Abstract
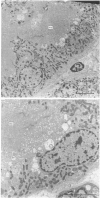
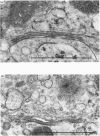
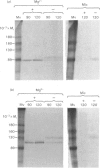
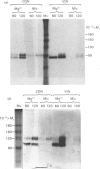
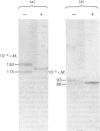
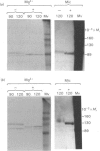
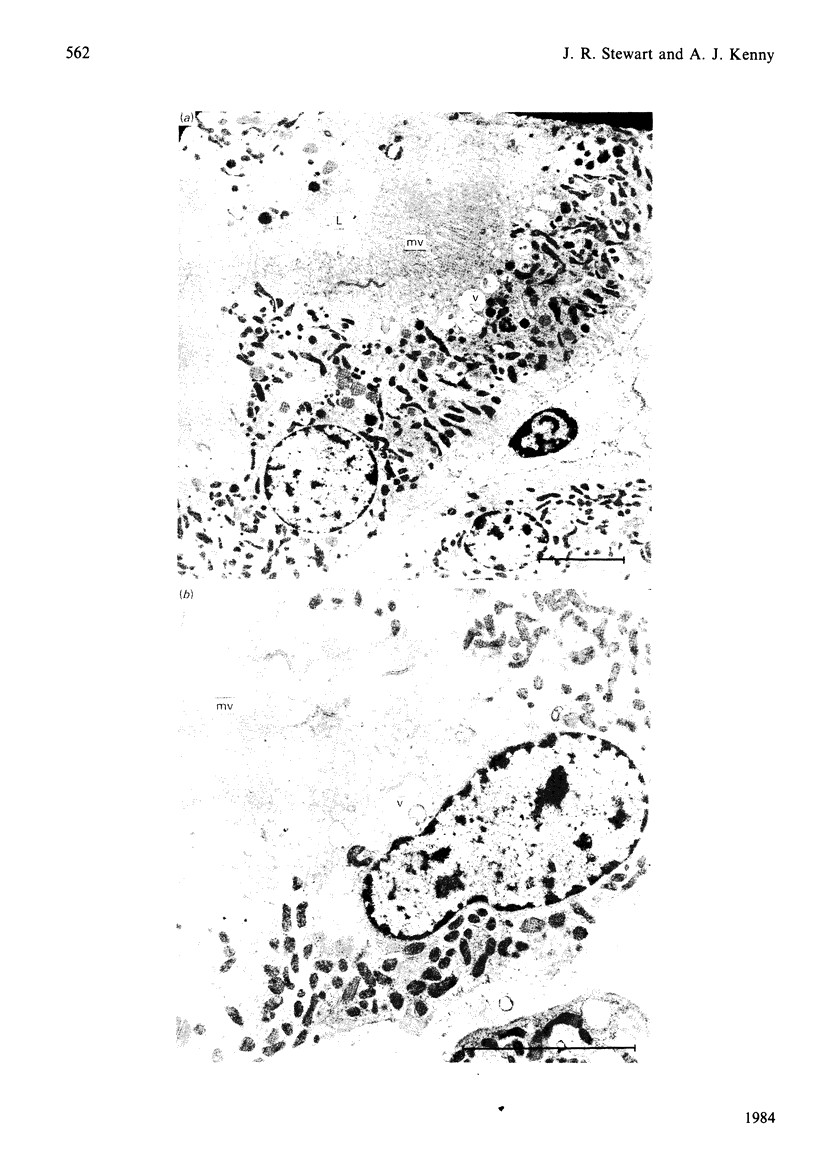
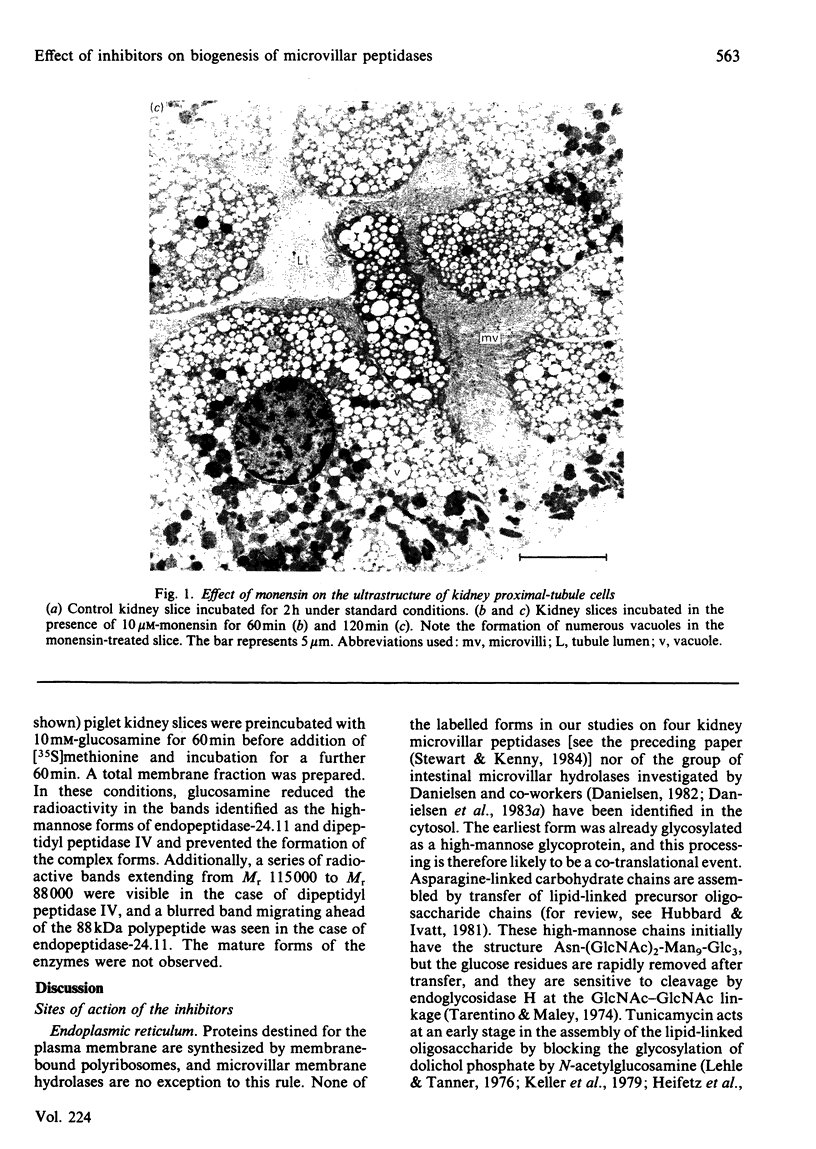
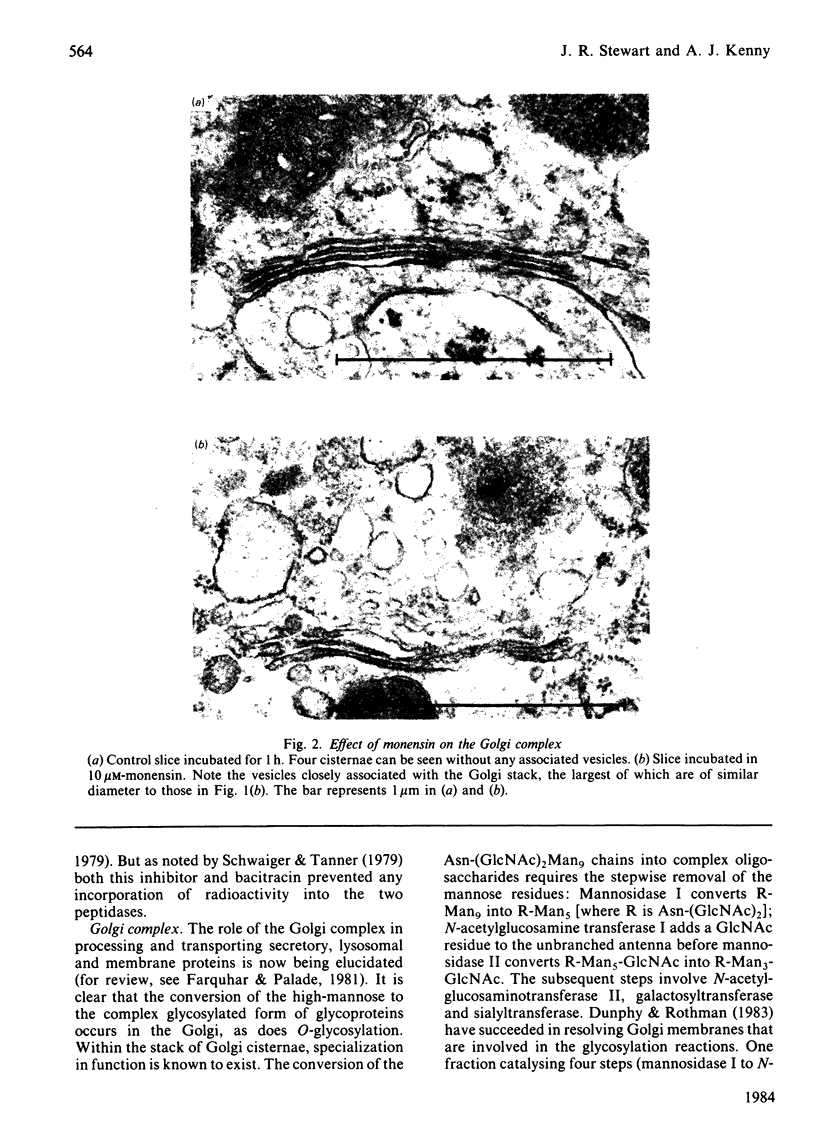
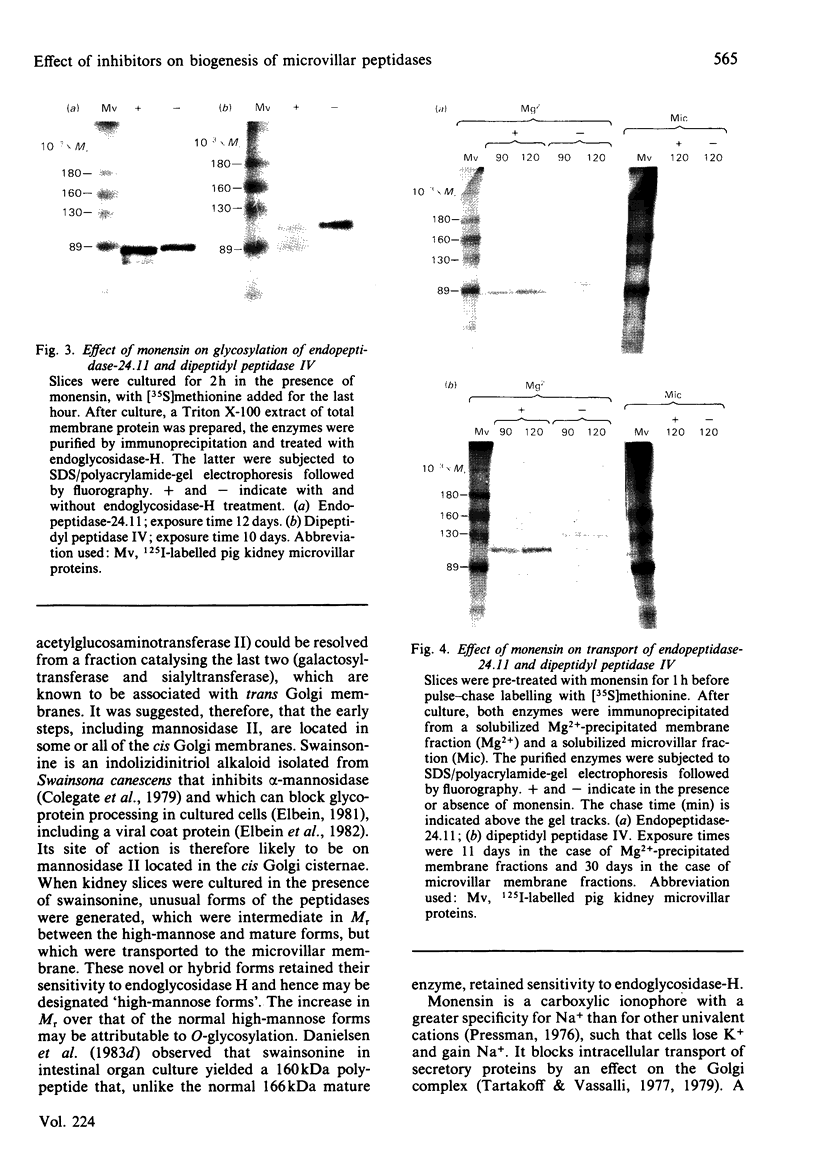
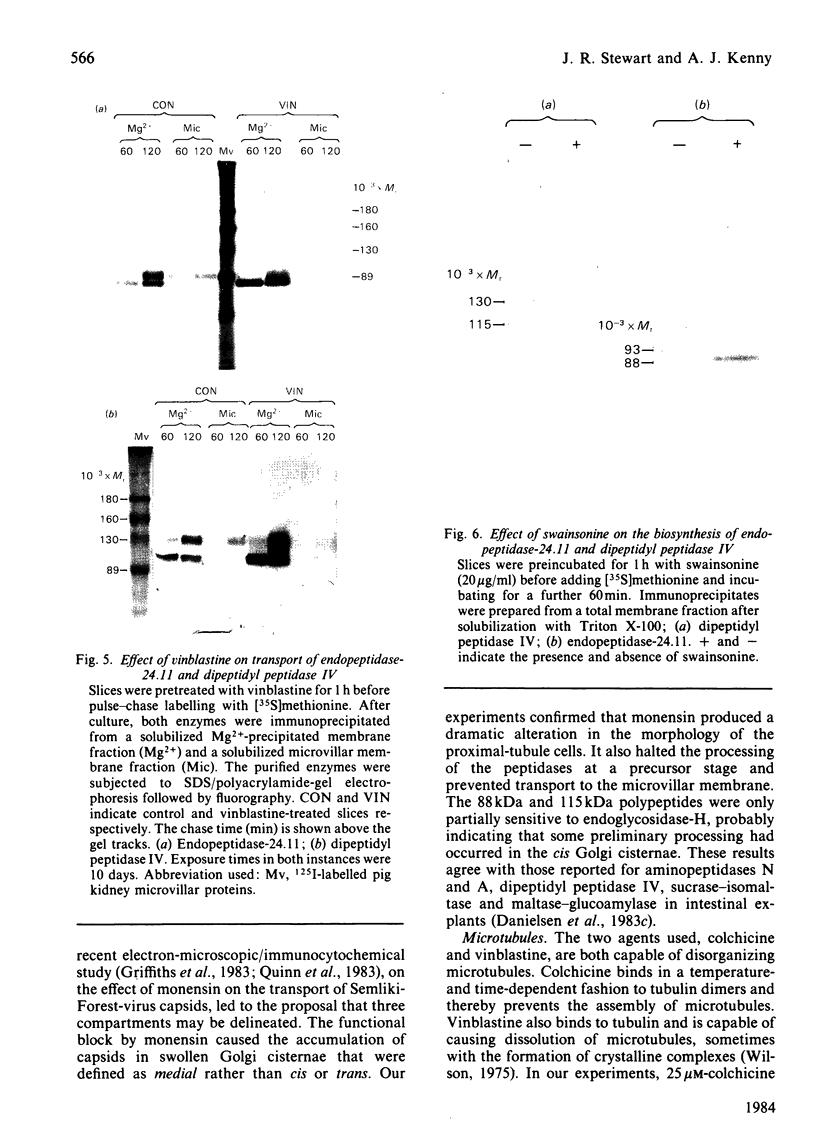
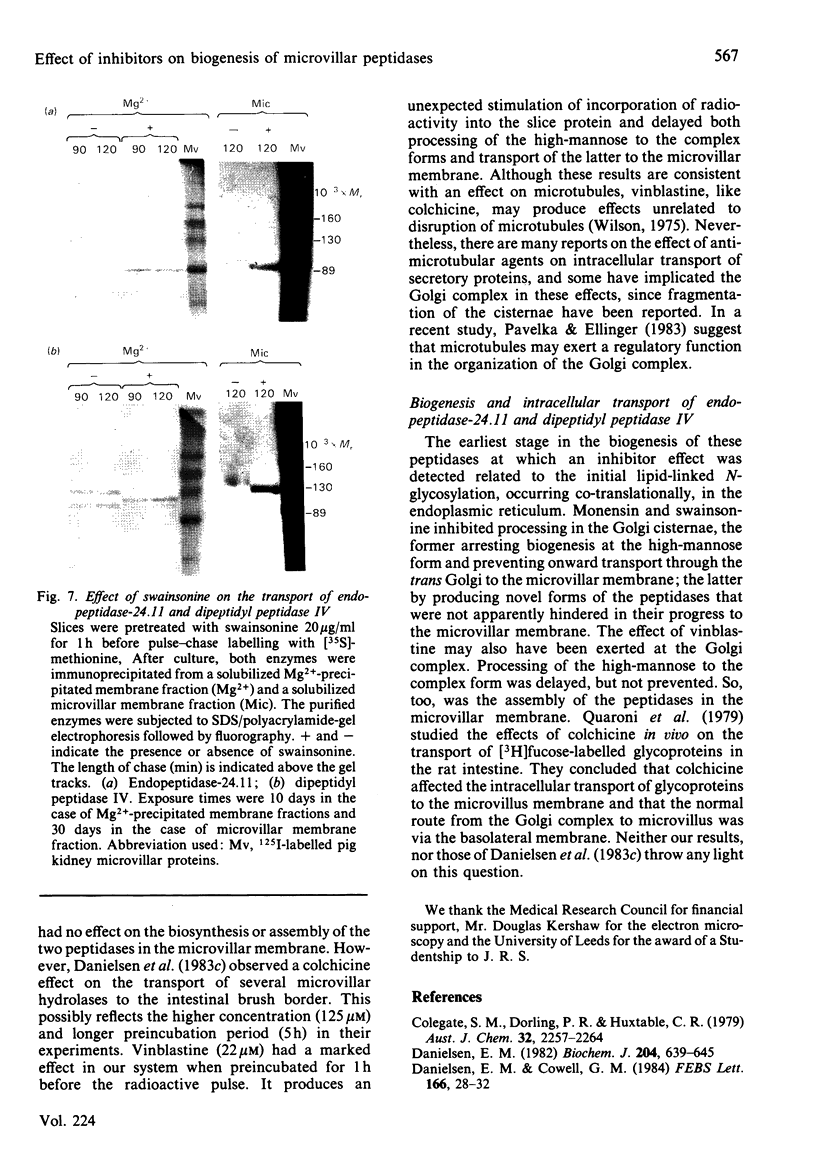
The effects of various inhibitors were studied on the biogenesis of endopeptidase-24.11 (EC 3.4.24.11) and dipeptidyl peptidase IV (EC 3.4.14.5) in slices of renal cortex, from piglets of the Yucatan strain, maintained in organ culture. These microvillar peptidases were synthesized within membrane compartments and underwent glycosylation to yield high-mannose and complex forms [the preceding paper, Stewart & Kenny (1984) Biochem. J. 224, 549-558]. Monensin caused very gross ultrastructural changes in the proximal-tubular cells, resulting from distension of the Golgi sacs. It blocked the processing of the high-mannose to the complex glycosylated forms of the peptidases and prevented their assembly in the microvillar membrane. Swainsonine, an inhibitor of alpha-mannosidase II, generated new 'hybrid' forms of the proteins, intermediate in Mr between the high-mannose and the complex forms, but did not prevent assembly of the hybrid forms in microvilli. Vinblastine, an agent that affects microtubules, delayed, but did not abolish, either the processing or the transport to microvilli. Glucosamine interfered with the initial glycosylation reactions and generated heterogeneous sets of partially glycosylated polypeptides of lower Mr than the high-mannose forms. These results are discussed in relation to the site and mechanism of glycosylation and the involvement of the Golgi complex and microtubules in the biogenesis of these membrane peptidases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Danielsen E. M. Biosynthesis of intestinal microvillar proteins. Pulse-chase labelling studies on aminopeptidase N and sucrase-isomaltase. Biochem J. 1982 Jun 15;204(3):639–645. doi: 10.1042/bj2040639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M., Cowell G. M. Biosynthesis of intestinal microvillar proteins. Further characterization of the intracellular processing and transport. FEBS Lett. 1984 Jan 23;166(1):28–32. doi: 10.1016/0014-5793(84)80038-1. [DOI] [PubMed] [Google Scholar]
- Danielsen E. M., Cowell G. M., Poulsen S. S. Biosynthesis of intestinal microvillar proteins. Role of the Golgi complex and microtubules. Biochem J. 1983 Oct 15;216(1):37–42. doi: 10.1042/bj2160037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M., Norén O., Sjöström H. Biosynthesis of intestinal microvillar proteins. Processing of aminopeptidase N by microsomal membranes. Biochem J. 1983 Apr 15;212(1):161–165. doi: 10.1042/bj2120161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M., Norén O., Sjöström H. Biosynthesis of intestinal microvillar proteins. Translational evidence in vitro that aminopeptidase N is synthesized as a Mr-115000 polypeptide. Biochem J. 1982 Apr 15;204(1):323–327. doi: 10.1042/bj2040323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M., Sjöström H., Norén O. Biosynthesis of intestinal microvillar proteins. Pulse-chase labelling studies on maltase-glucoamylase, aminopeptidase A and dipeptidyl peptidase IV. Biochem J. 1983 Feb 15;210(2):389–393. doi: 10.1042/bj2100389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datema R., Schwarz R. T. Interference with glycosylation of glycoproteins. Inhibition of formation of lipid-linked oligosaccharides in vivo. Biochem J. 1979 Oct 15;184(1):113–123. doi: 10.1042/bj1840113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy W. G., Rothman J. E. Compartmentation of asparagine-linked oligosaccharide processing in the Golgi apparatus. J Cell Biol. 1983 Jul;97(1):270–275. doi: 10.1083/jcb.97.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein A. D., Dorling P. R., Vosbeck K., Horisberger M. Swainsonine prevents the processing of the oligosaccharide chains of influenza virus hemagglutinin. J Biol Chem. 1982 Feb 25;257(4):1573–1576. [PubMed] [Google Scholar]
- Griffiths G., Quinn P., Warren G. Dissection of the Golgi complex. I. Monensin inhibits the transport of viral membrane proteins from medial to trans Golgi cisternae in baby hamster kidney cells infected with Semliki Forest virus. J Cell Biol. 1983 Mar;96(3):835–850. doi: 10.1083/jcb.96.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifetz A., Keenan R. W., Elbein A. D. Mechanism of action of tunicamycin on the UDP-GlcNAc:dolichyl-phosphate Glc-NAc-1-phosphate transferase. Biochemistry. 1979 May 29;18(11):2186–2192. doi: 10.1021/bi00578a008. [DOI] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Keller R. K., Boon D. Y., Crum F. C. N-Acetylglucosamine- 1 -phosphate transferase from hen oviduct: solubilization, characterization, and inhibition by tunicamycin. Biochemistry. 1979 Sep 4;18(18):3946–3952. doi: 10.1021/bi00585a016. [DOI] [PubMed] [Google Scholar]
- Koch H. U., Schwarz R. T., Scholtissek C. Glucosamine itself mediates reversible inhibition of protein glycosylation. A study of glucosamine metabolism at inhibitory concentrations in influenza-virus-infected cells. Eur J Biochem. 1979 Mar;94(2):515–522. doi: 10.1111/j.1432-1033.1979.tb12920.x. [DOI] [PubMed] [Google Scholar]
- Lehle L., Tanner W. The specific site of tunicamycin inhibition in the formation of dolichol-bound N-acetylglucosamine derivatives. FEBS Lett. 1976 Nov 15;72(1):167–170. doi: 10.1016/0014-5793(76)80922-2. [DOI] [PubMed] [Google Scholar]
- Mrsny R. J., Meizel S. Potassium ion influx and Na+,K+-ATPase activity are required for the hamster sperm acrosome reaction. J Cell Biol. 1981 Oct;91(1):77–82. [PMC free article] [PubMed] [Google Scholar]
- Pavelka M., Ellinger A. Effect of colchicine on the Golgi complex of rat pancreatic acinar cells. J Cell Biol. 1983 Sep;97(3):737–748. doi: 10.1083/jcb.97.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Quaroni A., Kirsch K., Weiser M. M. Synthesis of membrane glycoproteins in rat small-intestinal villus cells. Effect of colchicine on the redistribution of L-[1,5,6-3H]fucose-labelled membrane glycoproteins among Golgi, lateral basal and microvillus membranes. Biochem J. 1979 Jul 15;182(1):213–221. doi: 10.1042/bj1820213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn P., Griffiths G., Warren G. Dissection of the Golgi complex. II. Density separation of specific Golgi functions in virally infected cells treated with monensin. J Cell Biol. 1983 Mar;96(3):851–856. doi: 10.1083/jcb.96.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaiger H., Tanner W. Effects of gibberellic acid and of tunicamycin on glycosyl-transferase activities and on alpha-amylase secretion in barley. Eur J Biochem. 1979 Dec 17;102(2):375–381. doi: 10.1111/j.1432-1033.1979.tb04252.x. [DOI] [PubMed] [Google Scholar]
- Schwarz R. T., Schmidt M. F., Anwer U., Klenk H. D. Carbohydrates of influenza virus. I. Glycopeptides derived from viral glycoproteins after labeling with radioactive sugars. J Virol. 1977 Aug;23(2):217–226. doi: 10.1128/jvi.23.2.217-226.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. R., Kenny A. J. Proteins of the kidney microvillar membrane. Biosynthesis of endopeptidase-24.11, dipeptidylpeptidase IV and aminopeptidases N and A in pig kidney slices. Biochem J. 1984 Dec 1;224(2):549–558. doi: 10.1042/bj2240549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Tartakoff A. M., Vassalli P. Plasma cell immunoglobulin secretion: arrest is accompanied by alterations of the golgi complex. J Exp Med. 1977 Nov 1;146(5):1332–1345. doi: 10.1084/jem.146.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartakoff A., Vassalli P. Plasma cell immunoglobulin M molecules. Their biosynthesis, assembly, and intracellular transport. J Cell Biol. 1979 Nov;83(2 Pt 1):284–299. doi: 10.1083/jcb.83.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulsiani D. R., Harris T. M., Touster O. Swainsonine inhibits the biosynthesis of complex glycoproteins by inhibition of Golgi mannosidase II. J Biol Chem. 1982 Jul 25;257(14):7936–7939. [PubMed] [Google Scholar]
- Wilson L. Action of drugs on microtubules. Life Sci. 1975 Aug 1;17(3):303–309. doi: 10.1016/0024-3205(75)90476-2. [DOI] [PubMed] [Google Scholar]