phytonutrient modulation of cyclooxygenase-2 (COX-2) and inflammation related to cancer. Nutr Cancer 2018;70:350–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guan C, Zhou X, Li H, et al. NF-κB inhibitors gifted by nature: the anticancer promise of polyphenol compounds. Biomed Pharmacother 2022;156:113951. [DOI] [PubMed] [Google Scholar]
- 126.Hashem S, Ali TA, Akhtar S, et al. Targeting cancer signalling pathways by natural products: exploring promising anti-cancer agents. Biomed Pharmacother 2022;150:113054. [DOI] [PubMed] [Google Scholar]
- 127.Seeram NP. Berry fruits for cancer prevention: current status and future prospects. J Agric Food Chem 2008;56:630–635. [DOI] [PubMed] [Google Scholar]
- 128.Scalbert A, Johnson IT, Saltmarsh M. Polyphenols: antioxidants and beyond. Am J Clin Nutr 2005;81:215S–217S. [DOI] [PubMed] [Google Scholar]
- 129.de Kok TM, van Breda SG, Manson MM. Mechanisms of combined action of different chemopreventive dietary compounds: a review. Eur J Nutr 2008;47:51–59. [DOI] [PubMed] [Google Scholar]
- 130.Sik B, Kapcsándi V, Székelyhidi R, et al. Recent advances in the analysis of rosmarinic acid from herbs in the Lamiaceae family. Nat Prod Commun 2019;14:1934578X19864216. [Google Scholar]
- 131.Nunes S, Madureira AR, Campos D, et al. Therapeutic and nutraceutical potential of rosmarinic acid—cytoprotective properties and pharmacokinetic profile. Crit Rev Food Sci Nutr 2017;57:1799–1806. [DOI] [PubMed] [Google Scholar]
- 132.Shekarchi M, Hajimehdipoor H, Saeidnia S, et al. Comparative study of rosmarinic acid content in some plants of Labiatae family. Pharmacogn Mag 2012;8:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Costa DC, Costa HS, Albuquerque TG, et al. Advances in phenolic compounds analysis of aromatic plants and their potential applications. Trends Food Sci Technol 2015;45:336–354. [Google Scholar]
- 134.Kumar P Verma AK Umaraw P, et al. Plant phenolics as natural preservatives in food system. Plant Phenolics in Sustainable Agriculture, Vol. 1. Springer; 2020. pp. 367–406.
- 135.Afaq F. Natural agents: cellular and molecular mechanisms of photoprotection. Arch Biochem Biophys 2011;508:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pérez-Sánchez A, Barrajón-Catalán E, Herranz-López M, et al. Lemon balm extract (Melissa officinalis, L.) promotes melanogenesis and prevents UVB-induced oxidative stress and DNA damage in a skin cell model. J Dermatolog Sci 2016;84:169–177. [DOI] [PubMed] [Google Scholar]
- 137.Liu HM, Cheng MY, Xun MH, et al. Possible mechanisms of oxidative stress-induced skin cellular senescence, inflammation, and cancer and the therapeutic potential of plant polyphenols. Int J Mol Sci 2023;24:3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rodríguez-Luna A, Ávila-Román J, Oliveira H, et al. Fucoxanthin and rosmarinic acid combination has anti-inflammatory effects through regulation of NLRP3 inflammasome in UVB-exposed HaCaT keratinocytes. Mar Drugs 2019;17:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fernando PMDJ, Piao MJ, Kang KA, et al. Rosmarinic acid attenuates cell damage against UVB radiation-induced oxidative stress via enhancing antioxidant effects in human HaCaT cells. Biomol Ther (Seoul) 2016;24:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sánchez-Campillo M, Gabaldon JA, Castillo J, et al. Rosmarinic acid, a photo-protective agent against UV and other ionizing radiations. Food Chem Toxicol 2009;47:386–392. [DOI] [PubMed] [Google Scholar]
- 141.Pressi G, Bertaiola O, Guarnerio C, et al. In vitro cultured Melissa officinalis cells as effective ingredient to protect skin against oxidative stress, blue light, and infrared irradiations damages. Cosmetics 2021;8:23. [Google Scholar]
- 142.Han YH, Kee JY, Hong SH. Rosmarinic acid activates AMPK to inhibit metastasis of colorectal cancer. Front Pharmacol 2018;9:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xu Y, Xu G, Liu L, et al. Anti‐invasion effect of rosmarinic acid via the extracellular signal‐regulated kinase and oxidation–reduction pathway in Ls174‐T cells. J Cell Biochem 2010;111:370–379. [DOI] [PubMed] [Google Scholar]
- 144.Lee C, Park GH, Ahn EM, et al. Protective effect of Codium fragile against UVB-induced pro-inflammatory and oxidative damages in HaCaT cells and BALB/c mice. Fitoterapia 2013;86:54–63. [DOI] [PubMed] [Google Scholar]
- 145.Mengoni ES, Vichera G, Rigano LA, et al. Suppression of COX-2, IL-1β and TNF-α expression and leukocyte infiltration in inflamed skin by bioactive compounds from Rosmarinus officinalis L. Fitoterapia 2011;82:414–421. [DOI] [PubMed] [Google Scholar]
- 146.da Silva GB, Manica D, da Silva AP, et al. Rosmarinic acid decreases viability, inhibits migration and modulates expression of apoptosis-related CASP8/CASP3/NLRP3 genes in human metastatic melanoma cells. Chem Biol Interact 2023;375:110427. [DOI] [PubMed] [Google Scholar]
- 147.Zhao J, Xu L, Jin D, et al. Rosmarinic acid and related dietary supplements: potential applications in the prevention and treatment of cancer. Biomolecules 2022;12:1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Moore J, Yousef M, Tsiani E. Anticancer effects of rosemary (Rosmarinus officinalis L.) extract and rosemary extract polyphenols. Nutrients 2016;8:731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li H, Zhang Y, Chen HH, et al. Rosmarinic acid inhibits stem-like breast cancer through hedgehog and Bcl-2/Bax signalling pathways. Pharmacogny Mag 2019;15:600–606. [Google Scholar]
- 150.Jang YG, Hwang KA, Choi KC. Rosmarinic acid, a component of rosemary tea, induced the cell cycle arrest and apoptosis through modulation of HDAC2 expression in prostate cancer cell lines. Nutrients 2018;10:1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.da Silva GB, Yamauchi MA, Zanini D, et al. Novel possibility for cutaneous melanoma treatment by means of rosmarinic acid action on purinergic signalling. Purinergic Signal 2022;18:61–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Psotova J, Svobodova A, Kolarova H, et al. Photoprotective properties of Prunella vulgaris and rosmarinic acid on human keratinocytes. J Photochem Photobiol B Biol 2006;84:167–174. [DOI] [PubMed] [Google Scholar]
- 153.Huang SS, Zheng RL. Rosmarinic acid inhibits angiogenesis and its mechanism of action in vitro. Cancer Lett 2006;239:271–280. [DOI] [PubMed] [Google Scholar]
- 154.Gupta D, Sharma RR, Rashid H, et al. Rosmarinic acid alleviates ultraviolet mediated skin aging via attenuation of mitochondrial and ER stress responses. Exp Dermatol 2023;32:799–807. [DOI] [PubMed] [Google Scholar]
- 155.Huang L, Chen J, Quan J, et al. Rosmarinic acid inhibits proliferation and migration, promotes apoptosis and enhances cisplatin sensitivity of melanoma cells through inhibiting ADAM17/EGFR/AKT/GSK3β axis. Bioengineered 2021;12:3065–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sharmila R, Ghosh K, Manoharan S. Rewiring of chemically modified MAPK pathway and its downstream targets by rosmarinic acid in mice dermal cancer. J Inform Comput Sci 2020;10:1047–1064. [Google Scholar]
- 157.Lukmanul Hakkim F, Miura M, Matsuda N, et al. An in vitro evidence for caffeic acid, rosmarinic acid and trans cinnamic acid as a skin protectant against γ-radiation. Int J Low Radiat 2014;9:305–316. [Google Scholar]
- 158.Noor S, Mohammad T, Rub MA, et al. Biomedical features and therapeutic potential of rosmarinic acid. Arch Pharm Res 2022;45:205–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Esmeeta A, Adhikary S, Dharshnaa V, et al. Plant-derived bioactive compounds in colon cancer treatment: an updated review. Biomed Pharmacother 2022;153:113384. [DOI] [PubMed] [Google Scholar]
- 160.Taheri JB, Azimi S, Rafieian N, et al. Herbs in dentistry. Int Dent J 2011;61:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Veras KS, Fachel FNS, Teixeira HF, et al. Technological strategies applied for rosmarinic acid delivery through different routes – a review. J Drug Deliv Sci Technol 2022;68:103054. [Google Scholar]
- 162.Marchev AS, Vasileva LV, Amirova KM, et al. Rosmarinic acid – from bench to valuable applications in food industry. Trends Food Sci Technol 2021;117:182–193. [Google Scholar]
- 163.Swamy MK, Sinniah UR, Ghasemzadeh A. Anticancer potential of rosmarinic acid and its improved production through biotechnological interventions and functional genomics. Appl Microbiol Biotechnol 2018;102:7775–7793. [DOI] [PubMed] [Google Scholar]
- 164.Portz D, Koch E, Slusarenko AJ. Effects of garlic (Allium sativum) juice containing allicin on Phytophthora infestans and downy mildew of cucumber caused by Pseudoperonospora cubensis. Eur J Plant Pathol 2008;122:197–206. [Google Scholar]
- 165.Guillamón E, Andreo-Martínez P, Mut-Salud N, et al. Beneficial effects of organosulfur compounds from Allium cepa on gut health: a systematic review. Foods 2021;10:1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Marchese A, Barbieri R, Sanches-Silva A, et al. Antifungal and antibacterial activities of allicin: a review. Trends Food Sci Technol 2016;52:49–56. [Google Scholar]
- 167.Sbrana C, Avio L, Giovannetti M. Beneficial mycorrhizal symbionts affecting the production of health‐promoting phytochemicals. Electrophoresis 2014;35:1535–1546. [DOI] [PubMed] [Google Scholar]
- 168.Touloupakis E, Ghanotakis DF. Nutraceutical use of garlic sulfur-containing compounds. Adv Exp Med Biol 2010;698:110–121. [DOI] [PubMed] [Google Scholar]
- 169.Lawson LD, Hunsaker SM. Allicin bioavailability and bioequivalence from garlic supplements and garlic foods. Nutrients 2018;10:812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Amagase H. Clarifying the real bioactive constituents of garlic. J Nutr 2006;136:716S–725S. [DOI] [PubMed] [Google Scholar]
- 171.Wang HC, Pao J, Lin SY, et al. Molecular mechanisms of garlic‐derived allyl sulfides in the inhibition of skin cancer progression. Ann N Y Acad Sci 2012;1271:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jobani BM, Najafzadeh N, Mazani M, et al. Molecular mechanism and cytotoxicity of allicin and all-trans retinoic acid against CD44+ versus CD117+ melanoma cells. Phytomedicine 2018;48:161–169. [DOI] [PubMed] [Google Scholar]
- 173.Omar SH, Al-Wabel NA. Organosulfur compounds and possible mechanism of garlic in cancer. Saudi Pharm J 2010;18:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fatima N, Baqri SSR, Alsulimani A, et al. Phytochemicals from Indian ethnomedicines: promising prospects for the management of oxidative stress and cancer. Antioxidants 2021;10:1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chahal DS, Sivamani RK, Rivkah Isseroff R, et al. Plant‐based modulation of toll‐like receptors: an emerging therapeutic model. Phytother Res 2013;27:1423–1438. [DOI] [PubMed] [Google Scholar]
- 176.Chu YL, Ho CT, Chung JG, et al. Allicin induces anti-human liver cancer cells through the p53 gene modulating apoptosis and autophagy. J Agric Food Chem 2013;61:9839–9848. [DOI] [PubMed] [Google Scholar]
- 177.Dubey SK, ed. Natural Products and Nano-Formulations in Cancer Chemoprevention. CRC Press; 2023.
- 178.Hashemy SI, Amiri H, Hosseini H, et al. PEGylated lecithin–chitosan–folic acid nanoparticles as nanocarriers of allicin for in vitro controlled release and anticancer effects. Appl Biochem Biotechnol 2023;195:4036–4052. [DOI] [PubMed] [Google Scholar]
- 179.Hasanpourghadi M, Yeng Looi C, Kumar Pandurangan A, et al. Phytometabolites targeting the Warburg effect in cancer cells: a mechanistic review. Curr Drug Targets 2017;18:1086–1094. [DOI] [PubMed] [Google Scholar]
- 180.Catanzaro E, Canistro D, Pellicioni V, et al. Anticancer potential of allicin: a review. Pharmacol Res 2022;177:106118. [DOI] [PubMed] [Google Scholar]
- 181.Pandey MK, Gupta SC, Nabavizadeh A, et al. Regulation of cell signalling pathways by dietary agents for cancer prevention and treatment. Semin Cancer Biol 2017;46:158–181. [DOI] [PubMed] [Google Scholar]
- 182.Hitl M, Kladar N, Gavarić N, et al. Garlic burn injuries – a systematic review of reported cases. Am J Emerg Med 2021;44:5–10. [DOI] [PubMed] [Google Scholar]
- 183.Farías-Campomanes AM, Horita CN, Pollonio MA, et al. Allicin-rich extract obtained from garlic by pressurized liquid extraction: quantitative determination of allicin in garlic samples. Food Public Health 2014;4:272–278. [Google Scholar]
- 184.Clarke JD, Dashwood RH, Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett 2008;269:291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shapiro TA, Fahey JW, Wade KL, et al. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: metabolism and excretion in humans. Cancer Epidemiol Biomarkers Prevent 2001;10:501–508. [PubMed] [Google Scholar]
- 186.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci U S A 1997;94:10367–10372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Atwell LL, Hsu A, Wong CP, et al. Absorption and chemopreventive targets of sulforaphane in humans following consumption of broccoli sprouts or a myrosinase‐treated broccoli sprout extract. Mol Nutr Food Res 2015;59:424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang GC, Farnham M, Jeffery EH. Impact of thermal processing on sulforaphane yield from broccoli (Brassica oleracea L. ssp. italica). J Agric Food Chem 2012;60:6743–6748. [DOI] [PubMed] [Google Scholar]
- 189.Shokri S, Jegasothy H, Augustin MA, et al. Thermosonication for the production of sulforaphane rich broccoli ingredients. Biomolecules 2021;11:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chinembiri TN, Du Plessis LH, Gerber M, et al. Review of natural compounds for potential skin cancer treatment. Molecules 2014;19:11679–11721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jiang X, Liu Y, Ma L, et al. Chemopreventive activity of sulforaphane. Drug Des Devel Ther 2018;12:2905–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Giudice A, Montella M. Activation of the Nrf2–ARE signalling pathway: a promising strategy in cancer prevention. Bioessays 2006;28:169–181. [DOI] [PubMed] [Google Scholar]
- 193.Reddy SA, Shelar SB, Dang T-M, et al. Sulforaphane and its methylcarbonyl analogs inhibit the LPS-stimulated inflammatory response in human monocytes through modulating cytokine production, suppressing chemotactic migration and phagocytosis in a NF-κB- and MAPK-dependent manner. Int Immunopharmacol 2015;24(no. 2):440–450. [DOI] [PubMed] [Google Scholar]
- 194.Hamsa TP, Thejass P, Kuttan G. Induction of apoptosis by sulforaphane in highly metastatic B16F-10 melanoma cells. Drug Chem Toxicol 2011;34:332–340. [DOI] [PubMed] [Google Scholar]
- 195.Bhat TA, Singh RP. Tumor angiogenesis – a potential target in cancer chemoprevention. Food Chem Toxicol 2008;46:1334–1345. [DOI] [PubMed] [Google Scholar]
- 196.Balasubramanian S, Chew YC, Eckert RL. Sulforaphane suppresses polycomb group protein level via a proteasome-dependent mechanism in skin cancer cells. Mol Pharmacol 2011;80:870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pop S, Enciu AM, Tarcomnicu I, et al. Phytochemicals in cancer prevention: modulating epigenetic alterations of DNA methylation. Phytochem Rev 2019;18:1005–1024. [Google Scholar]
- 198.Park LK, Friso S, Choi SW. Nutritional influences on epigenetics and age-related disease. Proc Nutr Soc 2012;71:75–83. [DOI] [PubMed] [Google Scholar]
- 199.Eom YS, Shah FH, Kim SJ. Sulforaphane induces cell differentiation, melanogenesis and also inhibit the proliferation of melanoma cells. Eur J Pharmacol 2022;921:174894. [DOI] [PubMed] [Google Scholar]
- 200.Dickinson SE, Melton TF, Olson ER, et al. Inhibition of activator protein-1 by sulforaphane involves interaction with cysteine in the cFos DNA-binding domain: implications for chemoprevention of UVB-induced skin cancer. Cancer Res 2009;69:7103–7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Houghton CA, Fassett RG, Coombes JS. Sulforaphane: translational research from laboratory bench to clinic. Nutr Rev 2013;71:709–726. [DOI] [PubMed] [Google Scholar]
- 202.Cerdá B, Tomás-Barberán FA, Espín JC. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: identification of biomarkers and individual variability. J Agric Food Chem 2005;53:227–235. [DOI] [PubMed] [Google Scholar]
- 203.Bakkalbaşi E, Menteş Ö, Artik N. Food ellagitannins – occurrence, effects of processing and storage. Crit Rev Food Sci Nutr 2008;49:283–298. [DOI] [PubMed] [Google Scholar]
- 204.Hseu YC, Chou CW, Kumar KS, et al. Ellagic acid protects human keratinocyte (HaCaT) cells against UVA-induced oxidative stress and apoptosis through the upregulation of the HO-1 and Nrf-2 antioxidant genes. Food Chem Toxicol 2012;50:1245–1255. [DOI] [PubMed] [Google Scholar]
- 205.Devipriya N, Srinivasan M, Sudheer AR, et al. Effect of ellagic acid, a natural polyphenol, on alcohol-induced prooxidant and antioxidant imbalance: a drug dose dependent study. Singapore Med J 2007;48:311–8. [PubMed] [Google Scholar]
- 206.Umesalma S, Sudhandiran G. Differential inhibitory effects of the polyphenol ellagic acid on inflammatory mediators NF‐κB, iNOS, COX‐2, TNF‐α, and IL‐6 in 1,2‐dimethylhydrazine‐induced rat colon carcinogenesis. Basic Clin Pharmacol Toxicol 2010;107:650–655. [DOI] [PubMed] [Google Scholar]
- 207.Martin KR. Targeting apoptosis with dietary bioactive agents. Exp Biol Med 2006;231:117–129. [DOI] [PubMed] [Google Scholar]
- 208.Malik A, Afaq S, Shahid M, et al. Influence of ellagic acid on prostate cancer cell proliferation: a caspase-dependent pathway. Asian Pac J Trop Med 2011;4:550–555. [DOI] [PubMed] [Google Scholar]
- 209.D’Archivio M, Santangelo C, Scazzocchio B, et al. Modulatory effects of polyphenols on apoptosis induction: relevance for cancer prevention. Int J Mol Sci 2008;9:213–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang F, Chen J, Xiang D, et al. Ellagic acid inhibits cell proliferation, migration, and invasion in melanoma via EGFR pathway. Am J Transl Res 2020;12:2295. [PMC free article] [PubMed] [Google Scholar]
- 211.Bai S, Yu Y, An L, et al. Ellagic acid increases stress resistance via insulin/IGF-1 signalling pathway in Caenorhabditis elegans. Molecules 2022;27:6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zuccari G, Baldassari S, Ailuno G, et al. Formulation strategies to improve oral bioavailability of ellagic acid. Appl Sci 2020;10:3353. [Google Scholar]
- 213.Chatterjee S, Jain S, Jangid R, et al. Cytochrome P450 and P-gp mediated herb–drug interactions of some common Indian herbs. Stud Nat Prod Chem 2022;72:225–258. [Google Scholar]
- 214.Sharifi-Rad J, Quispe C, Castillo CMS, et al. Ellagic acid: a review on its natural sources, chemical stability, and therapeutic potential. Oxid Med Cell Longev 2022;2022:3848084. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 215.González-Sarrías A, García-Villalba R, Núñez-Sánchez MÁ, et al. Identifying the limits for ellagic acid bioavailability: a crossover pharmacokinetic study in healthy volunteers after consumption of pomegranate extracts. J Funct Foods 2015;19:225–235. [Google Scholar]
- 216.Espín JC, García-Conesa MT, Tomás-Barberán FA. Nutraceuticals: facts and fiction. Phytochemistry 2007;68:2986–3008. [DOI] [PubMed] [Google Scholar]
- 217.Hordyjewska A, Ostapiuk A, Horecka A, et al. Betulin and betulinic acid: triterpenoids derivatives with a powerful biological potential. Phytochem Rev 2019;18:929–951. [Google Scholar]
- 218.Mahajan RTCM, Chopda M. Phyto-pharmacology of Ziziphus jujuba Mill – a plant review. Pharmacogn Rev 2009;3:320. [Google Scholar]
- 219.Farneti B, Masuero D, Costa F, et al. Is there room for improving the nutraceutical composition of apple? J Agric Food Chem 2015;63:2750–2759. [DOI] [PubMed] [Google Scholar]
- 220.Ponou BK, Teponno RB, Tapondjou AL, et al. Steroidal saponins from the aerial parts of Cordyline fruticosa L. var. strawberries. Fitoterapia 2019;134:454–458. [DOI] [PubMed] [Google Scholar]
- 221.Demets OV, Takibayeva AT, Kassenov RZ, et al. Methods of betulin extraction from birch bark. Molecules 2022;27:3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Cichewicz RH, Kouzi SA. Chemistry, biological activity, and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med Res Rev 2004;24:90–114. [DOI] [PubMed] [Google Scholar]
- 223.Ríos JL, Máñez S. New pharmacological opportunities for betulinic acid. Planta Med 2018;84:8–19. [DOI] [PubMed] [Google Scholar]
- 224.Tewari D, Patni P, Bishayee A, et al. Natural products targeting the PI3K-Akt-mTOR signalling pathway in cancer: a novel therapeutic strategy. Semin Cancer Biol 2022;80:1–17. [DOI] [PubMed] [Google Scholar]
- 225.Gangwar V, Garg A, Lomore K, et al. Immunomodulatory effects of a concoction of natural bioactive compounds—mechanistic insights. Biomedicines 2021;9:1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Sugiura R, Satoh R, Takasaki T. ERK: a double-edged sword in cancer. ERK-dependent apoptosis as a potential therapeutic strategy for cancer. Cells 2021;10:2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sardar A, Gautam S, Sinha S, et al. Nanoparticles of naturally occurring PPAR-γ inhibitor betulinic acid ameliorates bone marrow adiposity and pathological bone loss in ovariectomized rats via Wnt/β-catenin pathway. Life Sci 2022;309:121020. [DOI] [PubMed] [Google Scholar]
- 228.Wróblewska-Łuczka P, Cabaj J, Bąk W, et al. Additive interactions between betulinic acid and two taxanes in in vitro tests against four human malignant melanoma cell lines. Int J Mol Sci 2022;23:9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ghiulai R, Mioc A, Racoviceanu R, et al. The anti-melanoma effect of betulinic acid functionalized gold nanoparticles: a mechanistic in vitro approach. Pharmaceuticals 2022;15:1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kallimanis P, Chinou I, Panagiotopoulou A, et al. Rosmarinus officinalis L. leaf extracts and their metabolites inhibit the aryl hydrocarbon receptor (AhR) activation in vitro and in human keratinocytes: potential impact on inflammatory skin diseases and skin cancer. Molecules 2022;27:2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bravo-Alfaro DA, Ochoa-Rodríguez LR, Villaseñor-Ortega F, et al. Self-nanoemulsifying drug delivery system (SNEDDS) improves the oral bioavailability of betulinic acid. J Mol Liquids 2022;364:119946. [Google Scholar]
- 232.Ameta RK, Soni K, Bhattarai A. Recent advances in improving the bioavailability of hydrophobic/lipophilic drugs and their delivery via self-emulsifying formulations. Colloids Interfaces 2023;7:16. [Google Scholar]
- 233.Wu X, Wei Z, Feng H, et al. Targeting effect of betulinic acid liposome modified by hyaluronic acid on hepatoma cells in vitro. J Pharm Sci 2022;111:3047–3053. [DOI] [PubMed] [Google Scholar]
- 234.Peng F, Jin Y, Wang K, et al. Glycosylated zein composite nanoparticles for efficient delivery of betulinic acid: fabrication, characterization, and in vitro release properties. Foods 2022;11:2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kumar V, Garg V, Dureja H. Nanoemulsion for delivery of anticancer drugs. Cancer Adv 2022;5:e22016. [Google Scholar]
- 236.Yogeeswari P, Sriram D. Betulinic acid and its derivatives: a review on their biological properties. Curr Med Chem 2005;12:657–666. [DOI] [PubMed] [Google Scholar]
- 237.Shankar E, Goel A, Gupta K, et al. Plant flavone apigenin: an emerging anticancer agent. Curr Pharmacol Rep 2017;3:423–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wang H, Yang L, Zu Y, et al. Microwave-assisted simultaneous extraction of luteolin and apigenin from tree peony pod and evaluation of its antioxidant activity. ScientificWorldJournal 2014;2014:506971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Michalaki A, Karantonis HC, Kritikou AS, et al. Ultrasound-assisted extraction of specific phenolic compounds from petroselinum crispum leaves using response surface methodology and HPLC-PDA and Q-TOF-MS/MS identification. Appl Sci 2023;13:798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Saotome Y, Imai M. Supercritical carbon dioxide extraction of apigenin from parsley leaves pre-treated to maximize yield. Food Sci Technol Res 2018;24:63–73. [Google Scholar]
- 241.Wang R, Ni N, Zhang QX, et al. Adsorption and separation of flavonoid aglycones and flavonol aglycones by using Zr (IV) immobilized collagen fiber adsorbent as column packing material. Sep Purif Technol 2022;289:120684. [Google Scholar]
- 242.Parihar A, Eubank TD, Doseff AI. Monocytes and macrophages regulate immunity through dynamic networks of survival and cell death. J Innate Immun 2010;2:204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol 2006;71:1397–1421. [DOI] [PubMed] [Google Scholar]
- 244.Ozbey U, Attar R, Romero MA, et al. Apigenin as an effective anticancer natural product: spotlight on TRAIL, WNT/β‐catenin, JAK‐STAT pathways, and microRNAs. J Cell Biochem 2019;120:1060–1067. [DOI] [PubMed] [Google Scholar]
- 245.L Suraweera T, Rupasinghe HV, Dellaire G, et al. Regulation of Nrf2/ARE pathway by dietary flavonoids: a friend or foe for cancer management? Antioxidants 2020;9:973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Montes de Oca MK, Pearlman RL, McClees SF, et al. Phytochemicals for the prevention of photocarcinogenesis. Photochem Photobiol 2017;93:956–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Bridgeman BB, Wang P, Ye B, et al. Inhibition of mTOR by apigenin in UVB-irradiated keratinocytes: a new implication of skin cancer prevention. Cell Signal 2016;28:460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Das S, Das J, Samadder A, et al. Strategic formulation of apigenin-loaded PLGA nanoparticles for intracellular trafficking, DNA targeting and improved therapeutic effects in skin melanoma in vitro. Toxicol Lett 2013;223:124–138. [DOI] [PubMed] [Google Scholar]
- 249.Jangdey MS, Gupta A, Saraf S, et al. Development and optimization of apigenin-loaded transfersomal system for skin cancer delivery: in vitro evaluation. Artif Cells Nanomed Biotechnol 2017;45:1452–1462. [DOI] [PubMed] [Google Scholar]
- 250.Waheed A, Zameer S, Sultana N, et al. Engineering of QbD driven and ultrasonically shaped lyotropic liquid crystalline nanoparticles for Apigenin in the management of skin cancer. Eur J Pharm Biopharm 2022;180:269–280. [DOI] [PubMed] [Google Scholar]
- 251.Fernández J, Silván B, Entrialgo-Cadierno R, et al. Antiproliferative and palliative activity of flavonoids in colorectal cancer. Biomed Pharmacother 2021;143:112241. [DOI] [PubMed] [Google Scholar]
- 252.Ali F, Rahul, Naz F, et al. Health functionality of apigenin: a review. Int J Food Prop 2017;20:1197–1238. [Google Scholar]
- 253.Zhou X, Fu L, Wang P, et al. Drug–herb interactions between Scutellaria baicalensis and pharmaceutical drugs: insights from experimental studies, mechanistic actions to clinical applications. Biomed Pharmacother 2021;138:111445. [DOI] [PubMed] [Google Scholar]
- 254.Tang D, Chen K, Huang L, et al. Pharmacokinetic properties and drug interactions of apigenin, a natural flavone. Exp Opin Drug Metab Toxicol 2017;13:323–330. [DOI] [PubMed] [Google Scholar]
- 255.Shahrajabian MH, Sun W, Cheng Q. Clinical aspects and health benefits of ginger (Zingiber officinale) in both traditional Chinese medicine and modern industry. Acta Agric Scand B Soil Plant Sci 2019;69:546–556. [Google Scholar]
- 256.Cosme P, Rodríguez AB, Espino J, et al. Plant phenolics: bioavailability as a key determinant of their potential health-promoting applications. Antioxidants 2020;9:1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Semwal RB, Semwal DK, Combrinck S, et al. Gingerols and shogaols: important nutraceutical principles from ginger. Phytochemistry 2015;117:554–568. [DOI] [PubMed] [Google Scholar]
- 258.Ali BH, Blunden G, Tanira MO, et al. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol 2008;46:409–420. [DOI] [PubMed] [Google Scholar]
- 259.Nafees S, Zafaryab M, Mehdi SH, et al. Anti-cancer effect of gingerol in cancer prevention and treatment. Anticancer Agents Med Chem 2021;21:428–432. [DOI] [PubMed] [Google Scholar]
- 260.Anbar EA, El-Naggar SA, Salama AF. The rationale of gingerol as a main phenolic compound of zingiber in improving hepatocellular carcinoma induced by dimethylaminoazobenzene: scientific implication. Egypt Acad J Biol Sci D Histol Histochem 2022;14:25–30. [Google Scholar]
- 261.White B. Ginger: an overview. Am Fam Physician 2007;75:1689–1691. [PubMed] [Google Scholar]
- 262.Sharifi-Rad M, Varoni EM, Salehi B, et al. Plants of the genus Zingiber as a source of bioactive phytochemicals: from tradition to pharmacy. Molecules 2017;22:2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer 2003;3:768–780. [DOI] [PubMed] [Google Scholar]
- 264.Park KK, Chun KS, Lee JM, et al. Inhibitory effects of [6]-gingerol, a major pungent principle of ginger, on phorbol ester-induced inflammation, epidermal ornithine decarboxylase activity and skin tumor promotion in ICR mice. Cancer Lett 1998;129:139–144. [DOI] [PubMed] [Google Scholar]
- 265.Nigam N, George J, Srivastava S, et al. Induction of apoptosis by [6]-gingerol associated with the modulation of p53 and involvement of mitochondrial signalling pathway in B[a]P-induced mouse skin tumorigenesis. Cancer Chemother Pharmacol 2010;65:687–696. [DOI] [PubMed] [Google Scholar]
- 266.Adikesavan M, Athiraja P, Divakar MBB. Investigation on the anticancer activity of [6]-gingerol of Zingiber officinale and its structural analogs against skin cancer. Curr Comput Aided Drug Des 2024;20:367–373. [DOI] [PubMed] [Google Scholar]
- 267.Abebe W. Review of herbal medications with the potential to cause bleeding: dental implications, and risk prediction and prevention avenues. EPMA J 2019;10:51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Alipour A, Baradaran Rahimi V, Askari VR. Promising influences of gingerols against metabolic syndrome: a mechanistic review. Biofactors 2022;48:993–1004. [DOI] [PubMed] [Google Scholar]
- 269.Tiran D. Ginger to reduce nausea and vomiting during pregnancy: evidence of effectiveness is not the same as proof of safety. Complement Ther Clin Pract 2012;18:22–25. [DOI] [PubMed] [Google Scholar]
- 270.Iqubal MK, Chaudhuri A, Iqubal A, et al. Targeted delivery of natural bioactives and lipid-nanocargos against signalling pathways involved in skin cancer. Curr Med Chem 2021;28:8003–8035. [DOI] [PubMed] [Google Scholar]
- 271.Lakhanpal P, Rai DK. Quercetin: a versatile flavonoid. Internet J Med Update 2007;2:22–37. [Google Scholar]
- 272.Slimestad R, Fossen T, Vågen IM. Onions: a source of unique dietary flavonoids. J Agric Food Chem 2007;55:10067–10080. [DOI] [PubMed] [Google Scholar]
- 273.Boyer J, Liu RH. Apple phytochemicals and their health benefits. Nutr J 2004;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Seeram NP. Berry fruits: compositional elements, biochemical activities, and the impact of their intake on human health, performance, and disease. J Agric Food Chem 2008;56:627–629. [DOI] [PubMed] [Google Scholar]
- 275.Franke AA, Custer LJ, Arakaki C, et al. Vitamin C and flavonoid levels of fruits and vegetables consumed in Hawaii. J Food Compos Anal 2004;17:1–35. [Google Scholar]
- 276.Jaganath IB, Crozier A. Dietary flavonoids and phenolic compounds. Plant Phenolics and Human Health: Biochemistry, Nutrition, and Pharmacology, 2010. pp. 1–49.
- 277.Ivana B, Marija L. Role of phenols in energy and functional beverages. Sports and Energy Drinks, 2019. pp. 229–268.
- 278.Lesjak M, Beara I, Simin N, et al. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J Funct Foods 2018;40:68–75. [Google Scholar]
- 279.Bądziul D, Jakubowicz-Gil J, Langner E, et al. The effect of quercetin and imperatorin on programmed cell death induction in T98G cells in vitro. Pharmacolog Rep 2014;66:292–300. [DOI] [PubMed] [Google Scholar]
- 280.Dhillon AS, Hagan S, Rath O, et al. MAP kinase signalling pathways in cancer. Oncogene 2007;26:3279–3290. [DOI] [PubMed] [Google Scholar]
- 281.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 2013;13:11–26. [DOI] [PubMed] [Google Scholar]
- 282.Li H, Chen C. Quercetin has antimetastatic effects on gastric cancer cells via the interruption of uPA/uPAR function by modulating NF-κb, PKC-δ, ERK1/2, and AMPKα. Integr Cancer Ther 2018;17:511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Imran M, Iqubal MK, Imtiyaz K, et al. Topical nanostructured lipid carrier gel of quercetin and resveratrol: formulation, optimization, in vitro and ex vivo study for the treatment of skin cancer. Int J Pharm 2020;587:119705. [DOI] [PubMed] [Google Scholar]
- 284.Caddeo C, Nacher A, Vassallo A, et al. Effect of quercetin and resveratrol co-incorporated in liposomes against inflammatory/oxidative response associated with skin cancer. Int J Pharm 2016;513:153–163. [DOI] [PubMed] [Google Scholar]
- 285.Paliwal S, Sundaram J, Mitragotri S. Induction of cancer-specific cytotoxicity towards human prostate and skin cells using quercetin and ultrasound. Br J Cancer 2005;92:499–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Jung M, Bu SY, Tak KH, et al. Anticarcinogenic effect of quercetin by inhibition of insulin-like growth factor (IGF)-1 signalling in mouse skin cancer. Nutr Res Pract 2013;7:439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.D’Andrea G. Quercetin: a flavonol with multifaceted therapeutic applications? Fitoterapia 2015;106:256–271. [DOI] [PubMed] [Google Scholar]
- 288.Di Bari L, Ripoli S, Pradhan S, et al. Interactions between quercetin and warfarin for albumin binding: a new eye on food/drug interference. Chirality 2010;22:593–596. [DOI] [PubMed] [Google Scholar]
- 289.Samuel T, Fadlalla K, Mosley L, et al. Dual-mode interaction between quercetin and DNA-damaging drugs in cancer cells. Anticancer Res 2012;32:61–71. [PMC free article] [PubMed] [Google Scholar]
- 290.Gupta A, Birhman K, Raheja I, et al. Quercetin: a wonder bioflavonoid with therapeutic potential in disease management. Asian Pac J Trop Dis 2016;6:248–252. [Google Scholar]
- 291.Simoes MF, Sousa JS, Pais AC. Skin cancer and new treatment perspectives: a review. Cancer Lett 2015;357:8–42. [DOI] [PubMed] [Google Scholar]
- 292.Montané X, Kowalczyk O, Reig-Vano B, et al. Current perspectives of the applications of polyphenols and flavonoids in cancer therapy. Molecules 2020;25:3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Liu Y, Cai X, Zhang Y, et al. Kaempferol alleviates renal fibrosis via NF-κB-mediated inhibition of epithelial-to-mesenchymal transition in vitro and in vivo. Food Fun 2020;11:2972–2985. [Google Scholar]
- 294.Xu W, Liu J, Li C, et al. Kaempferol-7-O-β-d-glucoside (KG) isolated from Smilax china L. rhizome induces G2/M phase arrest and apoptosis on HeLa cells in a p53-independent manner. Cancer Lett 2008;264:229–240. [DOI] [PubMed] [Google Scholar]
- 295.Lee HS, Cho HJ, Yu R, et al. Mechanisms underlying apoptosis-inducing effects of Kaempferol in HT-29 human colon cancer cells. Int J Mol Sci 2014;15:2722–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Nam SY, Jeong HJ, Kim HM. Kaempferol impedes IL-32-induced monocyte-macrophage differentiation. Chem Biol Interact 2017;274:107–115. [DOI] [PubMed] [Google Scholar]
- 297.Liao W, Chen L, Ma X, et al. Protective effects of kaempferol against reactive oxygen species-induced hemolysis and its antiproliferative activity on human cancer cells. Eur J Med Chem 2016;114:24–32. [DOI] [PubMed] [Google Scholar]
- 298.Farasat A, Ghorbani M, Gheibi N, et al. In silico assessment of the inhibitory effect of four flavonoids (Chrysin, Naringin, Quercetin, Kaempferol) on tyrosinase activity using the MD simulation approach. BioTechnologia J Biotechnol Comput Biol Bionanotechnol 2020;101:193–204. [Google Scholar]
- 299.Li C, Zhao Y, Yang D, et al. Inhibitory effects of kaempferol on the invasion of human breast carcinoma cells by downregulating the expression and activity of matrix metalloproteinase-9. Biochem Cell Biol 2015;93:16–27. [DOI] [PubMed] [Google Scholar]
- 300.Rajendran P, Rengarajan T, Nandakumar N, et al. Kaempferol, a potential cytostatic and cure for inflammatory disorders. Eur J Med Chem 2014;86:103–112. [DOI] [PubMed] [Google Scholar]
- 301.Yang J, Xiao P, Sun J, et al. Anticancer effects of kaempferol in A375 human malignant melanoma cells are mediated via induction of apoptosis, cell cycle arrest, inhibition of cell migration and downregulation of m-TOR/PI3K/AKT pathway. J BUON 2018;23:218–223. [PubMed] [Google Scholar]
- 302.Yao K, Chen H, Liu K, et al. Kaempferol targets RSK2 and MSK1 to suppress UV radiation-induced skin cancer. Cancer Prev Res (Phila) 2014;7:958–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Lee KM, Lee KW, Jung SK, et al. Kaempferol inhibits UVB-induced COX-2 expression by suppressing Src kinase activity. Biochem Pharmacol 2010;80:2042–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Kim SH, Choi KC. Anti-cancer effect and underlying mechanism(s) of kaempferol, a phytoestrogen, on the regulation of apoptosis in diverse cancer cell models. Toxicol Res 2013;29:229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Dabeek WM, Marra MV. Dietary quercetin and kaempferol: bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019;11:2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Lambert JD, Sang S, Yang CS. Possible controversy over dietary polyphenols: benefits vs risks. Chem Res Toxicol 2007;20:583–585. [DOI] [PubMed] [Google Scholar]
- 307.Hodek P, Trefil P, Stiborová M. Flavonoids-potent and versatile biologically active compounds interacting with cytochromes P450. Chem Biol Interact 2002;139:1–21. [DOI] [PubMed] [Google Scholar]
- 308.Igura K, Ohta T, Kuroda Y, et al. Resveratrol and quercetin inhibit angiogenesis in vitro. Cancer Lett 2001;171:11–16. [DOI] [PubMed] [Google Scholar]
- 309.Bouby L, Figueiral I, Bouchette A, et al. Bioarchaeological insights into the process of domestication of grapevine (Vitis vinifera L.) during Roman times in Southern France. PLoS One 2013;8:e63195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Moreno A, Castro M, Falqué E. Evolution of trans-and cis-resveratrol content in red grapes (Vitis vinifera L. cv Mencía, Albarello and Merenzao) during ripening. Eur Food Res Technol 2008;227:667–674. [Google Scholar]
- 311.Sales JM, Resurreccion AV. Resveratrol in peanuts. Crit Rev Food Sci Nutr 2014;54:734–770. [DOI] [PubMed] [Google Scholar]
- 312.Ector BJ, Magee JB, Hegwood CP, et al. Resveratrol concentration in muscadine berries, juice, pomace, purees, seeds, and wines. Am J Enol Vitic 1996;47:57–62. [Google Scholar]
- 313.Chen H, Tuck T, Ji X, et al. Quality assessment of Japanese knotweed (Fallopia japonica) grown on Prince Edward Island as a source of resveratrol. J Agric Food Chem 2013;61:6383–6392. [DOI] [PubMed] [Google Scholar]
- 314.Charles DJ. Sources of natural antioxidants and their activities. Antioxidant Properties of Spices, Herbs and Other Sources, 2013. pp. 65–138.
- 315.Jeandet P, Bessis R, Sbaghi M, et al. Production of the phytoalexin resveratrol by grapes as a response to Botrytis attack under natural conditions. J Phytopathol 1995;143:135–139. [Google Scholar]
- 316.Kuršvietienė L, Stanevičienė I, Mongirdienė A, et al. Multiplicity of effects and health benefits of resveratrol. Medicina 2016;52:148–155. [DOI] [PubMed] [Google Scholar]
- 317.Švajger U, Jeras M. Anti-inflammatory effects of resveratrol and its potential use in therapy of immune-mediated diseases. Int Rev Immunol 2012;31:202–222. [DOI] [PubMed] [Google Scholar]
- 318.Dybkowska E, Sadowska A, Swiderski F, et al. The occurrence of resveratrol in foodstuffs and its potential for supporting cancer prevention and treatment. A review. Rocz Państw Zakł Hig 2018;69:5–14. [PubMed] [Google Scholar]
- 319.Lee IT, Yang CM. Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem Pharmacol 2012;84:581–590. [DOI] [PubMed] [Google Scholar]
- 320.Kundu JK, Surh YJ. Inflammation: gearing the journey to cancer. Mutat Res 2008;659:15–30. [DOI] [PubMed] [Google Scholar]
- 321.Zhao H, Han L, Jian Y, et al. Resveratrol induces apoptosis in human melanoma cell through negatively regulating Erk/PKM2/Bcl-2 axis. Onco Targets Ther 2018;11:8995–9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Gali-Muhtasib H, Hmadi R, Kareh M, et al. Cell death mechanisms of plant-derived anticancer drugs: beyond apoptosis. Apoptosis 2015;20:1531–1562. [DOI] [PubMed] [Google Scholar]
- 323.Yan Y, Yang JY, Mou YH, et al. Differences in the activities of resveratrol and ascorbic acid in protection of ethanol-induced oxidative DNA damage in human peripheral lymphocytes. Food Chem Toxicol 2012;50:168–174. [DOI] [PubMed] [Google Scholar]
- 324.Pratheeshkumar P, Sreekala C, Zhang Z, et al. Cancer prevention with promising natural products: mechanisms of action and molecular targets. Anticancer Agents Med Chem 2012;12:1159–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Osmond GW, Augustine CK, Zipfel PA, et al. Enhancing melanoma treatment with resveratrol. J Surg Res 2012;172:109–115. [DOI] [PubMed] [Google Scholar]
- 326.Aziz SW, Aziz MH. Protective molecular mechanisms of resveratrol in UVR‐induced skin carcinogenesis. Photodermatol Photoimmunol Photomed 2018;34:35–41. [DOI] [PubMed] [Google Scholar]
- 327.Berman AY, Motechin RA, Wiesenfeld MY, et al. The therapeutic potential of resveratrol: a review of clinical trials. NPJ Precis Oncol 2017;1:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Kundu JK, Shin YK, Kim SH, et al. Resveratrol inhibits phorbol ester-induced expression of COX-2 and activation of NF-κB in mouse skin by blocking IκB kinase activity. Carcinogenesis 2006;27:1465–1474. [DOI] [PubMed] [Google Scholar]
- 329.Reagan-Shaw S, Afaq F, Aziz MH, et al. Modulations of critical cell cycle regulatory events during chemoprevention of ultraviolet B-mediated responses by resveratrol in SKH-1 hairless mouse skin. Oncogene 2004;23:5151–5160. [DOI] [PubMed] [Google Scholar]
- 330.Wu Z, Uchi H, Morino-Koga S, et al. Resveratrol inhibition of human keratinocyte proliferation via SIRT1/ARNT/ERK dependent downregulation of aquaporin 3. J Dermatolog Sci 2014;75:16–23. [DOI] [PubMed] [Google Scholar]
- 331.Chiba T, Kimura Y, Suzuki S, et al. Trans-resveratrol enhances the anticoagulant activity of warfarin in a mouse model. J Atheroscler Thromb 2016;23:1099–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Gescher A. Polyphenolic phytochemicals versus non-steroidal anti-inflammatory drugs: which are better cancer chemopreventive agents? J Chemother 2004;16:3–6. [DOI] [PubMed] [Google Scholar]
- 333.Lu C, Lin HZ, Li YY, et al. Making room for specific molecules in the treatment of depression. Sci J Depress Anxiety 2017;1:20–22. [Google Scholar]
- 334.Jiang Z, Chen K, Cheng L, et al. Resveratrol and cancer treatment: updates. Ann N Y Acad Sci 2017;1403:59–69. [DOI] [PubMed] [Google Scholar]
- 335.Ren B, Kwah MX-Y, Liu C, et al. Resveratrol for cancer therapy: challenges and future perspectives. Cancer Lett 2021;515:63–72. [DOI] [PubMed] [Google Scholar]
- 336.Chedea VS, Vicaş SI, Sticozzi C, et al. Resveratrol: from diet to topical usage. Food Funct 2017;8:3879–3892. [DOI] [PubMed] [Google Scholar]
- 337.Fibigr J, Šatínský D, Solich P. Current trends in the analysis and quality control of food supplements based on plant extracts. Anal Chim Acta 2018;1036:1–15. [DOI] [PubMed] [Google Scholar]
- 338.Akram M, Shahab-Uddin AA, Usmanghani K, et al. Curcuma longa and curcumin: a review article. Rom J Biol Plant Biol 2010;55:65–70. [Google Scholar]
- 339.Debjit Bhowmik C, Kumar KS, Chandira M, et al. Turmeric: a herbal and traditional medicine. Arch Appl Sci Res 2009;1:86–108. [Google Scholar]
- 340.Abd El‐Hack ME, El‐Saadony MT, Swelum AA, et al. Curcumin, the active substance of turmeric: its effects on health and ways to improve its bioavailability. J Sci Food Agric 2021;101:5747–5762. [DOI] [PubMed] [Google Scholar]
- 341.Ak T, Gülçin I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact 2008;174:27–37. [DOI] [PubMed] [Google Scholar]
- 342.Menon VP and Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. In: Aggarwal BB, Surh Y-J, Shishodia S, eds. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease. Springer; 2007. pp. 105–25. [DOI] [PubMed]
- 343.Perrone D, Ardito F, Giannatempo G, et al. Biological and therapeutic activities, and anticancer properties of curcumin. Exp Ther Med 2015;10:1615–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Jobin C, Bradham CA, Russo MP, et al. Curcumin blocks cytokine-mediated NF-κB activation and proinflammatory gene expression by inhibiting inhibitory factor I-κB kinase activity. J Immunol 1999;163:3474–3483. [PubMed] [Google Scholar]
- 345.Reuter S, Eifes S, Dicato M, et al. Modulation of anti-apoptotic and survival pathways by curcumin as a strategy to induce apoptosis in cancer cells. Biochem Pharmacol 2008;76:1340–1351. [DOI] [PubMed] [Google Scholar]
- 346.Zhao G, Han X, Zheng S, et al. Curcumin induces autophagy, inhibits proliferation and invasion by downregulating AKT/mTOR signalling pathway in human melanoma cells. Oncol Rep 2016;35:1065–1074. [DOI] [PubMed] [Google Scholar]
- 347.Wu J, Lu WY, Cui LL. Inhibitory effect of curcumin on invasion of skin squamous cell carcinoma A431 cells. Asian Pac J Cancer Prev 2015;16:2813–2818. [DOI] [PubMed] [Google Scholar]
- 348.Kunnumakkara AB, Diagaradjane P, Anand P, et al. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin Cancer Res 2008;14:2128–2136. [DOI] [PubMed] [Google Scholar]
- 349.Rein MJ, Renouf M, Cruz‐Hernandez C, et al. Bioavailability of bioactive food compounds: a challenging journey to bioefficacy. Br J Clin Pharmacol 2013;75:588–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Wang Z, Sun W, Huang CK, et al. Inhibitory effects of curcumin on activity of cytochrome P450 2C9 enzyme in human and 2C11 in rat liver microsomes. Drug Dev Ind Pharm 2015;41:613–616. [DOI] [PubMed] [Google Scholar]
- 351.Hu C, Kitts DD. Evaluation of antioxidant activity of epigallocatechin gallate in biphasic model systems in vitro. Mol Cell Biochem 2001;218:147–155. [DOI] [PubMed] [Google Scholar]
- 352.Zhong Y, Chiou YS, Pan MH, et al. Anti-inflammatory activity of lipophilic epigallocatechin gallate (EGCG) derivatives in LPS-stimulated murine macrophages. Food Chem 2012;134:742–748. [DOI] [PubMed] [Google Scholar]
- 353.Namita P, Mukesh R, Vijay KJ. Camellia sinensis (green tea): a review. Glob J Pharmacol 2012;6:52–59. [Google Scholar]
- 354.Łuczaj W, Skrzydlewska E. Antioxidative properties of black tea. Prev Med 2005;40:910–918. [DOI] [PubMed] [Google Scholar]
- 355.Pastoriza S, Mesías M, Cabrera C, et al. Healthy properties of green and white teas: an update. Food Funct 2017;8:2650–2662. [DOI] [PubMed] [Google Scholar]
- 356.Lee RJ, Lee VS, Tzen JT, et al. Study of the release of gallic acid from (–)‐epigallocatechin gallate in old oolong tea by mass spectrometry. Rapid Commun Mass Spectrom 2010;24:851–858. [DOI] [PubMed] [Google Scholar]
- 357.Ferrari E, Bettuzzi S, Naponelli V. The potential of epigallocatechin gallate (EGCG) in targeting autophagy for cancer treatment: a narrative review. Int J Mol Sci 2022;23:6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Eng QY, Thanikachalam PV, Ramamurthy S. Molecular understanding of epigallocatechin gallate (EGCG) in cardiovascular and metabolic diseases. J Ethnopharmacol 2018;210:296–310. [DOI] [PubMed] [Google Scholar]
- 359.Wightman EL, Haskell CF, Forster JS, et al. Epigallocatechin gallate, cerebral blood flow parameters, cognitive performance and mood in healthy humans: a double‐blind, placebo‐controlled, crossover investigation. Hum Psychopharmacol 2012;27:177–186. [DOI] [PubMed] [Google Scholar]
- 360.Oz HS. Chronic inflammatory diseases and green tea polyphenols. Nutrients 2017;9:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.H Farzaei M, Rahimi R, Abdollahi M. The role of dietary polyphenols in the management of inflammatory bowel disease. Curr Pharm Biotechnol 2015;16:196–210. [DOI] [PubMed] [Google Scholar]
- 362.Liu Q, Qian Y, Chen F, et al. EGCG attenuates pro-inflammatory cytokines and chemokines production in LPS-stimulated L02 hepatocyte. Acta Biochim Biophys Sin (Shanghai) 2014;46:31–39. [DOI] [PubMed] [Google Scholar]
- 363.Gan R-Y, Li H-B, Sui Z-Q, et al. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): an updated review. Crit Rev Food Sci Nutr 2018;58:924–941. [DOI] [PubMed] [Google Scholar]
- 364.El-Kayal M, Nasr M, Elkheshen S, et al. Colloidal (–)-epigallocatechin-3-gallate vesicular systems for prevention and treatment of skin cancer: a comprehensive experimental study with preclinical investigation. Eur J Pharm Sci 2019;137:104972. [DOI] [PubMed] [Google Scholar]
- 365.Zhang J, Lei Z, Huang Z, et al. Epigallocatechin-3-gallate (EGCG) suppresses melanoma cell growth and metastasis by targeting TRAF6 activity. Oncotarget 2016;7:79557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Ellis LZ, Liu W, Luo Y, et al. Green tea polyphenol epigallocatechin-3-gallate suppresses melanoma growth by inhibiting inflammasome and IL-1β secretion. Biochem Biophys Res Commun 2011;414:551–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Lambert JD, Kennett MJ, Sang S, et al. Hepatotoxicity of high oral dose (–)-epigallocatechin-3-gallate in mice. Food Chem Toxicol 2010;48:409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Yang CS, Wang X, Lu G, et al. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer 2009;9:429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Andreu Fernandez V, Almeida Toledano L, Pizarro Lozano N, et al. Bioavailability of epigallocatechin gallate administered with different nutritional strategies in healthy volunteers. Antioxidants 2020;9:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Harati K, Behr B, Wallner C, et al. Anti‑proliferative activity of epigallocatechin‑3‑gallate and silibinin on soft tissue sarcoma cells. Mol Med Rep 2017;15:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Zhang Y, Owusu L, Duan W, et al. Anti-metastatic and differential effects on protein expression of epigallocatechin-3-gallate in HCCLM6 hepatocellular carcinoma cells. Int J Mol Med 2013;32:959–964. [DOI] [PubMed] [Google Scholar]
- 372.Guzman I, Hamby S, Romero J, et al. Variability of carotenoid biosynthesis in orange colored Capsicum spp. Plant Sci 2010;179:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Olson JA. Provitamin A function of carotenoids: the conversion of β-carotene into vitamin A. J Nutr 1989;119:105–108. [DOI] [PubMed] [Google Scholar]
- 374.Johnson EJ. The role of carotenoids in human health. Nutr Clin Care 2002;5:56–65. [DOI] [PubMed] [Google Scholar]
- 375.Gul K, Tak A, Singh AK, et al. Chemistry, encapsulation, and health benefits of β-carotene - a review. Cogent Food Agric 2015;1:1018696. [Google Scholar]
- 376.Wood SM, Beckham C, Yosioka A, et al. β-Carotene and selenium supplementation enhances immune response in aged humans. Integr Med 2000;2:85–92. [DOI] [PubMed] [Google Scholar]
- 377.Pritwani R, Mathur P. β-carotene content of some commonly consumed vegetables and fruits available in Delhi, India. J Nutr Food Sci 2017;7:1–7. [Google Scholar]
- 378.Devasagayam TPA, Tilak JC, Boloor KK, et al. Free radicals and antioxidants in human health: current status and future prospects. J Assoc Physicians India 2004;52:794–804. [PubMed] [Google Scholar]
- 379.Godic A, Poljšak B, Adamic M, et al. The role of antioxidants in skin cancer prevention and treatment. Oxid Med Cell Longev 2014;2014:860479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Greenberg ER, Baron JA, Stukel TA, et al. A clinical trial of beta carotene to prevent basal-cell and squamous-cell cancers of the skin. N Engl J Med 1990;323:789–795. [DOI] [PubMed] [Google Scholar]
- 381.Kune GA, Bannerman S, Field B, et al. Diet, alcohol, smoking, serum β‐carotene, and vitamin A in male nonmelanocytic skin cancer patients and controls. Nutr Cancer 1992;18:237–44. [DOI] [PubMed] [Google Scholar]
- 382.Black HS, Boehm F, Edge R, et al. The benefits and risks of certain dietary carotenoids that exhibit both anti- and pro-oxidative mechanisms - a comprehensive review. Antioxidants (Basel) 2020;9:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Stahl W, Sies H. β-Carotene and other carotenoids in protection from sunlight. Am J Clin Nutr 2012;96:1179S–1184S. [DOI] [PubMed] [Google Scholar]
- 384.Seifried HE, McDonald SS, Anderson DE, et al. The antioxidant conundrum in cancer. Cancer Res 2003;63:4295–4298. [PubMed] [Google Scholar]
- 385.Tsai J, Chien AL. Photoprotection for skin of color. Am J Clin Dermatol 2022;23:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Alam MA, Subhan N, Hossain H, et al. Hydroxycinnamic acid derivatives: a potential class of natural compounds for the management of lipid metabolism and obesity. Nutr Metab 2016;13:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Crozier A Yokota T Jaganath IB, et al. Secondary metabolites in fruits, vegetables, beverages and other plant based dietary components. In: Crozier A, Clifford MN, Ashihara H, eds. Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet. Blackwell Publishing Ltd; 2006. pp. 208–302.
- 388.Gülçin İ. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006;217:213–220. [DOI] [PubMed] [Google Scholar]
- 389.Yang WS, Jeong D, Yi YS, et al. IRAK1/4-targeted anti-inflammatory action of caffeic acid. Mediators Inflamm 2013;2013:518183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Guo D, Dou D, Ge L, et al. A caffeic acid mediated facile synthesis of silver nanoparticles with powerful anti-cancer activity. Colloids Surf B Biointerfaces 2015;134:229–234. [DOI] [PubMed] [Google Scholar]
- 391.Mirzaei S, Gholami MH, Zabolian A, et al. Caffeic acid and its derivatives as potential modulators of oncogenic molecular pathways: new hope in the fight against cancer. Pharmacolog Res 2021;171:105759. [DOI] [PubMed] [Google Scholar]
- 392.Kabała-Dzik A, Rzepecka-Stojko A, Kubina R, et al. Comparison of two components of propolis: caffeic acid (CA) and caffeic acid phenethyl ester (CAPE) induce apoptosis and cell cycle arrest of breast cancer cells MDA-MB-231. Molecules 2017;22:1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Dziedzic A, Kubina R, Kabała-Dzik A, et al. Induction of cell cycle arrest and apoptotic response of head and neck squamous carcinoma cells (Detroit 562) by caffeic acid and caffeic acid phenethyl ester derivative. Evid Based Complement Alternat Med 2017;2017:6793456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Rangwala S, Tsai KY. Roles of the immune system in skin cancer. Br J Dermatol 2011;165:953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Hofbauer GF, Bavinck JNB, Euvrard S. Organ transplantation and skin cancer: basic problems and new perspectives. Exp Dermatol 2010;19:473–482. [DOI] [PubMed] [Google Scholar]
- 396.Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg 2006;391:499–510. [DOI] [PubMed] [Google Scholar]
- 397.Zhou Y, Jiang Z, Lu H, et al. Recent advances of natural polyphenols activators for Keap1‐Nrf2 signalling pathway. Chem Biodivers 2019;16:e1900400. [DOI] [PubMed] [Google Scholar]
- 398.Scapagnini G, Sonya V, Nader AG, et al. Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol Neurobiol 2011;44:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Magesh S, Chen Y, Hu L. Small molecule modulators of Keap1‐Nrf2‐ARE pathway as potential preventive and therapeutic agents. Med Res Rev 2012;32:687–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Yang G, Fu Y, Malakhova M, et al. Caffeic acid directly targets ERK1/2 to attenuate solar UV-induced skin carcinogenesis. Cancer Prev Res (Phila) 2014;7:1056–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Kudugunti SK, Vad NM, Ekogbo E, et al. Efficacy of caffeic acid phenethyl ester (CAPE) in skin B16-F0 melanoma tumor bearing C57BL/6 mice. Invest New Drugs 2011;29:52–62. [DOI] [PubMed] [Google Scholar]
- 402.Balupillai A, Prasad RN, Ramasamy K, et al. Caffeic acid inhibits UVB‐induced inflammation and photocarcinogenesis through activation of peroxisome proliferator‐activated receptor‐γ in mouse skin. Photochem Photobiol 2015;91:1458–1468. [DOI] [PubMed] [Google Scholar]
- 403.Balupillai A, Nagarajan RP, Ramasamy K, et al. Caffeic acid prevents UVB radiation induced photocarcinogenesis through regulation of PTEN signalling in human dermal fibroblasts and mouse skin. Toxicol Appl Pharmacol 2018;352:87–96. [DOI] [PubMed] [Google Scholar]
- 404.Agilan B, Rajendra Prasad N, Kanimozhi G, et al. Caffeic acid inhibits chronic UVB‐induced cellular proliferation through JAK‐STAT 3 signalling in mouse skin. Photochem Photobiol 2016;92:467–474. [DOI] [PubMed] [Google Scholar]
- 405.Mileo AM, Miccadei S. Polyphenols as modulator of oxidative stress in cancer disease: new therapeutic strategies. Oxid Med Cell Longev 2016;2016:6475624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Wang SJ, Zeng J, Yang BK, et al. Bioavailability of caffeic acid in rats and its absorption properties in the Caco-2 cell model. Pharm Biol 2014;52:1150–1157. [DOI] [PubMed] [Google Scholar]
- 407.D’Archivio M, Filesi C, Di Benedetto R, et al. Polyphenols, dietary sources and bioavailability. Ann Ist Super Sanità 2007;43:348–361. [PubMed] [Google Scholar]
- 408.Setty AR, Sigal LH. Herbal medications commonly used in the practice of rheumatology: mechanisms of action, efficacy, and side effects. Semin Arthr Rheum 2005;34:773–784. [DOI] [PubMed] [Google Scholar]
- 409.Boz H. Ferulic acid in cereals - a review. Czech J Food Sci 2015;33:1–7. [Google Scholar]
- 410.Kähkönen MP, Hopia AI, Vuorela HJ, et al. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem 1999;47:3954–3962. [DOI] [PubMed] [Google Scholar]
- 411.Kumar N, Goel N. Phenolic acids: natural versatile molecules with promising therapeutic applications. Biotechnol Rep 2019;24:e00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem 2001;49:5165–5170. [DOI] [PubMed] [Google Scholar]
- 413.Peterson J, Dwyer J, Adlercreutz H, et al. Dietary lignans: physiology and potential for cardiovascular disease risk reduction. Nutr Rev 2010;68:571–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Özcan MM, Chalchat JC. Chemical composition and antifungal activity of rosemary (Rosmarinus officinalis L.) oil from Turkey. Int J Food Sci Nutr 2008;59:691–698. [DOI] [PubMed] [Google Scholar]
- 415.Rose DJ, Patterson JA, Hamaker BR. Structural differences among alkali-soluble arabinoxylans from maize (Zea mays), rice (Oryza sativa), and wheat (Triticum aestivum) brans influence human fecal fermentation profiles. J Agric Food Chem 2010;58:493–499. [DOI] [PubMed] [Google Scholar]
- 416.Srinivasan M, Sudheer AR, Menon VP. Ferulic acid: therapeutic potential through its antioxidant property. J Clin Biochem Nutr 2007;40:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Lin XF, Min W, Luo D. Anticarcinogenic effect of ferulic acid on ultraviolet-B irradiated human keratinocyte HaCaT cells. J Med Plants Res 2010;4:1686–1694. [Google Scholar]
- 418.Rajendran I, Ponrasu T, Rajaram R, et al. The apoptotic effect of ferulic acid-synthesized gold nanoparticles against human epidermoid carcinoma (A431) cells via activation of caspase-3 pathway. J Drug Deliv Sci Technol 2021;63:102478. [Google Scholar]
- 419.Khalid EB, Ayman EMEK, Rahman H, et al. Natural products against cancer angiogenesis. Tumor Biol 2016;37:14513–14536. [DOI] [PubMed] [Google Scholar]
- 420.Alias LM, Manoharan S, Vellaichamy L, et al. Protective effect of ferulic acid on 7,12-dimethylbenz[a]anthracene-induced skin carcinogenesis in Swiss albino mice. Exp Toxicolog Pathol 2009;61:205–214. [DOI] [PubMed] [Google Scholar]
- 421.Choi YS, Sohn YW. Anticancer effect of ferulic acid on cultured human skin melanoma cells. J Exp Biomed Sci 2006;12:457–461. [Google Scholar]
- 422.Murakami A, Nakamura Y, Koshimizu K, et al. FA15, a hydrophobic derivative of ferulic acid, suppresses inflammatory responses and skin tumor promotion: comparison with ferulic acid. Cancer Lett 2002;180:21–129. [DOI] [PubMed] [Google Scholar]
- 423.Diaconeasa Z, Stirbu I, Xiao J, et al. Anthocyanins, vibrant color pigments, and their role in skin cancer prevention. Biomedicines 2020;8:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Korać RR, Khambholja KM. Potential of herbs in skin protection from ultraviolet radiation. Pharmacogn Rev 2011;5:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Hamid AA, Aiyelaagbe OO, Usman LA, et al. Antioxidants: its medicinal and pharmacological applications. Afr J Pure Appl Chem 2010;4:142–151. [Google Scholar]
- 426.Offord EA, Gautier JC, Avanti O, et al. Photoprotective potential of lycopene, β-carotene, vitamin E, vitamin C and carnosic acid in UVA-irradiated human skin fibroblasts. Free Radic Biol Med 2002;32:1293–1303. [DOI] [PubMed] [Google Scholar]
- 427.Pullar JM, Carr AC, Vissers MC. The roles of vitamin C in skin health. Nutrients 2017;9:866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Grune T, Lietz G, Palou A, et al. β-Carotene is an important vitamin A source for humans. J Nutr 2010;140:2268S–2285S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Amao I. Health benefits of fruits and vegetables: review from Sub-Saharan Africa. In: Asaduzzaman M, Asao T, eds. Vegetables: Importance of Quality Vegetables to Human Health. Springer Nature; 2018. pp. 33–53.
- 430.Bulló M, Casas-Agustench P, Amigó-Correig P, et al. Inflammation, obesity and comorbidities: the role of diet. Public Health Nutr 2007;10(10A):1164–1172. [DOI] [PubMed] [Google Scholar]
- 431.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Rolls BJ, Drewnowski A, Ledikwe JH. Changing the energy density of the diet as a strategy for weight management. J Am Diet Assoc 2005;105:98–103. [DOI] [PubMed] [Google Scholar]
- 433.Karimi K, Lindgren TH, Koch CA, et al. Obesity as a risk factor for malignant melanoma and non-melanoma skin cancer. Rev Endocr Metab Dis 2016;17:389–403. [DOI] [PubMed] [Google Scholar]
- 434.Williams Merten J, King JL, Walsh-Childers K, et al. Skin cancer risk and other health risk behaviors: a scoping review.. Am J Lifestyle Med 2016;11:182–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Iqbal J, Abbasi BA, Ahmad R, et al. Potential phytochemicals in the fight against skin cancer: current landscape and future perspectives. Biomed Pharmacother 2019;109:1381–1393. [DOI] [PubMed] [Google Scholar]
- 436.Chamcheu JC, Roy T, Uddin MB, et al. Role and therapeutic targeting of the PI3K/Akt/mTOR signalling pathway in skin cancer: a review of current status and future trends on natural and synthetic agents therapy. Cells 2019;8:803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Athar M, Tang X, Lee JL, et al. Hedgehog signalling in skin development and cancer. Exp Dermatol 2006;15:667–677. [DOI] [PubMed] [Google Scholar]
- 438.George BP, Chandran R, Abrahamse H. Role of phytochemicals in cancer chemoprevention: insights. Antioxidants 2021;10:1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Cháirez-Ramírez MH, de la Cruz-López KG, García-Carrancá A. Polyphenols as antitumor agents targeting key players in cancer-driving signalling pathways. Front Pharmacol 2021;12:710304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Pandey P, Khan F. A mechanistic review of the anticancer potential of hesperidin, a natural flavonoid from citrus fruits. Nutr Res 2021;92:21–31. [DOI] [PubMed] [Google Scholar]
- 441.Choi J, Lee DH, Jang H, et al. Naringenin exerts anticancer effects by inducing tumor cell death and inhibiting angiogenesis in malignant melanoma. Int J Med Sci 2020;17:3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Mintie CA, Singh CK, Ahmad N. Whole fruit phytochemicals combating skin damage and carcinogenesis. Transl Oncol 2020;13:146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Neagu M, Constantin C, Caruntu C, et al. Inflammation: a key process in skin tumorigenesis. Oncol Lett 2019;17:4068–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Li Y, Yao J, Han C, et al. Quercetin, inflammation and immunity. Nutrients 2016;8:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Al Robaee AA. Awareness to sun exposure and use of sunscreen by the general population. Bosn J Basic Med Sci 2010;10:314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Chadni M, Isidore E, Lagalle F, et al. Optimization of the supercritical extraction of rosmarinic acid from clary sage residue and the antioxidant activity of the extracts. J Supercrit Fluids 2023;193:105830. [Google Scholar]
- 447.Erkan N, Ayranci G, Ayranci E. Antioxidant activities of rosemary (Rosmarinus Officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem 2008;110:76–82. [DOI] [PubMed] [Google Scholar]
- 448.Wang H, Provan GJ, Helliwell K. Determination of rosmarinic acid and caffeic acid in aromatic herbs by HPLC. Food Chem 2004;87:307–311. [Google Scholar]
- 449.Kiferle C, Lucchesini M, Mensuali-Sodi A, et al. Rosmarinic acid content in basil plants grown in vitro and in hydroponics. Open Life Sci 2011;6:946–957. [Google Scholar]
- 450.Perry PL, Shetty K. A model for involvement of proline during pseudomonas‐mediated stimulation of rosmarinic acid levels in oregano shoot clones. Food Biotechnol 1999;13:137–154. [Google Scholar]
- 451.Ashoush YAM, Ali AMF, Abozid MM, et al. Comparative study between celery leaves and broccoli flowers for their chemical composition and amino acids as well as phenolic and flavonoid compounds. Menoufia Jf Agric Biotechnol 2017;2:1–13. [Google Scholar]
- 452.Gomes PG, Veloso AF, Maynard IF, et al. Integrative process to extract chlorophyll and purify rosmarinic acid from rosemary leaves (Rosmarinus officialis). J Chem Technol Biotechnol 2020;95:1503–1510. [Google Scholar]
- 453.Carnat AP, Carnat A, Fraisse D, et al. The aromatic and polyphenolic composition of lemon balm (Melissa officinalis L. subsp. officinalis) tea. Pharm Acta Helv 1998;72:301–305. [Google Scholar]
- 454.David IG, Buleandră M, Popa DE, et al. Voltammetric determination of polyphenolic content as rosmarinic acid equivalent in tea samples using pencil graphite electrodes. J Food Sci Technol 2016;53:2589–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Andrade P, Ferreres F, Gil MI, et al. Determination of phenolic compounds in honeys with different floral origin by capillary zone electrophoresis. Food Chem 1997;60:79–84. [Google Scholar]
- 456.Mandal SK, Das A, Dey S, et al. Bioactivities of allicin and related organosulfur compounds from garlic: overview of the literature since 2010. Egypt J Chem 62 (Special Issue - Part 1 Innovat Chem) 2019;62:1–11. [Google Scholar]
- 457.Prati P, Henrique CM, Souza ASD, et al. Evaluation of allicin stability in processed garlic of different cultivars. Food Sci Technol 2014;34:623–628. [Google Scholar]
- 458.Yin MC, Cheng WS. Antioxidant activity of several Allium members. J Agric Food Chem 1998;46:4097–4101. [Google Scholar]
- 459.Melouk SA, Hassan MA, Elwan MWM, et al. Horticultural, chemical and genetic diversity using SSR markers in Leek germplasm collection. Sci Hortic 2023;311:111782. [Google Scholar]
- 460.Ghodrati Azadi H, Ghaffari SM, Riazi GH, et al. Antiproliferative activity of chloroformic extract of Persian Shallot, Allium hirtifolium, on tumor cell lines. Cytotechnology 2008;56:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Gu C, Ma H, Tuly JA, et al. Effects of catalytic infrared drying in combination with hot air drying and freeze drying on the drying characteristics and product quality of chives. LWT 2022;161:113363. [Google Scholar]
- 462.de Figueiredo SM, Binda NS, Nogueira-Machado JA, et al. The antioxidant properties of organosulfur compounds (sulforaphane). Recent Pat Endocr Metab Immune Drug Discov 2015;9:24–39. [DOI] [PubMed] [Google Scholar]
- 463.Moon JK, Kim JR, Ahn YJ, et al. Analysis and anti-Helicobacter activity of sulforaphane and related compounds present in broccoli (Brassica oleracea L.) sprouts. J Agric Food Chem 2010;58:6672–6677. [DOI] [PubMed] [Google Scholar]
- 464.Čekey N, Šlosár M, Uher A, et al. The effect of nitrogen and sulphur fertilization on the yield and content of sulforaphane and nitrates in cauliflower. Acta Univ Agric Silvic Mendel Brun 2011;59:17–22. [Google Scholar]
- 465.Bryant CS, Kumar S, Chamala S, et al. Sulforaphane induces cell cycle arrest by protecting RB-E2F-1 complex in epithelial ovarian cancer cells. Mol Cancer 2010;9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Sasaki K, Neyazaki M, Shindo K, et al. Quantitative profiling of glucosinolates by LC–MS analysis reveals several cultivars of cabbage and kale as promising sources of sulforaphane. J Chromatogr B Analyt Technol Biomed Life Sci 2012;903:171–176. [DOI] [PubMed] [Google Scholar]
- 467.Kim J. Pre-clinical neuroprotective evidences and plausible mechanisms of sulforaphane in Alzheimer’s disease. Int J Mol Sci 2021;22:2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Herr I, Lozanovski V, Houben P, et al. Sulforaphane and related mustard oils in focus of cancer prevention and therapy. Wien Med Wochenschr 2013;163:80–88. [DOI] [PubMed] [Google Scholar]
- 469.Dosz EB, Jeffery EH. Modifying the processing and handling of frozen broccoli for increased sulforaphane formation. J Food Sci 2013;78:H1459–H1463. [DOI] [PubMed] [Google Scholar]
- 470.Belyakova T Morozova O Antonceva E, et al. Perspective processing of turnip root (Brassica rapa) as a source of sulforafan. In: E3S Web of Conferences, Vol. 164. EDP Sciences; 2020. 06028. 6pp.
- 471.Rosillo MA, Sanchez-Hidalgo M, Cárdeno A, et al. Protective effect of ellagic acid, a natural polyphenolic compound, in a murine model of Crohn’s disease. Biochem Pharmacol 2011;82:737–745. [DOI] [PubMed] [Google Scholar]
- 472.Zafrilla P, Ferreres F, Tomás-Barberán FA. Effect of processing and storage on the antioxidant ellagic acid derivatives and flavonoids of red raspberry (Rubus idaeus) jams. J Agric Food Chem 2001;49:3651–3655. [DOI] [PubMed] [Google Scholar]
- 473.Đurić M, Maškovic P, Murtić S, et al. Quantitation of ellagic acid in blackberries. Hem Ind 2014;68:241–245. [Google Scholar]
- 474.Usta C, Ozdemir S, Schiariti M, et al. The pharmacological use of ellagic acid-rich pomegranate fruit. Int J Food Sci Nutr 2013;64:907–913. [DOI] [PubMed] [Google Scholar]
- 475.Vattem DA, Shetty K. Ellagic acid production and phenolic antioxidant activity in cranberry pomace (Vaccinium macrocarpon) mediated by Lentinus edodes using a solid-state system. Process Biochem 2003;39:367–379. [Google Scholar]
- 476.Flores-Cordova M, Muñoz-Márquez E, Muñoz-Márquez E, et al. Phytochemical composition and antioxidant capacity in Mexican pecan nut. Emir J Food Agric 2017;29:346–350. [Google Scholar]
- 477.Han DH, Lee MJ, Kim JH. Antioxidant and apoptosis-inducing activities of ellagic acid. Anticancer Res 2006;26(5A):3601–3606. [PubMed] [Google Scholar]
- 478.Weisburg JH, Schuck AG, Reiss SE, et al. Ellagic acid, a dietary polyphenol, selectively cytotoxic to HSC-2 oral carcinoma cells. Anticancer Res 2013;33:1829–1836. [PubMed] [Google Scholar]
- 479.de Melo CL, Queiroz MGR, Arruda Filho ACV, et al. Betulinic acid, a natural pentacyclic triterpenoid, prevents abdominal fat accumulation in mice fed a high-fat diet. J Agric Food Chem 2009;57:8776–8781. [DOI] [PubMed] [Google Scholar]
- 480.Alhazmi H. Extraction of phytochemicals betulin and betulinic acid from the chaga mushroom and their effect on MCF-7 cells [Doctoral dissertation], 2018.
- 481.Hossain U, Das AK, Ghosh S, et al. An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem Toxicol 2020;145:111738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Liu J, Chen P, Yao W, et al. Subcritical water extraction of betulinic acid from birch bark. Ind Crops Prod 2015;74:557–565. [Google Scholar]
- 483.Madunić J, Madunić IV, Gajski G, et al. Apigenin: a dietary flavonoid with diverse anticancer properties. Cancer Lett 2018;413:11–22. [DOI] [PubMed] [Google Scholar]
- 484.Poureini F, Najafpour GD, Nikzad M, et al. Loading of apigenin extracted from parsley leaves on colloidal core-shell nanocomposite for bioavailability enhancement. Colloids Surf A Physicochem Eng Aspects 2021;625:126867. [Google Scholar]
- 485.Srivastava JK, Shankar E, Gupta S. Chamomile: a herbal medicine of the past with bright future. Mol Med Rep 2010;3:895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Kruma Z, Andjelkovic M, Verhe R, et al. Phenolic compounds in basil, oregano and thyme. Foodbalt 2008;5:99–103. [Google Scholar]
- 487.Oyenihi OR, Oyenihi AB, Alabi TD, et al. Reactive oxygen species: key players in the anticancer effects of apigenin? J Food Biochem 2022;46:e14060. [DOI] [PubMed] [Google Scholar]
- 488.Namsi A, Nury T, Hamdouni H, et al. Induction of neuronal differentiation of murine N2a cells by two polyphenols present in the Mediterranean diet mimicking neurotrophins activities: resveratrol and apigenin. Diseases 2018;6:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: progress, potential and promise. Int J Oncol 2007;30:233–245. [PubMed] [Google Scholar]
- 490.Pandino G, Courts FL, Lombardo S, et al. Caffeoylquinic acids and flavonoids in the immature inflorescence of globe artichoke, wild cardoon, and cultivated cardoon. J Agric Food Chem 2010;58:1026–1031. [DOI] [PubMed] [Google Scholar]
- 491.Pająk P, Socha R, Gałkowska D, et al. Phenolic profile and antioxidant activity in selected seeds and sprouts. Food Chem 2014;143:300–306. [DOI] [PubMed] [Google Scholar]
- 492.Meckelmann SW, Riegel DW, Van Zonneveld MJ, et al. Compositional characterization of native Peruvian chili peppers (Capsicum spp.). J Agric Food Chem 2013;61:2530–2537. [DOI] [PubMed] [Google Scholar]
- 493.Rowell KM, Beisel CG. Isolation of gram quantities of a rhamnoglucoside of apigenin from grapefruit. J Food Sci 1963;28:195–197. [Google Scholar]
- 494.Szczepaniak O, Ligaj M, Stuper-Szablewska K, et al. Genoprotective effect of cornelian cherry (Cornus mas L.) phytochemicals, electrochemical and ab initio interaction study. Biomed Pharmacother 2022;152:113216. [DOI] [PubMed] [Google Scholar]
- 495.McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phytother Res 2006;20:519–530. [DOI] [PubMed] [Google Scholar]
- 496.Pais JM, Pereira B, Paz FAA, et al. Solid γ-cyclodextrin inclusion compound with gingerols, a multi-component guest: preparation, properties and application in yogurt. Biomolecules 2020;10:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Schwertner HA, Rios DC, Pascoe JE. Variation in concentration and labeling of ginger root dietary supplements. Obstet Gynecol 2006;107:1337–1343. [DOI] [PubMed] [Google Scholar]
- 498.David AVA, Arulmoli R, Parasuraman S. Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacogn Rev 2016;10:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Hollman PC, Van Trijp JM, Buysman MN, et al. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett 1997;418:152–156. [DOI] [PubMed] [Google Scholar]
- 500.Wilms LC, Hollman PC, Boots AW, et al. Protection by quercetin and quercetin-rich fruit juice against induction of oxidative DNA damage and formation of BPDE-DNA adducts in human lymphocytes. Mutat Res 2005;582:155–162. [DOI] [PubMed] [Google Scholar]
- 501.Neto CC, Dao CA, Salvas MR, et al. Variation in concentration of phenolic acid derivatives and quercetin glycosides in foliage of cranberry that may play a role in pest deterrence. J Am Soc Hortic Sci 2010;135:494–500. [Google Scholar]
- 502.Schmitzer V, Veberic R, Stampar F. European elderberry (Sambucus nigra L.) and American Elderberry (Sambucus canadensis L.): botanical, chemical and health properties of flowers, berries and their products. Berries Prop Consum Nutr 2012;2012:127–144. [Google Scholar]
- 503.Snyder SM, Zhao B, Luo T, et al. Consumption of quercetin and quercetin-containing apple and cherry extracts affects blood glucose concentration, hepatic metabolism, and gene expression patterns in obese C57BL/6J high fat-fed mice. J Nutr 2016;146:1001–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Eftekhari M, Alizadeh M, Ebrahimi P. Evaluation of the total phenolics and quercetin content of foliage in mycorrhizal grape (Vitis vinifera L.) varieties and effect of postharvest drying on quercetin yield. Ind Crop Prod 2012;38:160–165. [Google Scholar]
- 505.Careri M, Elviri L, Mangia A, et al. Spectrophotometric and coulometric detection in the high-performance liquid chromatography of flavonoids and optimization of sample treatment for the determination of quercetin in orange juice. J Chromatogr A 2000;881:449–460. [DOI] [PubMed] [Google Scholar]
- 506.Farag RS, Abdel-Latif MS, Emam SS, et al. Phytochemical screening and polyphenol constituents of pomegranate peels and leave juices. Agric Soil Sci 2014;1:86–93. [Google Scholar]
- 507.McAnlis GT, McEneny J, Pearce J, et al. Absorption and antioxidant effects of quercetin from onions, in man. Eur J Clin Nutr 1999;53:92–96. [DOI] [PubMed] [Google Scholar]
- 508.Wiczkowski W, Romaszko J, Bucinski A, et al. Quercetin from shallots (Allium cepa L. var. aggregatum) is more bioavailable than its glucosides. J Nutr 2008;138:885–888. [DOI] [PubMed] [Google Scholar]
- 509.Yang I, Jayaprakasha GK, Patil B. In vitro digestion with bile acids enhances the bioaccessibility of kale polyphenols. Food Funct 2018;9:1235–1244. [DOI] [PubMed] [Google Scholar]
- 510.Zein N, Yassin F, Othman M. Quercetin extracted from broccoli attenuates the renewal of hepatic cells via downregulation of TGFβ-1 and arresting of HSCs activation in mice. Biointerface Res Appl Chem 2021;11:12348–12363. [Google Scholar]
- 511.Bozack A. Effect of organic and conventional farming practices on quercetin content in spinach, Spinacia oleracea. Quercetin Content Spinach 2006;5:1–15. [Google Scholar]
- 512.Hirose Y, Fujita T, Ishii T, et al. Antioxidative properties and flavonoid composition of Chenopodium quinoa seeds cultivated in Japan. Food Chem 2010;119:1300–1306. [Google Scholar]
- 513.Zhao G, Zou L, Wang Z, et al. Pharmacokinetic profile of total quercetin after single oral dose of tartary buckwheat extracts in rats. J Agric Food Chem 2011;59:4435–4441. [DOI] [PubMed] [Google Scholar]
- 514.Kim IS, Yang MR, Lee OH, et al. Antioxidant activities of hot water extracts from various spices. Int J Mol Sci 2011;12:4120–4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Jin D, Hakamata H, Takahashi K, et al. Determination of quercetin in human plasma after ingestion of commercial canned green tea by semi‐micro HPLC with electrochemical detection. Biomed Chromatogr 2004;18:662–666. [DOI] [PubMed] [Google Scholar]
- 516.McDonald MS, Hughes M, Burns J, et al. Survey of the free and conjugated myricetin and quercetin content of red wines of different geographical origins. J Agric Food Chem 1998;46:368–375. [DOI] [PubMed] [Google Scholar]
- 517.Guo AJ, Choi RC, Zheng KY, et al. Kaempferol as a flavonoid induces osteoblastic differentiation via estrogen receptor signalling. Chin Med 2012;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Dehkharghanian M, Adenier H, Vijayalakshmi MA. Study of flavonoids in aqueous spinach extract using positive electrospray ionisation tandem quadrupole mass spectrometry. Food Chem 2010;121:863–870. [Google Scholar]
- 519.Gliszczyńska-Świgło A, Kałużewicz A, Lemańska K, et al. The effect of solar radiation on the flavonol content in broccoli inflorescence. Food Chem 2007;100:241–245. [Google Scholar]
- 520.Nirmala P, Ramanathan M. Effect of kaempferol on lipid peroxidation and antioxidant status in 1,2-dimethyl hydrazine induced colorectal carcinoma in rats. Eur J Pharmacol 2011;654:75–79. [DOI] [PubMed] [Google Scholar]
- 521.Nielsen JK, Nørbæk R, Olsen CE. Kaempferol tetraglucosides from cabbage leaves. Phytochemistry 1998;49:2171–2176. [DOI] [PubMed] [Google Scholar]
- 522.Castillo-Muñoz N, Gómez-Alonso S, García-Romero E, et al. Flavonol profiles of Vitis vinifera red grapes and their single-cultivar wines. J Agric Food Chem 2007;55:992–1002. [DOI] [PubMed] [Google Scholar]
- 523.Cho HJ, Park JHY. Kaempferol induces cell cycle arrest in HT-29 human colon cancer cells. J Cancer Prev 2013;18:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Karabagias IK, Dimitriou E, Kontakos S, et al. Phenolic profile, colour intensity, and radical scavenging activity of Greek unifloral honeys. Eur Food Res Technol 2016;242:1201–1210. [Google Scholar]
- 525.Park JS, Rho HS, Kim DH, et al. Enzymatic preparation of kaempferol from green tea seed and its antioxidant activity. J Agric Food Chem 2006;54:2951–2956. [DOI] [PubMed] [Google Scholar]
- 526.Wang H, Helliwell K. Determination of flavonols in green and black tea leaves and green tea infusions by high-performance liquid chromatography. Food Res Int 2001;34:223–227. [Google Scholar]
- 527.Singh A, Jain A, Sarma BK, et al. Rhizosphere competent microbial consortium mediates rapid changes in phenolic profiles in chickpea during Sclerotium rolfsii infection. Microbiol Res 2014;169:353–360. [DOI] [PubMed] [Google Scholar]
- 528.Manoj BS, Gupta M, Jeelani MI, et al. Metabolic footprints of chitosan primed red kidney bean under restricted irrigation. Int J Biol Macromol 2022;208:367–380. [DOI] [PubMed] [Google Scholar]
- 529.Frémont L. Biological effects of resveratrol. Life Sci 2000;66:663–673. [DOI] [PubMed] [Google Scholar]
- 530.Leyva-Porras C, Saavedra-Leos MZ, Cervantes-González E, et al. Spray drying of blueberry juice-maltodextrin mixtures: evaluation of processing conditions on content of resveratrol. Antioxidants 2019;8:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Rocha‐González HI, Ambriz‐Tututi M, Granados‐Soto V. Resveratrol: a natural compound with pharmacological potential in neurodegenerative diseases. CNS Neurosci Ther 2008;14:234–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Counet C, Callemien D, Collin S. Chocolate and cocoa: new sources of trans-resveratrol and trans-piceid. Food Chem 2006;98:649–657. [Google Scholar]
- 533.Ragab AS, Van Fleet J, Jankowski B, et al. Detection and quantitation of resveratrol in tomato fruit (Lycopersicon esculentum Mill.). J Agric Food Chem 2006;54:7175–7179. [DOI] [PubMed] [Google Scholar]
- 534.Shankar S, Srivastava RK. Bax and Bak genes are essential for maximum apoptotic response by curcumin, a polyphenolic compound and cancer chemopreventive agent derived from turmeric, Curcuma longa. Carcinogenesis 2007;28:1277–1286. [DOI] [PubMed] [Google Scholar]
- 535.Weiss DJ, Anderton CR. Determination of catechins in matcha green tea by micellar electrokinetic chromatography. J Chromatogr A 2003;1011:173–180. [DOI] [PubMed] [Google Scholar]
- 536.Sanlier N, Atik İ, Atik A. A minireview of effects of white tea consumption on diseases. Trends Food Sci Technol 2018;82:82–88. [Google Scholar]
- 537.Oliveira A, Almeida DP, Pintado M. Changes in phenolic compounds during storage of pasteurized strawberry. Food Bioprocess Technol 2014;7:1840–1846. [Google Scholar]
- 538.Pavlović AV, Papetti A, Zagorac DČD, et al. Phenolics composition of leaf extracts of raspberry and blackberry cultivars grown in Serbia. Ind Crops Prod 2016;87:304–314. [Google Scholar]
- 539.Varo MÁ, Martín-Gómez J, Mérida J, et al. Bioactive compounds and antioxidant activity of highbush blueberry (Vaccinium corymbosum) grown in southern Spain. Eur Food Res Technol 2021;247:1199–1208. [Google Scholar]
- 540.Souquet JM, Cheynier V, Brossaud F, et al. Polymeric proanthocyanidins from grape skins. Phytochemistry 1996;43:509–512. [Google Scholar]
- 541.Casanova E, Salvadó J, Crescenti A, et al. Epigallocatechin gallate modulates muscle homeostasis in type 2 diabetes and obesity by targeting energetic and redox pathways: a narrative review. Int J Mol Sci 2019;20:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Yoo KS, Bang H, Pike L, et al. Comparing carotene, anthocyanins, and terpenoid concentrations in selected carrot lines of different colors. Hortic Environ Biotechnol 2020;61:385–393. [Google Scholar]
- 543.Wu X, Sun C, Yang L, et al. β-Carotene content in sweet potato varieties from China and the effect of preparation on β-carotene retention in the Yanshu No. 5. Innov Food Sci Emerg Technol 2008;9:581–586. [Google Scholar]
- 544.Koh SH, Loh SP. In vitro bioaccessibility of β-carotene in pumpkin and butternut squash subjected to different cooking methods. Int Food Res J 2018;25:188–195. [Google Scholar]
- 545.Tsao R, Yang R. Lutein in selected Canadian crops and agri-food processing by-products and purification by high-speed counter-current chromatography. J Chromatogr A 2006;1112:202–208. [DOI] [PubMed] [Google Scholar]
- 546.Rock CL, Lovalvo JL, Emenhiser C, et al. Bioavailability of β-carotene is lower in raw than in processed carrots and spinach in women. J Nutr 1998;128:913–916. [DOI] [PubMed] [Google Scholar]
- 547.Chen X, Wu J, Zhou S, et al. Application of near-infrared reflectance spectroscopy to evaluate the lutein and β-carotene in Chinese kale. J Food Compos Anal 2009;22:148–153. [Google Scholar]
- 548.Ren Y, Bang H, Lee EJ, et al. Levels of phytoene and β-carotene in transgenic honeydew melon (Cucumis melo L. inodorus). Plant Cell Tissue Organ Cult (PCTOC) 2013;113:291–301. [Google Scholar]
- 549.Ornelas-Paz JDJ, Failla ML, Yahia EM, et al. Impact of the stage of ripening and dietary fat on in vitro bioaccessibility of β-carotene in ‘Ataulfo’ mango. J Agric Food Chem 2008;56:1511–1516. [DOI] [PubMed] [Google Scholar]
- 550.Karabulut I, Topcu A, Duran A, et al. Effect of hot air drying and sun drying on color values and β-carotene content of apricot (Prunus armenica L.). LWT-Food Sci Technol 2007;40:753–758. [Google Scholar]
- 551.Espíndola KMM, Ferreira RG, Narvaez LEM, et al. Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front Oncol 2019;9:541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Reinders RD, Biesterveld S, Bijker PG. Survival of Escherichia coli O157: H7 ATCC 43895 in a model apple juice medium with different concentrations of proline and caffeic acid. Appl Environ Microbiol 2001;67:2863–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Öztürk A, Demirsoy L, Demirsoy H, et al. Phenolic compounds and chemical characteristics of pears (Pyrus Communis L.). Int J Food Prop 2015;18:536–546. [Google Scholar]
- 554.Sellappan S, Akoh CC, Krewer G. Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J Agric Food Chem 2002;50:2432–2438. [DOI] [PubMed] [Google Scholar]
- 555.Levaj B, Dragović-Uzelac V, Delonga K, et al. Polyphenols and volatiles in fruits of two sour cherry cultivars, some berry fruits and their jams. Food Technol Biotechnol 2010;48:538–547. [Google Scholar]
- 556.Nia NN, Hadjmohammadi MR. Amino acids-based hydrophobic natural deep eutectic solvents as a green acceptor phase in two-phase hollow fiber-liquid microextraction for the determination of caffeic acid in coffee, green tea, and tomato samples. Microchem J 2021;164:06021. [Google Scholar]
- 557.Singletary K. Oregano: overview of the literature on health benefits. Nutr Today 2010;45:129–138. [Google Scholar]
- 558.Peng Y, Yuan J, Liu F, et al. Determination of active components in rosemary by capillary electrophoresis with electrochemical detection. J Pharm Biomed Anal 2005;39:431–437. [DOI] [PubMed] [Google Scholar]
- 559.Areias F, Valentão P, Andrade PB, et al. Flavonoids and phenolic acids of sage: influence of some agricultural factors. J Agric Food Chem 2000;48:6081–6084. [DOI] [PubMed] [Google Scholar]
- 560.Banjanin T, Nikolic D, Uslu N, et al. Physicochemical properties, fatty acids, phenolic compounds, and mineral contents of 12 Serbia regional and commercial almond cultivars. J Food Process Preserv 2021;45:e15015. [Google Scholar]
- 561.Pedrosa MM, Muzquiz M, García‐Vallejo C, et al. Determination of caffeic and chlorogenic acids and their derivatives in different sunflower seeds. J Sci Food Agric 2000;80:459–464. [Google Scholar]
- 562.Li M, Luo J, Nawaz MA, et al. Phytochemistry, bioaccessibility, and bioactivities of sesame seeds: an overview. Food Rev Int 2023;40:309–335. [Google Scholar]
- 563.Ferri M, Happel A, Zanaroli G, et al. Advances in combined enzymatic extraction of ferulic acid from wheat bran. New Biotechnol 2020;56:38–45. [DOI] [PubMed] [Google Scholar]
- 564.Liu RH. Whole grain phytochemicals and health. J Cereal Sci 2007;46:207–219. [Google Scholar]
- 565.Kelebek H, Selli S, Canbas A, et al. HPLC determination of organic acids, sugars, phenolic compositions and antioxidant capacity of orange juice and orange wine made from a Turkish cv. Kozan. Microchem J 2009;91:187–192. [Google Scholar]
- 566.Lee J, Chan BLS, Mitchell AE. Identification/quantification of free and bound phenolic acids in peel and pulp of apples (Malus domestica) using high resolution mass spectrometry (HRMS). Food Chem 2017;215:301–310. [DOI] [PubMed] [Google Scholar]
- 567.Smith BG, Harris PJ. Ferulic acid is esterified to glucuronoarabinoxylans in pineapple cell walls. Phytochemistry 2001;56:513–519. [DOI] [PubMed] [Google Scholar]
- 568.Bursal E, Köksal E, Gülçin İ, et al. Antioxidant activity and polyphenol content of cherry stem (Cerasus avium L.) determined by LC–MS/MS. Food Res Int 2013;51:66–74. [Google Scholar]
- 569.Lichtenthaler HK, Schweiger J. Cell wall bound ferulic acid, the major substance of the blue-green fluorescence emission of plants. J Plant Physiol 1998;152:272–282. [Google Scholar]
- 570.Rodríguez García SL, Raghavan V. Microwave-assisted extraction of phenolic compounds from broccoli (Brassica oleracea) stems, leaves, and florets: optimization, characterization, and comparison with maceration extraction. Rec Progr Nutr 2022;2:1–20. [Google Scholar]
- 571.Szczepańska J, Barba FJ, Skąpska S, et al. High pressure processing of carrot juice: effect of static and multi-pulsed pressure on the polyphenolic profile, oxidoreductases activity and colour. Food Chem 2020;307:125549. [DOI] [PubMed] [Google Scholar]
- 572.Min JY, Kang SM, Park DJ, et al. Enzymatic release of ferulic acid from Ipomoea batatas L.(sweet potato) stem. Biotechnol Bioprocess Eng 2006;11:372–376. [Google Scholar]
- 573.Vallverdú-Queralt A, Regueiro J, Martínez-Huélamo M, et al. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem 2014;154:299–307. [DOI] [PubMed] [Google Scholar]
- 574.Eliasson C, Kamal-Eldin A, Andersson R, et al. High-performance liquid chromatographic analysis of secoisolariciresinol diglucoside and hydroxycinnamic acid glucosides in flaxseed by alkaline extraction. J Chromatogr A 2003;1012:151–159. [DOI] [PubMed] [Google Scholar]
- 575.Khezeli T, Daneshfar A, Sahraei R. A green ultrasonic-assisted liquid–liquid microextraction based on deep eutectic solvent for the HPLC-UV determination of ferulic, caffeic and cinnamic acid from olive, almond, sesame and cinnamon oil. Talanta 2016;150:577–585. [DOI] [PubMed] [Google Scholar]
- 576.Chen L, Liu Y, Liu H, et al. Identification and expression analysis of MATE genes involved in flavonoid transport in blueberry plants. PLoS One 2015;10:e0118578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Die JV, Jones RW, Ogden EL, et al. Characterization and analysis of anthocyanin-related genes in wild-type blueberry and the pink-fruited mutant cultivar ‘Pink Lemonade’: new insights into anthocyanin biosynthesis. Agronomy 2020;10:1296. [Google Scholar]
- 578.Kähkönen MP, Heinonen M. Antioxidant activity of anthocyanins and their aglycons. J Agric Food Chem 2003;51:628–633. [DOI] [PubMed] [Google Scholar]
- 579.Lee YM, Yoon Y, Yoon H, et al. Dietary anthocyanins against obesity and inflammation. Nutrients 2017;9:1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Cassidy A. Berry anthocyanin intake and cardiovascular health. Mol Aspects Med 2018;61:76–82. [DOI] [PubMed] [Google Scholar]
- 581.Wang D, Guo H, Yang H, et al. Pterostilbene, an active constituent of blueberries, suppresses proliferation potential of human cholangiocarcinoma via enhancing the autophagic flux. Front Pharmacol 2019;10:1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Campelo APBS. The use of pterostilbene in the treatment of skin cancer: a literature review. Clin Surg 2022;8:1–4. [Google Scholar]
- 583.Shivembe A, Ojinnaka D. Determination of vitamin C and total phenolic in fresh and freeze dried blueberries and the antioxidant capacity of their extracts. Integr Food Nutr Metab 2017;4:1–5. [Google Scholar]
- 584.Maya-Cano DA, Arango-Varela S, Santa-Gonzalez GA. Phenolic compounds of blueberries (Vaccinium spp.) as a protective strategy against skin cell damage induced by ROS: a review of antioxidant potential and antiproliferative capacity. Heliyon 2021;7:e06297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci 2016;5:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Coates EM, Popa G, Gill CI, et al. Colon-available raspberry polyphenols exhibit anti-cancer effects on in vitro models of colon cancer. J Carcinog 2007;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Katta R, Brown DN. Diet and skin cancer: the potential role of dietary antioxidants in nonmelanoma skin cancer prevention. J Skin Cancer 2015;2015:893149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Duncan FJ, Martin JR, Wulff BC, et al. Topical treatment with black raspberry extract reduces cutaneous UVB-induced carcinogenesis and inflammation. Cancer Prev Res (Phila) 2009;2:665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Oberyszyn T. Effects of black raspberries on UV-induced cutaneous inflammation and tumor development. In: Seeram NP, Stoner GD, eds. Berries and Cancer Prevention. Springer; 2011. pp. 131–42.
- 590.Mace TA, King SA, Ameen Z, et al. Bioactive compounds or metabolites from black raspberries modulate T lymphocyte proliferation, myeloid cell differentiation and Jak/STAT signalling. Cancer Immunol Immunother 2014;63:889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Satpal D, Kaur J, Bhadariya V, et al. Actinidia deliciosa (Kiwi fruit): a comprehensive review on the nutritional composition, health benefits, traditional utilization, and commercialization. J Food Process Preserv 2021;45:e15588. [Google Scholar]
- 592.Kim YM, Park YS, Park YK, et al. Characterization of bioactive ligands with antioxidant properties of kiwifruit and persimmon cultivars using in vitro and in silico studies. Appl Sci 2020;10:4218. [Google Scholar]
- 593.Azeem M, Hanif M, Mahmood K, et al. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: a review. Polym Bull 2023;80:241–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Wang J, Fang X, Ge L, et al. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS One 2018;13:e0197563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Ampomah-Dwamena C, McGhie T, Wibisono R, et al. The kiwifruit lycopene beta-cyclase plays a significant role in carotenoid accumulation in fruit. J Exp Bot 2009;60:3765–3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Nishiyama I, Fukuda T, Oota T. Cultivar difference in chlorophyll, lutein and β-carotene content in the fruit of kiwifruit and other Actinidia species. Acta Horticult 2006;753:473–478. [Google Scholar]
- 597.Sommerburg O, Keunen JE, Bird AC, et al. Fruits and vegetables that are sources for lutein and zeaxanthin: the macular pigment in human eyes. Br J Ophthalmol 1998;82:907–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Chauhan M, Garg V, Zia G, et al. Potential role of phytochemicals of fruits and vegetables in human diet. Res J Pharm Technol 2020;13:1587–1591. [Google Scholar]
- 599.El-Raey MA, Ibrahim GE, Eldahshan OA. Lycopene and Lutein; A review for their chemistry and medicinal uses. J Pharmacogn Phytochem 2013;2:245–254. [Google Scholar]
- 600.Kim YE, Cho CH, Kang H, et al. Kiwifruit of Actinidia eriantha cv. Bidan has in vitro antioxidative, anti-inflammatory and immunomodulatory effects on macrophages and splenocytes isolated from male BALB/c mice. Food Sci Biotechnol 2018;27:1503–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Kou L, Zhu Z, Redington C, et al. Potential use of kiwifruit extract for treatment of melanoma. Med Oncol 2021;38:1–7. [DOI] [PubMed] [Google Scholar]
- 602.Kou L, Zhu Z, Fajardo E, et al. Harnessing the power of kiwifruit for radiosensitization of melanoma. Anticancer Res 2021;41:5945–5951. [DOI] [PubMed] [Google Scholar]
- 603.Maoto MM, Beswa D, Jideani AI. Watermelon as a potential fruit snack. Int J Food Prop 2019;22:355–370. [Google Scholar]
- 604.Kim CH, Park MK, Kim SK, et al. Antioxidant capacity and anti‐inflammatory activity of lycopene in watermelon. Int J Food Sci Technol 2014;49:2083–2091. [Google Scholar]
- 605.van Breemen RB, Pajkovic N. Multitargeted therapy of cancer by lycopene. Cancer Lett 2008;269:339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Manivannan A, Lee ES, Han K, et al. Versatile nutraceutical potentials of watermelon - a modest fruit loaded with pharmaceutically valuable phytochemicals. Molecules 2020;25:5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Lester G. Melon (Cucumis melo L.) fruit nutritional quality and health functionality. HortTechnology 1997;7:222–227. [Google Scholar]
- 608.Itoh T, Fujita S, Koketsu M, et al. Citrulluside H and citrulluside T from young watermelon fruit attenuate ultraviolet B radiation‐induced matrix metalloproteinase expression through the scavenging of generated reactive oxygen species in human dermal fibroblasts. Photodermatol Photoimmunol Photomed 2021;37:386–394. [DOI] [PubMed] [Google Scholar]
- 609.Ascenso A, Ribeiro H, Marques HC, et al. Chemoprevention of photocarcinogenesis by lycopene. Exp Dermatol 2014;23:874–878. [DOI] [PubMed] [Google Scholar]
- 610.Mirza B, Croley CR, Ahmad M, et al. Mango (Mangifera indica L.): a magnificent plant with cancer preventive and anticancer therapeutic potential. Crit Rev Food Sci Nutr 2021;61:2125–2151. [DOI] [PubMed] [Google Scholar]
- 611.Saha S, Sadhukhan P, Sil PC. Mangiferin: a xanthonoid with multipotent anti‐inflammatory potential. Biofactors 2016;42:459–474. [DOI] [PubMed] [Google Scholar]
- 612.Shi W, Deng J, Tong R, et al. Molecular mechanisms underlying mangiferin-induced apoptosis and cell cycle arrest in A549 human lung carcinoma cells. Mol Med Rep 2016;13:3423–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Schagen SK, Zampeli VA, Makrantonaki E, et al. Discovering the link between nutrition and skin aging. Dermatoendocrinology 2012;4:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Balić A, Mokos M. Do we utilize our knowledge of the skin protective effects of carotenoids enough? Antioxidants 2019;8:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Li J, Malakhova M, Mottamal M, et al. Norathyriol suppresses skin cancers induced by solar ultraviolet radiation by targeting ERK kinases. Cancer Res 2012;72:260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Saleem M, Afaq F, Adhami VM, et al. Lupeol modulates NF-κB and PI3K/Akt pathways and inhibits skin cancer in CD-1 mice. Oncogene 2004;23:5203–5214. [DOI] [PubMed] [Google Scholar]
- 617.Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr 2004;134:3479S–3485S. [DOI] [PubMed] [Google Scholar]
- 618.Ahmad T, Cawood M, Iqbal Q, et al. Phytochemicals in Daucus carota and their health benefits. Foods 2019;8:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Hughes DA. Dietary carotenoids and human immune function. Nutrition 2001;17:823–827. [DOI] [PubMed] [Google Scholar]
- 620.Boelsma E, Hendriks HF, Roza L. Nutritional skin care: health effects of micronutrients and fatty acids. Am J Clin Nutr 2001;73:853–864. [DOI] [PubMed] [Google Scholar]
- 621.Alfurayhi R, Huang L, Brandt K. Pathways affected by falcarinol-type polyacetylenes and implications for their anti-inflammatory function and potential in cancer chemoprevention. Foods 2023;12:1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Cooper DA, Eldridge AL, Peters JC. Dietary carotenoids and certain cancers, heart disease, and age-related macular degeneration: a review of recent research. Nutr Rev 1999;57:201–214. [DOI] [PubMed] [Google Scholar]
- 623.Shebaby WN, Mroueh MA, Boukamp P, et al. Wild carrot pentane-based fractions suppress proliferation of human HaCaT keratinocytes and protect against chemically-induced skin cancer. BMC Complement Altern Med 2017;17:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624.Natarajmurthy SH, Dharmesh SM. Amelioration of skin cancer in mice by β-carotene and phenolics of carrot (Daucus carota). Am J Biopharmacol Biochem Life Sci 2015;4:1–25. [Google Scholar]
- 625.Islam SU, Ahmed MB, Ahsan H, et al. An update on the role of dietary phytochemicals in human skin cancer: new insights into molecular mechanisms. Antioxidants (Basel) 2020;9:916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Otto A, Du Plessis J, Wiechers JW. Formulation effects of topical emulsions on transdermal and dermal delivery. Int J Cosmet Sci 2009;31:1–19. [DOI] [PubMed] [Google Scholar]
- 627.Bitew M, Desalegn T, Demissie TB, et al. Pharmacokinetics and drug-likeness of antidiabetic flavonoids: molecular docking and DFT study. PLoS One 2021;16:e0260853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Puglia C, Lauro MR, Tirendi GG, et al. Modern drug delivery strategies applied to natural active compounds. Expert Opin Drug Deliv 2017;14:755–768. [DOI] [PubMed] [Google Scholar]
- 629.More MP, Pardeshi SR, Pardeshi CV, et al. Recent advances in phytochemical-based nano-formulation for drug-resistant cancer. Med Drug Discov 2021;10:100082. [Google Scholar]
- 630.Thakur L, Ghodasra U, Patel N, et al. Novel approaches for stability improvement in natural medicines. Pharmacogn Rev 2011;5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 631.Bonifacio BV, da Silva PB, Ramos MADS, et al. Nanotechnology-based drug delivery systems and herbal medicines: a review. Int J Nanomedicine 2014;9:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable to this article.


