Abstract
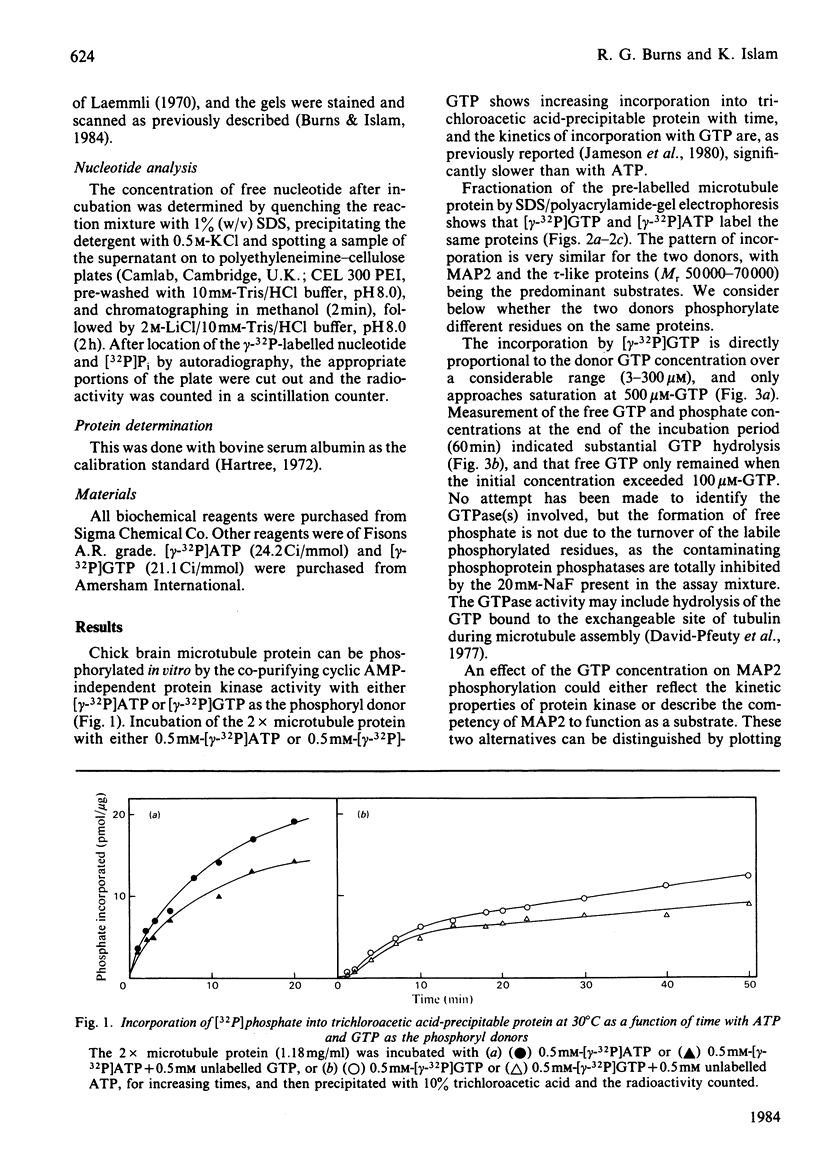
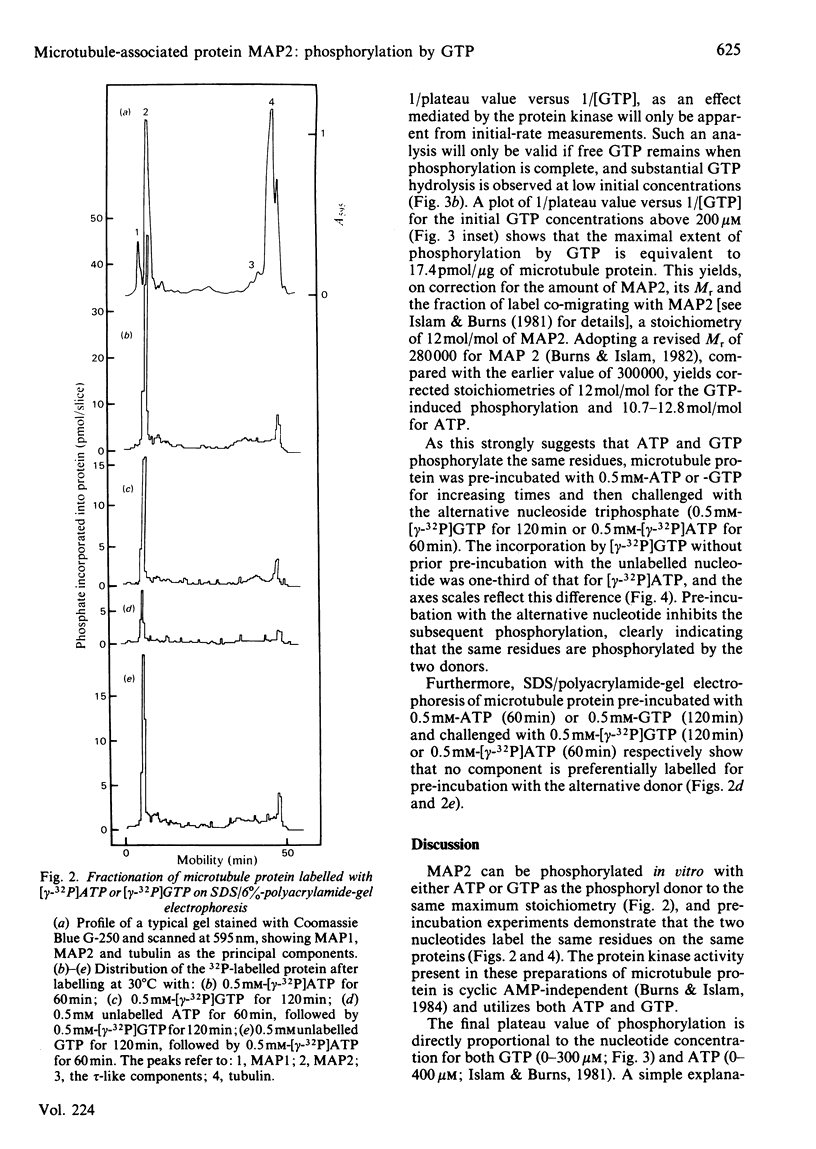
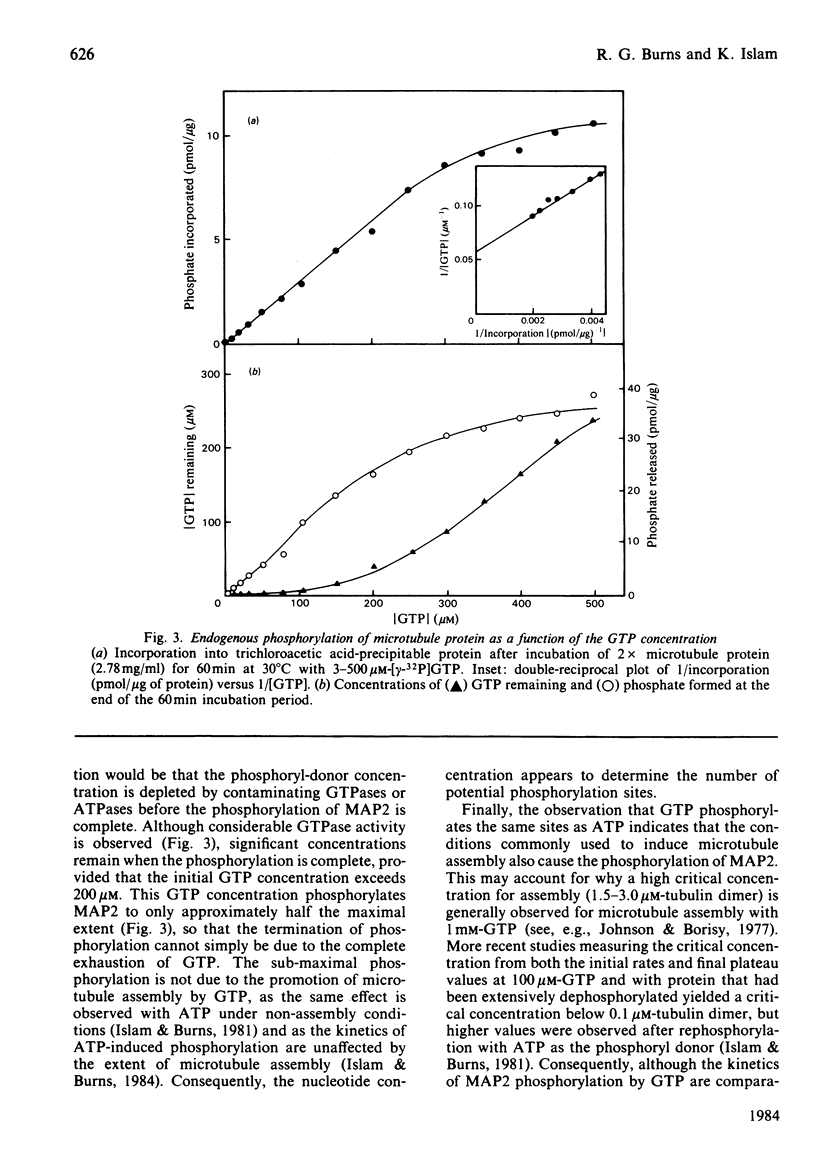
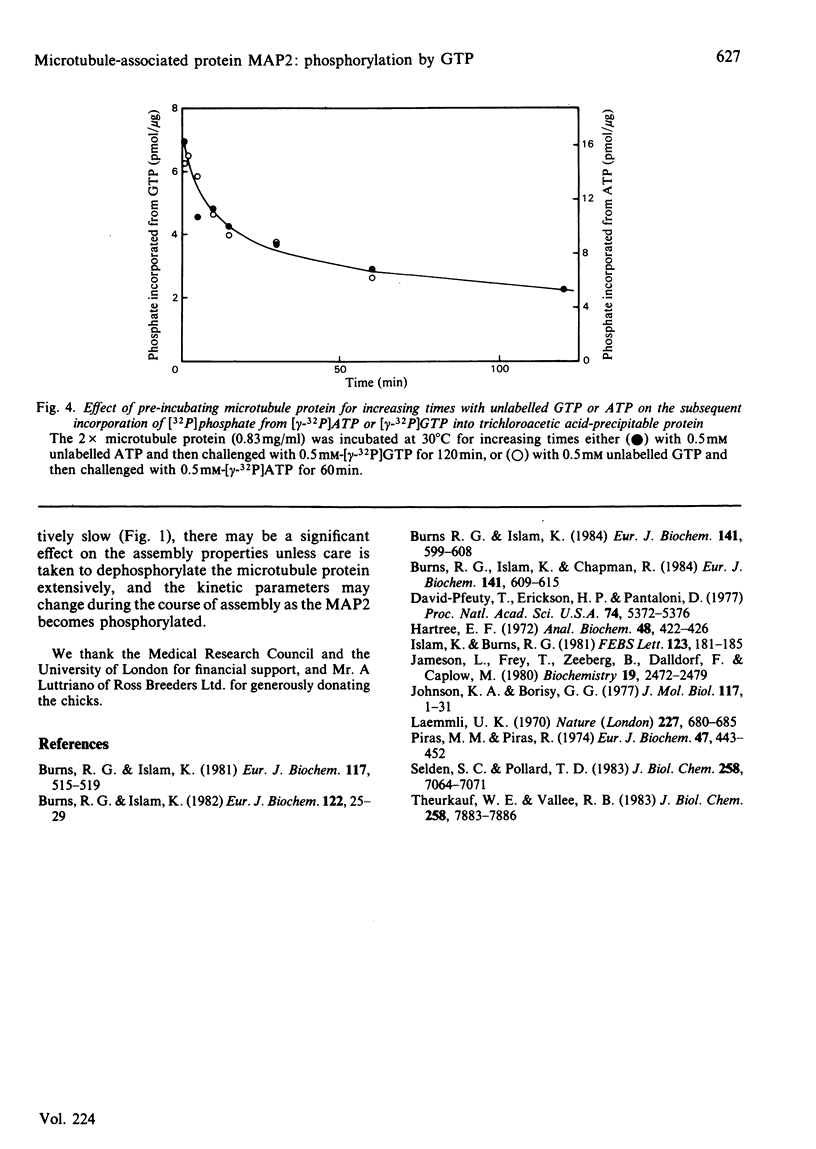
The chick brain microtubule-associated protein MAP2 can be phosphorylated in vitro to the extent of 12 mol/mol with GTP at the same sites as can be labelled by the cyclic AMP-independent protein kinase utilizing [gamma-32P]ATP as the phosphoryl donor. Consequently, the microtubule protein is chemically modified by the conditions usually employed for studies of microtubule assembly, so that the derived kinetic parameters may not relate to steady-state conditions.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burns R. G., Islam K., Chapman R. The multiple phosphorylation of the microtubule-associated protein MAP2 controls the MAP2:tubulin interaction. Eur J Biochem. 1984 Jun 15;141(3):609–615. doi: 10.1111/j.1432-1033.1984.tb08236.x. [DOI] [PubMed] [Google Scholar]
- Burns R. G., Islam K. Characterisation of the chick brain high molecular weight multiply phosphorylated microtubule associated protein. Eur J Biochem. 1982 Feb;122(1):25–29. doi: 10.1111/j.1432-1033.1982.tb05843.x. [DOI] [PubMed] [Google Scholar]
- Burns R. G., Islam K. Nucleosidediphosphate kinase associates with rings but not with assembled microtubules. Eur J Biochem. 1981 Jul;117(3):515–519. doi: 10.1111/j.1432-1033.1981.tb06367.x. [DOI] [PubMed] [Google Scholar]
- Burns R. G., Islam K. Stoichiometry of microtubule-associated protein (MAP2):tubulin and the localisation of the phosphorylation and cysteine residues along the MAP2 primary sequence. Eur J Biochem. 1984 Jun 15;141(3):599–608. doi: 10.1111/j.1432-1033.1984.tb08235.x. [DOI] [PubMed] [Google Scholar]
- David-Pfeuty T., Erickson H. P., Pantaloni D. Guanosinetriphosphatase activity of tubulin associated with microtubule assembly. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5372–5376. doi: 10.1073/pnas.74.12.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Islam K., Burns R. Multiple phosphorylation sites of microtubule-associated protein (MAP2) observed at high ATP concentrations. FEBS Lett. 1981 Jan 26;123(2):181–185. doi: 10.1016/0014-5793(81)80282-7. [DOI] [PubMed] [Google Scholar]
- Jameson L., Frey T., Zeeberg B., Dalldorf F., Caplow M. Inhibition of microtubule assembly by phosphorylation of microtubule-associated proteins. Biochemistry. 1980 May 27;19(11):2472–2479. doi: 10.1021/bi00552a027. [DOI] [PubMed] [Google Scholar]
- Johnson K. A., Borisy G. G. Kinetic analysis of microtubule self-assembly in vitro. J Mol Biol. 1977 Nov 25;117(1):1–31. doi: 10.1016/0022-2836(77)90020-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Piras M. M., Piras R. Phosphorylation of vinblastine-isolated microtubules from chick-embryonic muscles. Eur J Biochem. 1974 Sep 16;47(3):443–452. doi: 10.1111/j.1432-1033.1974.tb03711.x. [DOI] [PubMed] [Google Scholar]
- Selden S. C., Pollard T. D. Phosphorylation of microtubule-associated proteins regulates their interaction with actin filaments. J Biol Chem. 1983 Jun 10;258(11):7064–7071. [PubMed] [Google Scholar]
- Theurkauf W. E., Vallee R. B. Extensive cAMP-dependent and cAMP-independent phosphorylation of microtubule-associated protein 2. J Biol Chem. 1983 Jun 25;258(12):7883–7886. [PubMed] [Google Scholar]


