Abstract
Matricellular proteins are integral non-structural components of the extracellular matrix. They serve as essential modulators of immunometabolism and tissue homeostasis, playing critical roles in physiological and pathological conditions. These extracellular matrix proteins including thrombospondins, osteopontin, tenascins, the secreted protein acidic and rich in cysteine (SPARC) family, the Cyr61, CTGF, NOV (CCN) family, and fibulins have multi-faceted functions in regulating immune cell functions, metabolic pathways, and tissue homeostasis. They are involved in immune-metabolic regulation and influence processes such as insulin signaling, adipogenesis, lipid metabolism, and immune cell function, playing significant roles in metabolic disorders such as obesity and diabetes. Furthermore, their modulation of tissue homeostasis processes including cellular adhesion, differentiation, migration, repair, and regeneration is instrumental for maintaining tissue integrity and function. The importance of these proteins in maintaining physiological equilibrium is underscored by the fact that alterations in their expression or function often coincide with disease manifestation. This review contributes to our growing understanding of these proteins, their mechanisms, and their potential therapeutic applications.
Keywords: Immunometabolism, Inflammation, Matricellular proteins, Metabolic disorders, Tissue homeostasis
INTRODUCTION
Matricellular proteins are non-structural extracellular matrix (ECM) components that play a crucial role in modulating cell-matrix interactions and mediating a multitude of biological processes (1). Although they are not involved in physical formation of the ECM, they can act as regulators of cell behavior, offering a unique perspective on tissue development, homeostasis, and disease.
The term “matricellular” was introduced in the 1990s by Paul Bornstein. Since then, it has been expanded to include a variety of protein families such as thrombospondins (TSPs), tenascins (TNs), osteopontin (OPN), the secreted protein acidic and rich in cysteine (SPARC) family, the Cyr61, CTGF, NOV (CCN) family, and the fibulins (2, 3). Expression levels of these proteins are typically upregulated during development, tissue repair, and in response to stress or injury, further reinforcing their critical roles in modulating biological processes (4).
In recent years, immunometabolism, which explores the intersection of immune responses and metabolic processes, has gained considerable attention (5, 6). Matricellular proteins have been implicated in immunometabolism as they play crucial roles in modulating immune cell function, driving metabolic adaptation, and mediating inflammatory responses. Furthermore, dysregulation of these proteins is associated with numerous pathological conditions such as inflammation, fibrosis, obesity, cardiovascular diseases, and cancer, rendering them potential therapeutic targets (1, 7, 8).
Given the breadth and complexity of matricellular protein functions, this review aims to provide a comprehensive overview of their roles in immunometabolism and tissue homeostasis, with a focus on their pathological implications.
BIOLOGY OF MATRICELLULAR PROTEINS
Matricellular proteins serve as crucial mediators of cell-matrix interactions, affecting cellular behavior. Although they are not major structural components of the ECM, matricellular proteins are indispensable for fine-tuning the cellular microenvironment. They contribute to a wide variety of biological processes such as cellular adhesion, migration, and differentiation (1). Each matricellular protein has a unique structure and function. Collectively, they contribute to the regulation of cell-matrix interactions and intracellular signaling pathways. This section presents a deeper understanding of several representative matricellular proteins, including TSPs, TNs, OPN, SPARC family, CCN family, and fibulins (Table 1).
Table 1.
Summarized biological functions of matricellular proteins and their subtypes
| Matricellular proteins | Subtypes of matricellular proteins | Cell/tissue | Biological functions |
|---|---|---|---|
| TSPs | TSP-1 | Endothelial cell | Increases cell apoptosis (16) |
| Modulates cell adhesion (19) | |||
| Modulates cell migration (23) | |||
| Osteoclast | Promotes differentiation (25) | ||
| TSP-2 | Endothelial cell | Suppresses angiogenesis (24) | |
| TNs | TNC | Endothelial cell | Increases cell migration (41) |
| Astrocyte | Promotes cell proliferation and migration (42) | ||
| Osteosarcoma | Promotes cell migration and lung metastasis (43) | ||
| Neural progenitor cell | Regulates cell differentiation (44) | ||
| Mesodermal cell | Promotes cell differentiation (45) | ||
| TNX | Bovine foetal tissue | Increases collagen fibrillogenesis (46) | |
| OPN | OPN | Osteoclast | Promotes cell migration (59) |
| Breast cancer cell | Induces cell migration and invasion, inhibits cell apoptosis (60) | ||
| Macrophage | Increases cell migration (61) | ||
| Inhibits macrophage-to-osteoclast differentiation and decrease inflammation (64) | |||
| Gastric cancer cell | Increases cell proliferation, invasion, and migration, inhibits apoptosis (62) | ||
| Cancer cell | Stimulates cancer cell migration (63) | ||
| Adipocyte | Decreases adipogenesis (65) | ||
| SPARC family | SPARC | Embryo | Promotes basal lamina maturation (76) |
| Endothelial cell | Regulates fibrinolysis and angiogenesis (77) | ||
| Glioma cell | Promotes cell invasion (79) | ||
| Bone | Promotes osteoblast differentiation (81) | ||
| SPARCL1 | Endothelial cell | Decreases cell attachment (78) | |
| Muscle-derived satellite cell | Promotes cell differentiation and migration (80) | ||
| CCN family | CCN1 | Fibroblast | Promotes cell adhesion (104) |
| Endothelial cell | Promotes cell adhesion and migration (98) | ||
| CCN2 | Keratinocyte | Promotes cell adhesion and migration (105) | |
| Chondrocyte | Promotes cell differentiation and proliferation (106) | ||
| Osteoblast | Promotes cell differentiation and proliferation (107) | ||
| Fibulins | Fibulin-1 | Platelet | Promotes cell adhesion (109) |
| Neural crest cell | Promotes cell migration and survival for development (132) | ||
| Fibulin-3 | Endothelial cell | Inhibits cell migration (131) | |
| Fibulin-4 | Smooth muscle cells/fibroblast | Promotes development of arterial elastic lamina (133) | |
| Fibulin-5 | Endothelial cell | Inhibits cell migration (131) |
TSPs belong to a family of matricellular proteins. There are five TSPs, TSP-1 to TSP-5 coded by separate genes. TSP-1 and TSP-2 have been mainly investigated in the TSP family regarding their biological functions. Multiple cell types are known to produce and secrete TSPs. For example, platelets are releasing TSP-1 upon activation, which subsequently aids in wound healing and inflammation regulation (9). Endothelial cells express TSP-1, influencing processes such as angiogenesis and vascular homeostasis (10). Additionally, macrophages secrete TSP-1, which modulate immune responses in tissue injury (11). Fibroblasts synthesize TSP-2 during tissue repair, influencing fibrosis and tissue integrity (12). As vital matricellular proteins, TSPs are regulated in their expression by various external cues. Transforming growth factor-β (TGF-β) is a key signaling molecule that induces the expression of TSP-1, contributing to the control of tissue remodeling (13). Hypoxia is another crucial trigger and TSP-1 expression is upregulated under hypoxic condition, thereby modulating angiogenesis and tissue repair (14). Furthermore, oxidative stress has been demonstrated to enhance TSP-2 expression, reflecting the protein’s role in response to stress (15). TSPs engage in numerous interactions with diverse molecules, modulating various cellular processes. Cell membrane glycoprotein CD36 is one of the interaction partners. The binding of TSP-1 to CD36 inhibits angiogenesis by inducing endothelial cell apoptosis (16). Another interaction partner is the integrin family of cell adhesion receptors. The interaction between TSPs and integrins such as α3β1 is essential for cell-to-matrix adhesion, migration, and modulation of growth factor signaling (17). TSP-1 affects αvβ3 function by interacting with integrin-associated protein CD47 (18). TSPs also bind to proteoglycans, like syndecan-4, impacting cell adhesion and angiogenesis (19). Additionally, TSP-1’s interaction with TGF-β plays a significant role in the activation of the latent form of TGF-β, which is a crucial mediator in various pathophysiological processes like inflammation and fibrosis (20). TSPs are multifunctional proteins involved in a range of biological processes such as cellular adhesion, migration, and differentiation. TSPs exhibit diversity in their structures, which can influence their functional roles. For instance, TSP-1 and TSP-2 are trimeric, while TSP-3, TSP-4, and TSP-5 are pentameric (21). TSP-1 mediate cellular adhesion by interacting with a variety of cell surface receptors including CD36 and CD47 (16, 22). Migration of cells is a complex process. TSPs play a key role in regulating this process. TSP-1 can modulate the migration of endothelial cells by upregulating metalloproteinase-9 (MMP-9). TSPs inhibits the angiogenesis by changing ECM composition and expression of MMPs (23, 24). TSPs are also critical in cellular differentiation processes. TSP-1 in particular is known to support the differentiation of osteoclasts and Treg cells (25).
Tenascins are a family of matricellular proteins. There are four members of tenasins: tenascin-C, -R, -X, and -W. They are produced by a variety of cell types. Tenascin-C (TNC) is produced by fibroblasts in the stroma of various tumors, and its elevated levels often correlate with tumor aggressiveness (26). Furthermore, in developing and adult nervous tissue, astrocytes and neurons express TNC, implicating its role in neural plasticity (27). Tenascin-R (TNR), predominantly found in the nervous system, is produced by oligodendrocytes and some subsets of neurons, playing a role in regulating neural cell adhesion and neurite outgrowth (28). Tenascin-X (TNX) production is primarily attributed to fibroblasts within the connective tissues, influencing tissue elasticity and structure (29). Tenascin-W (TNW) has been detected in certain types of cancer, suggesting endothelial cells in blood vessels might be its main producers (30). Tenascins respond to an array of external stimuli which modulate their expression levels. For example, cytokines such as TGF-β and TNF-α have been demonstrated to enhance the expression of TNC in fibroblasts, suggesting its involvement in wound healing and inflammatory processes (31, 32). Furthermore, mechanical stress, a key component in tissue remodeling, significantly induces TNC expression in musculoskeletal tissues, pointing to its role in tissue repair and remodeling (33). During brain neural development, nerve growth factor (NGF), augment the production of TNR, highlighting its role in central nervous system (34). The expression of TNX can be induced by brain-derived neurotrophic factor (BDNF) in human endothelial cells, implying its importance in tissue regeneration (35). Given the increased levels of TNW in malignant tumors, bone morphogenic protein (BMP)-2 stimulated the expression of TNW in cells that locate in tumor stroma (36). The tenascins engage in multiple interactions which are pivotal to their diverse biological functions. TNC interacts with fibronectin, thereby influencing cell adhesion and tumor cell proliferation (37). Additionally, TNC has been found to bind with syndecans, modulating cell-matrix adhesions and influencing cell signaling (38). TNR, largely found in the nervous system, interacts with contactin-1, contributing to the modulation of neural cell adhesion and neurite outgrowth (39). While the interaction partners for TNX and TNW are less extensively studied, their interplay with various collagens and integrins in the extracellular matrix is being unraveled, suggesting their roles in tissue structure and integrity (36, 40). Tenascins have been implicated in a variety of biological processes including cellular adhesion, migration, and differentiation. TNC and TNX are among prominent family members contributing to these biological activities. Tenascins, particularly TNC, are recognized as anti-adhesive molecules. They can disrupt adhesion of cells to the extracellular matrix, promoting cell detachment and facilitating cell migration (41). Migration of cells, especially during wound healing and cancer metastasis, is another key cellular process regulated by tenascins (42, 43). Tenascins also play significant roles in cellular differentiation processes. TNC has been implicated in the differentiation of various cell types, including neural progenitor cells and muscle cells (44, 45). TNX, on the other hand, has been associated with differentiation of connective tissues, notably influencing collagen fibrillogenesis (46).
OPN is a multifunctional matricellular protein that plays crucial roles in various physiological and pathological processes. Its name, osteopontin, derived from its initial identification in bone, reflects its critical role in osteogenesis, with osteoblasts and osteoclasts being primary producers (47). However, its expression is not restricted to bone tissues. Many immune cells, including activated T cells, macrophages, and dendritic cells, produce OPN, where it modulates immune responses and inflammatory reactions (48, 49). In the process of wound healing and tissue repair, fibroblasts have been shown to produce OPN (50). Additionally, in pathological contexts such as cancer, tumor cells themselves can upregulate and secrete OPN, promoting tumor progression and metastasis (51). The regulation of OPN expression is intricate, responding to various external stimuli to fulfill its roles in physiological and pathological contexts. Inflammatory cytokines, such as interleukin-1 (IL-1) can enhance OPN expression in dendritic cells, highlighting its involvement in immune and inflammatory responses (52). Hormonal signals, particularly 1,25-dihydroxyvitamin D3 induces OPN expression in osteoblast, linking it to bone metabolism (53). Additionally, growth factors such as TGF-β and platelet-derived growth factor (PDGF) have been identified as upregulators of OPN, indicating its participation in tissue repair (50, 54). OPN exhibits an array of binding interactions that underpin its diverse roles in cellular processes. One of the key interaction partners of OPN is the integrin family. For example, OPN can bind to αvβ1, αvβ3, and αvβ5 in tumor cells (55). Another major binding partner is CD44. The binding of OPN to CD44 variants facilitates migration and invasion of cancer cells, suggesting a potential mechanism by which OPN contributes to tumor progression (56). Calcium binding is another critical interaction for OPN, considering its role in mineralized tissues, and this interaction modulates crystal growth and biomineralization processes (57). Moreover, OPN’s interaction with thrombin creates a cleaved form that exposes cryptic sites, enhancing integrin-binding activity, a process that plays a role in wound healing and tissue remodeling (58). OPN is a multifunctional matricellular protein that can modulate cellular adhesion, migration, and differentiation. OPN has a significant role in cellular adhesion, primarily mediated by its interaction with several integrins such as αvβ3 and α4β1 (59, 60). It can enhance the adhesion of a variety of cells including immune cells, endothelial cells, and tumor cells, contributing to processes such as wound healing, immune response, and tumor metastasis (61, 62). Migration of cells is another process where OPN plays a crucial role. It can stimulate cell migration in various physiological and pathological contexts, including cancer progression (63). Molecular mechanisms of the migration involve OPN-integrin interaction, leading to the activation of downstream signaling pathways such as PI3K/Akt/mTOR pathway (60). Differentiation processes are also influenced by OPN. OPN has been implicated in the differentiation of immune cells, osteoclasts, and adipocytes. It often contributes to pathological conditions such as inflammation, bone diseases, and obesity (64, 65).
The SPARC family includes SPARC (Osteonectin) and other matricellular proteins such as SPARC-like 1 (SPARCL1)/Hevin, and SPARC/osteonectin, cwcv, and kazal-like domains proteoglycan (testican) 1 (SPOCK1) (66). SPARC, a well-investigated member of this family, is produced by various cell types, including osteoblasts, contributing to bone mineralization (67). It is also expressed in fibroblasts, endothelial cells, and certain cancer cells, suggesting its role in wound healing, angiogenesis, and tumor biology (68). SPARCL1 is predominantly found in the brain and is expressed in astrocytes (69). The SPARC family proteins are modulated by a myriad of external signals. For SPARC, TGF-β has been demonstrated as a potent stimulator of its expression in fibroblasts, emphasizing its role in wound healing and fibrosis (70). Furthermore, SPARC expression is elevated by treatment of vascular endothelial growth factor (VEGF) in endothelial cells, implying its involvement in angiogenesis (71). SPARCL1 has been shown to be upregulated in astrocytes in response to injury-induced cues, suggesting its active role in synaptic modifications and neural repair (72). The SPARC family interact with various proteins influencing cellular behavior. SPARC prominently interacts with collagens, including collagen type I, II, III, IV, and V, modulating collagen fibrillogenesis and influencing tissue remodeling (66). It also binds to various integrins such as integrin β1 (73). Additionally, SPARC’s interaction with VEGF hinders angiogenesis, shedding light on its potential anti-tumorigenic properties (74). SPARCL1 forms complexes with the proteoglycan decorin, which is vital for its role in extracellular matrix synthesis and collagen fibril formation (75). These proteins play a critical role in cellular adhesion, migration, and differentiation. Cellular adhesion is largely influenced by the SPARC family. SPARC has been shown to modulate cell-matrix interactions by binding to various ECM proteins such as collagens, vitronectin, and fibrinogen (76, 77). SPARCL1 similarly can inhibit cell adhesion by affecting organization of the ECM (78). In terms of cell migration, SPARC and SPARCL1 can regulate the migratory behavior of various cell types. SPARC influences cell migration by modulating the ECM, activating intracellular signaling pathways and affecting the expression and activity of matrix MMPs (79). Similarly, SPARCL1 influences cell migration by interacting with ECM components and integrins (80). SPARC’s role in differentiation is widely recognized. For example, in bone, SPARC is considered essential for the differentiation of osteoblasts, thereby playing a critical role in bone formation and remodeling (81).
The CCN family (CCN1-6) is a group of cysteine-rich regulatory proteins known to play critical roles in diverse cellular processes. The CCN family was named after the first three members identified, Cyr61 (CCN1), CTGF (CCN2), and NOV (CCN3). It is a group of secreted, cysteine-rich, regulatory proteins. Other members include WISP-1 (CCN4), WISP-2 (CCN5), and WISP-3 (CCN6) (82). CCN1 is produced by a variety of cells, including fibroblasts, endothelial cells, pericytes, and certain cancer cells, playing critical roles in processes like angiogenesis, wound healing, and tumorigenesis (83, 84). CCN2 is prominently synthesized by chondrocytes, fibroblasts, endothelial cells, and vascular smooth muscle cells, highlighting its functions in skeletal development, fibrosis, and vascular biology (85-87). CCN3 is mainly expressed by cells in the vascular system, including vascular smooth muscle cells, as well as certain tumor cells (88, 89). CCN4, CCN5, and CCN6 are expressed in various cells, including those in skeletal tissues and tumors, indicating potential roles in bone biology and oncogenesis (90-92). The CCN family’s expression is influenced by numerous external signals. CCN1 expression is significantly upregulated by growth factors such as PDGF and VEGF, underlining its role in processes like angiogenesis and wound healing (93, 94). CCN2 is upregulated in response to TGF-β in various cell types, especially fibroblasts (95). While CCN3 was identified as a nephroblastoma overexpressed gene, CCN3 has been downregulated by PDGF in mesangial cells of kidney (96). As the WISP means the Wnt1-inducible signaling pathway proteins, CCN4 (WISP-1), CCN5 (WISP-2), and CCN6 (WISP-3) are upregulated in the mouse mammary epithelial cells transformed by Wnt-1 and they are considered to play a pivotal roles in tumorigenesis (97). The CCN proteins are recognized for their ability to interact with a variety of partners. CCN1 has been shown to bind integrins, specifically αvβ3 and α6β1 in endothelial cells (98). CCN2 forms complexes with various molecules, notably the VEGF, modulating angiogenesis, and also with the αvβ3 (99, 100). CCN3 predominantly interacts with integrins like αvβ3 and α5β1 (101). CCN4, CCN5, and CCN6 interact with components of the ECM such as decorin, biglycan, and integrin αvβ3, suggesting their involvement in ECM organization (102, 103). CCN proteins have been widely recognized for their important roles in cellular adhesion, migration, and differentiation. In relation to cellular adhesion, CCN proteins have been found to modulate cell-matrix interactions mainly through binding with various ECM proteins and surface proteins. For example, CCN1 can promote cell adhesion by binding directly to integrins, leading to activation of downstream signaling pathways (104). In terms of cell migration, CCN proteins can act as chemoattractants and induce changes in the cytoskeleton to facilitate migration. Both CCN1 and CCN2 have been reported to promote cell migration in endothelial cells and keratinocytes through their interactions with integrins (98, 105). Finally, CCN proteins contribute to cell differentiation. CCN2 is known for its pivotal role in chondrogenesis and osteogenesis by modulating the differentiation of mesenchymal stem cells into chondrocytes and osteoblasts (106, 107).
The fibulin family comprises seven members: Fibulin-1 to -7. It is another essential group within matricellular proteins (108). They are produced by various cell types, depending on the specific family member and tissue context. Fibulin-1, a widely expressed protein, is expressed in fibroblasts, and smooth muscle cells, playing roles in vascular remodeling and platelet adhesion (109). Fibulin-2 is highly expressed in cardiovascular system, primarily produced by endothelial cells and certain epithelial cells (110, 111). Fibulin-3 is abundantly expressed in epithelial and endothelial cells implicating its role in angiogenesis (112). Expression of fibulin-4 and fibulin-5 is prominent in vascular smooth muscle cells which are integral to elastogenesis (113, 114). Fibulin-6 is prominently expressed in epithelial cells, endothelial cell, and vascular smooth muscle cells (115). Fibulin-7 has been reported to be expressed in endothelial cells, odontoblasts, and chondrocytes in multiple vital tissues (116). The expression of fibulins is sensitive to several external signals. Fibulin-1 expression can be downregulated by TGF-β, a potent regulator of extracellular matrix proteins, particularly in airway smooth muscle cells (117). Meanwhile fibulin-2 expression is enhanced by Angiotensin II together with TGF-β stimulation in cardiac fibroblast, suggesting its role in processes like fibrosis and tissue remodeling (118). Fibulin-3 has its expression increased under pathological conditions, including malignant glioma and cardiac fibroblast with heart failure (119, 120). Fibulin-4 levels are elevated in colon cancer tumors compared to the adjacent normal tissues (121). Fibulin-5 has its expression increased under hypoxic conditions in endothelial cells, suggesting its role in the adaptive survival response (122). Fibulin-6 levels are enhanced in cardiac fibroblasts and fibulin-7 is upregulated in glioblastomas modulating neovascularization (115, 123). The fibulin family interact with partners that orchestrate various cellular functions. Fibulin-1 can bind to ECM proteins, including fibronectin, laminin, and nidogen (124). Fibulin-2 associates with ECM proteins such as aggrecan, versican, and brevican, playing a role in matrix assembly (125). Fibulin-2 also interacts with fibrillin-1, highlighting its role in fiber assembly in some tissues (126). Fibulin-3 binds to elastin precursor, tropoelastin, impacting processes like elastogenesis and tissue remodeling (127). Fibulin-4 also interacts closely with tropoelastin, aiding in the proper assembly of elastic fibers (128). Fibulin-5 binds to elastic fiber components, such as lysyl oxidase-like 1 and fibrillin-1, ensuring correct elastic fiber formation (129). Fibulin-6 can bind to the basement membrane protein nidogen-2 implying an essential role in basement membrane integrity (130). Fibulin-7 interacts with matrix proteins, including fibronectin, dentin sialophosphoprotein, and fibulin-1 (116). Each of these proteins has unique characteristics. However, they share common functions in cellular adhesion, migration, and differentiation. In terms of cellular adhesion, fibulins are crucial as they can facilitate cell-to-ECM interactions. For instance, fibulin-1 can promote cellular adhesion by binding ECM component fibronectin (109). The role of fibulins in cell migration is also well-documented. Fibulin-3 and fibulin-5 can inhibit endothelial cell migration antagonizing angiogenesis (131). Finally, in terms of cell differentiation, fibulin-1 and fibulin-4 play crucial roles in morphogenesis of neural crest-derived structures and elastogenesis, respectively (132, 133).
Overall, these matricellular proteins exert a broad range of regulatory functions on cellular adhesion, migration, and differentiation to respond appropriately to environmental cues. These diverse roles highlight the complexity and versatility of matricellular proteins in tissue homeostasis and disease process.
MATRICELLULAR PROTEINS AND IMMUNOMETABOLISM
Matricellular proteins play a central role in the regulation of immunometabolic processes. The emerging field of immunometabolism investigates the intersection between the immune system and metabolic processes, with matricellular proteins acting as significant contributors. In this review, roles of matricellular proteins in immune and metabolic responses and their possible integration at the systemic level rather than metabolic regulation of immune cell function at the cellular level are mainly described (Table 2).
Table 2.
Summarized roles of matricellular proteins in immunometabolism and tissue homeostasis
| Matricellular proteins | Immunometabolism | Tissue homeostasis |
|---|---|---|
| TSP-1 |
Activates pro-inflammatory macrophage via CD47 (134) Increases adipocyte proliferation and inflammation in obesity (135) Induces mitochondrial dysregulation and cell death in T cell (137) Regulates T cell activation through LRP1 signaling (139) Inhibits dendritic cell activation (140) |
Inhibits angiogenesis in wound healing and tissue regeneration by binding to CD36 (175) Inhibits angiogenesis by impeding VEGF signaling and matrix metalloproteinases (176) Inhibits wound healing by reducing angiogenesis in transgenic mice (177) Promotes angiogenesis by interacting with α3β1 integrin (17) |
| TSP-2 | Inhibits adipocyte proliferation (136) |
Regulates angiogenesis (24) Increases vascular density and accelerates wound healing in KO mice (12, 178) |
| TSP-5 | Regulates structural integrity of cartilage ECM (179) | |
| TNC |
Associates with increased inflammation in obese adipose tissue (141) Activates inflammation via TLR4 on macrophage in arthritic joint disease (142) Blocks the migration of T cell infiltration in tumor (143) |
Interrupts cell-matrix interaction through inhibiting cell-fibronectin interaction (180) Regulates cell migration during wound healing after myocardial injury (181) Contributes to corneal wound healing by enhancing fibronectin expression (182) Upregulated in newly formed blood vessels for vascular remodeling (183) |
| TNX | Maintains tissue elasticity, induces hypermobility of joints and hyperelasticity by deficiency (184) | |
| OPN |
Activates type 1 T cell immune response (48, 144) Induces migration and inflammation of macrophage via CD44 and integrin receptor (145) Associates with increased inflammation and insulin resistance in obese adipose tissue (8, 146) Induces adipocyte insulin resistance and inflammation in differentiated adipocyte (147) Contributes to progression of hepatic inflammation and fibrosis (149) |
Regulates bone formation and resorption by interacting with the αvβ3 integrin receptor on osteoclasts (186) Contributes to skin wound healing by stimulating migration of MSCs (187) Regulates smooth muscle cell activity by inhibiting differentiation in cardiovascular tissues (188) Regulates glial cell activity and modulates phagocytic activity in a brain injury model (189) |
| SPARC |
Upregulated in obese adipose tissue (150) Induces inflammatory response and insulin resistance in obesity and type 2 diabetes (151) Interferes with insulin signaling in adipocyte (152) Inhibits adipogenesis by enhancing beta-catenin signaling (153) Induces adipocyte hypertrophy in HFD-fed KO mice (154) Reduces aging- and obesity-induced weight gain and inflammation in adipocyte KO mice (155, 156) |
Regulates development and differentiation of lens cells (190) Contributes to bone mineralization and collagen binding (81) Involved in formation of the dermal ECM and collagen fibrillogenesis (191) Regulates cutaneous wound healing process (192) |
| SPARCL1 |
Contributes to synaptic plasticity and neuronal tissue homeostasis (193) Regulates corneal wound healing and fibrosis processes (194) |
|
| CCN1 |
Upregulated in steatosis of NASH patients and obese mice and induces inflammation (158) Induces pro-inflammatory macrophage and recruitment of monotype in inflammatory vasculature (159, 160) |
Regulates wound healing and tissue repair, and induces fibroblast senescence by binding integrin α6β1 and heparin sulphate proteoglycans (196) |
| CCN2 |
Upregulated in obesity and associates with inflammation (161) Associates with macrophage-mediated inflammation and insulin resistance in adipose tissue (162) Contributes to T cell differentiation and induction of pro-inflammatory Th17 cell responses (163) |
Contributes to connective tissue formation and wound healing, recruiting Sox2-expressiing progenitor cells and myofibroblast differentiation (197) |
| CCN3 |
Positively associates with obesity in human and upregulated in obese adipose tissue in mouse (164) Inhibits glycolysis in chondrocytes (166) |
Regulates wound healing and tissue repair by skin fibroblast adhesion and chemotaxis through integrin receptors (198) |
| CCN4 | Upregulated in obese adipose tissue in mouse and inhibition of adipogenesis through inactivation of PPARγ (167) | Promotes wound healing by modulating proliferation, migration, and ECM expression in dermal fibroblast (199) |
| Fibulin-1 | Upregulated in diabetes and cardiovascular diseases and downregulated by taking antidiabetic drug (168, 169) |
Contributes to structuring tissues facilitating cell adhesion by binding to fibronectin (201) Contributes to stiffness with aggrecan modulating vascular and cardiac remodeling (202) |
| Fibulin-2 |
Upregulated in obese adipose tissue in mouse (170) Regulates immune dysfunction in bone trauma model (171) |
Regulates mineralized bone formation and bone resorption (203) |
| Fibulin-3 |
Upregulated in osteoarthritis in obese women (172) Upregulated circulating levels in obese middle-aged individuals (173) |
Maintains vascular tissue by acting as a growth factor for arteries and inhibiting MMP-2, MMP-9, and oxidative stress (204) |
| Fibulin-4 | Maintains tissue elasticity by interaction with elastin-cross-linking enzymes (133) | |
| Fibulin-5 | Regulates insulin secretion in pancreatic beta cell through glucokinase-mediated signaling (174) |
TSPs, especially TSP-1 and TSP-2, have been found to play crucial roles in immunometabolism. TSP-1 can activate CD47 on macrophages, affecting pro-inflammatory IL-1β maturation, a critical process for pathogen-induced and metabolic dysregulation-induced inflammation (134). The involvement of TSPs in lipogenesis is also significant. TSP-1 has capabilities to increase proliferation of adipocytes and uptake of fatty acid by adipocytes. It can also modulate inflammation in adipose tissues. Depletion of TSP-1 can protect mice from diet-induced obesity (135). In contrast, TSP-2 can inhibit adipocyte proliferation. TSP-2 knock-out mice show increased weight gain with large amounts of non-visceral adipose tissues (136). TSP-1 and TSP-2 expression can modulate fat storage and release, affecting metabolic syndromes such as obesity and diabetes. Further, TSP-1’s binding with CD47 can induce mitochondrial dysregulation and T cell death (137). As mitochondria are primary sites of cellular energy production, their regulation by these proteins can affect immune cell activation and differentiation, thus modulating the immune response. TSPs also play a role in modulating angiogenesis and inflammation. It has been shown that TSP-1 can regulate mononuclear cell adhesion and migration, causing vascular inflammation in abdominal aortic aneurysm (138). TSP-1 plays a significant role in immune modulation by influencing the activation and function of various immune cells. It has been demonstrated that TSP-1 can regulate motility, adhesion, and activation of T cells through lipoprotein receptor-related protein (LRP1) signaling, thereby modulating immune responses (139). Moreover, TSP-1 can also inhibit dendritic cell activation in an autocrine manner, thus influencing the nature of the initiation of immune responses (140).
Tenascins are known to have critical roles in tissue repair and inflammation. Their regulation of immunometabolic processes is gaining recognition. TNC, the most extensively studied protein of the tenascin family, has been linked to various pathophysiological processes, including immune regulation and metabolic disorders. For example, elevated TNC expression has been observed in visceral adipose tissues of obese individuals. It has been associated with increased ECM remodeling and inflammation (141). TNC can modulate the activation and function of immune cells. TNC is known as an endogenous activator of toll-like receptor 4 (TLR4) on macrophages. It can mediate inflammatory response in arthritic joint disease (142). It also plays a crucial role in immune cell recruitment. TNC can induce CXCL12 and block the migration of infiltrating T lymphocytes (TIL) in tumor microenvironment (143).
OPN plays a crucial role in the immune system by modulating the function of various immune cells. In T cells, OPN can promote the production of IFN-γ and IL-12, driving type 1 T cell immune responses, respectively (48, 144). In macrophages, OPN is involved in the induction of migration, skewing macrophages toward a pro-inflammatory phenotype by interacting with CD44 and integrin receptor (145). In the context of immunometabolism, OPN’s role in adipose tissue inflammation and insulin resistance has been well documented. OPN is upregulated in adipose tissues during obesity. It contributes to the recruitment of pro-inflammatory macrophages, leading to chronic low-grade inflammation and insulin resistance (8, 146). Furthermore, OPN can directly interfere with insulin sensitivity in human differentiated adipocytes, inducing insulin resistance and inflammatory signaling (147). In addition, high levels of OPN have been observed in human atherosclerotic lesions of the aorta (148). Another noteworthy role of OPN in immunometabolism is its involvement in hepatic steatosis and non-alcoholic fatty liver disease (NAFLD). A previous study has shown that OPN expression is increased in these conditions and that OPN contributes to the progression of hepatic inflammation and fibrosis (149).
Roles of SPARC family proteins in immunometabolism have increasingly become an area of focus, especially regarding inflammation, tissue remodeling, and metabolic diseases. Among the SPARC family members, SPARC has been studied extensively. It has been identified as being highly expressed in adipose tissues, particularly under conditions of obesity (150). SPARC contributes significantly to inflammatory response and insulin resistance associated with metabolic disorders such as obesity and type 2 diabetes (151). SPARC’s interaction with insulin signaling has been identified as a critical point of impact. Studies have shown that SPARC can interfere with insulin signaling pathways in adipocytes, leading to a decrease in insulin sensitivity and contributing to systemic insulin resistance, a key feature of metabolic syndrome and type 2 diabetes (152). Moreover, SPARC has been implicated in the regulation of adipogenesis, a process by which pre-adipocytes mature into adipocytes. It has been demonstrated that SPARC can inhibit adipogenesis by enhancing the signaling of beta-catenin, a central player in the Wnt signaling pathway, and the activated Wnt signaling negatively regulates adipocyte differentiation (153). In animal studies, SPARC-deficient mice exhibited enlarged fat pad size and adipocyte hypertrophy upon high-fat diet feeding compared to wild-type mice, suggesting a potential role for SPARC in lipid mobilization (154). Furthermore, SPARC critically influences immunometabolism in aging. Adipocyte-specific SPARC depletion in mice alleviated weight gain and fat mass increase during obesity and aging. Such effects enhanced health span, improved metabolic functions such as insulin sensitivity and glucose tolerance, and reduced inflammation (155, 156). SPARC is also involved in the modulation of inflammatory responses. It has been reported to influence macrophage polarization, thus affecting the balance between pro-inflammatory and anti-inflammatory responses (155, 157).
Recently, an emergent role of the CCN family in immunometabolism, an interface between immune responses and metabolic processes, has come to light. CCN1 is highly expressed in steatosis of NASH (non-alcoholic steatohepatitis) patients and hepatocytes of obese mice (158). Upregulation of CCN1 worsened steatosis and inflammation of NASH mouse model. In addition to its effects on metabolic dysregulation, CCN1 can contribute to the recruitment and activation of immune cells. CCN1 treatment induced pro-inflammatory M1 murine macrophages. CCN1 was responsible for recruitment of Ly6Clow monocytes in inflammatory vasculature (159, 160). These might link immune response, inflammation, and metabolic control. CCN2, a protein implicated in tissue remodeling and fibrosis, shows increased expression in the context of obesity, which is characterized by chronic low-grade inflammation (161). In adipose tissue macrophages, CCN2 upregulation is associated with inflammation and fibrosis, subsequently leading to metabolic dysregulation including insulin resistance (162). C-terminal module of CCN2 also contributes to the differentiation of T cells and induces pro-inflammatory Th17 cell responses, further establishing its role in immune interactions (163). CCN3 plasma levels are positively associated with obesity in human and expression of CCN3 in adipose tissues of high-fat diet fed obese mice is increased (164). CCN3 knockout mice were protected from obesity, insulin resistance, and adipose tissue macrophage-derived inflammation (165). Moreover, there is evidence to suggest CCN3’s involvement in metabolic processes. CCN3 expression was induced by inhibition of glycolysis in chondrocytes, suggesting metabolic regulation of CCN3 (166). CCN4 has been implicated in adipocyte differentiation that is a key aspect of metabolism. CCN4 was upregulated in obese mice models. The increase of CCN4 inhibited adipogenesis through inactivation of PPARγ (167).
In the context of immunometabolic processes, fibulin family’s role is becoming increasingly apparent. Fibulin-1, the first identified member of the fibulin family, has been extensively studied for its role in tissue integrity maintenance. In fact, increased plasma fibulin-1 has been associated with diabetes and cardiovascular diseases. Taking antidiabetic drug metformin attenuated the increase of fibulin-1 levels in type 2 diabetes patients (168, 169). Similarly, fibulin-2 has also been implicated in immunometabolic processes. Increased expression of fibulin-2 has been noted in white adipose tissues of a mouse model in the context of obesity (170). Furthermore, fibulin-2 has been shown to regulate immune dysfunction in bone trauma mouse model, further suggesting a potential role in immune responses (171). Fibulin-3 fragments’ serum levels were associated with osteoarthritis in obese women (172). Increased levels of circulating fibulin-3 are associated with obesity in middle-aged individuals (173). Fibulin-5 expression in pancreatic beta cell is responsible for glucose-stimulated insulin secretion through glucokinase-mediated signaling (174), which is pivotal for maintaining metabolic homeostasis.
In summary, matricellular proteins exhibits a growing importance in the context of immunometabolism (Fig. 1). Comprehensive research is required to elucidate the molecular mechanisms underlying their roles in this intertwined system of immune and metabolic regulation.
Fig. 1.
The interplay of matricellular proteins and immunometabolism. Matricellular proteins play diverse and intricate roles in the field of immunometabolism. The schematic diagram demonstrates the influence of six major matricellular proteins: thrombospondins (TSPs), tenascins (TNs), osteopontin (OPN), SPARC family, CCN family, and the fibulin family. They are involved in crucial metabolic processes, including glucose metabolism, lipid metabolism, and insulin signaling. These proteins also influence the immune response by activating specific immune cell types, such as macrophages, T cells, and dendritic cells, and inducing the recruitment of monocytes. In obesity and NAFLD, the matricellular proteins contribute to immunometabolic regulation in adipose tissue and liver, respectively, which represents the complex interplay and regulatory mechanisms of matricellular proteins within the immune and metabolic systems.
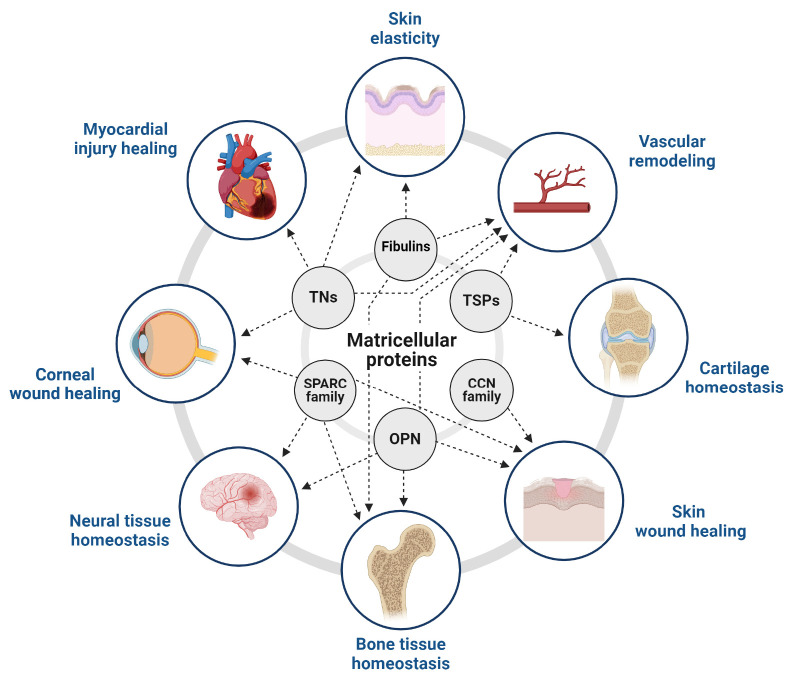
MATRICELLULAR PROTEINS IN TISSUE HOMEOSTASIS
Matricellular proteins play crucial roles in maintaining tissue homeostasis by modulating cell-ECM interactions, cell behavior, and signaling pathways. These proteins can modulate a variety of cellular functions, including cell proliferation, adhesion, migration, and differentiation, thereby maintaining the normal structure and function of various tissues (2).
TSP-1 is a well-known inhibitor of angiogenesis, a critical process in wound healing, tissue regeneration, and various pathological conditions. It exerts its function by binding to the CD36 receptor on endothelial cells, inhibiting their migration, proliferation, and survival (175). Moreover, TSP-1 can impede VEGF signaling and the action of matrix metalloproteinases, thereby inhibiting degradation of the extracellular matrix and reducing angiogenesis (176). TSP-1 can inhibit wound healing process by reducing angiogenesis in TSP-1 overexpressing transgenic mice (177). Interestingly, TSP-1 is not uniformly anti-angiogenic. It can promote angiogenesis by interacting with α3β1 integrin under specific circumstances (17). Other members of the TSP family also play vital roles in tissue homeostasis. For instance, TSP-2, similar to TSP-1, can regulate angiogenesis. It is essential for proper wound healing and tissue repair. TSP-2 null mice show increased vascular density and accelerated wound healing (12, 178). TSP-5 (also known as COMP) contributes to maintaining cartilage homeostasis by regulating structural integrity of the cartilage extracellular matrix crucial for joint function (179).
TNC is primarily expressed in the ECM during embryonic development, inflammation, wound healing, and tumorigenesis. TNC can modulate cell behavior by interrupting cell-matrix interactions, primarily by inhibiting the binding of cells to fibronectin (180). Furthermore, TNC can influence cell migration during wound healing after myocardial injury. It plays a critical role in corneal wound healing by enhancing fibronectin expression (181, 182). Additionally, TNC is involved in angiogenesis. It has been observed that TNC is highly expressed in newly formed blood vessels, indicating its potential role in vascular remodeling, an essential aspect of tissue homeostasis (183). Tenascin-X is widely distributed in connective tissues. It is crucial for maintaining tissue elasticity. Deficiencies in TNX can result in hypermobility of joints and hyperelasticity of the skin, which are characteristic symptoms of the Ehlers-Danlos syndrome (184).
OPN, also known as secreted phosphoprotein 1 (SPP1), is a multifunctional matricellular protein involved in diverse biological processes. OPN plays an instrumental role in maintaining tissue homeostasis through its involvement in cell adhesion, migration, immune response modulation, and ECM remodeling (185). One of the primary sites of OPN activity is the bone tissue, where it is involved in the homeostatic balance between bone formation and resorption. By interacting with the αvβ3 integrin receptor on osteoclasts, OPN can promote cell adhesion, migration, and survival, thereby driving bone resorption (186). In addition to its role in bone homeostasis, OPN has a critical function in the wound healing process. It can stimulate migration and differentiation of mesenchymal stem cells (MSCs) during skin wound repair (187). Moreover, in cardiovascular tissues, OPN has a profound impact on tissue homeostasis as it regulates the activity of vascular smooth muscle cells by inhibiting differentiation and modulates inflammatory responses by inducing accumulation of mononuclear cells (188). In the central nervous system, OPN can influence glial cell activity and modulate phagocytic activity in a brain injury model, contributing to neurological homeostasis (189).
SPARC is widely expressed in various tissues. It exerts its biological effects mainly through interactions with the extracellular matrix and various cell surface receptors (66). SPARC plays a critical role in the development and differentiation of lens cells (190). SPARC, also referred to as osteonectin, plays a crucial role in bone mineralization and collagen binding, thereby having a significant influence on the integrity and function of various tissues. SPARC-deficient mice demonstrate a decreased bone formation, suggesting that SPARC is essential for controlling osteoblast and osteoclast activity (81). SPARC is also important for maintaining skin homeostasis. It is involved in the formation of the dermal ECM. It can also regulate collagen fibrillogenesis. SPARC knockout mice display thinner skin with a disorganized ECM and collagen fibrils, emphasizing its vital role in skin homeostasis (191). Furthermore, SPARC is involved in regulation of cutaneous wound healing process (192). SPARCL1 (Hevin), also known as hevin, has been shown to be involved in tissue remodeling and homeostasis. SPARCL1 has demonstrated involvement in synaptic plasticity, a mechanism essential for neuronal tissue homeostasis. SPARCL1-deficient mice display impaired synaptic plasticity, indicating a critical role in maintaining synaptic homeostasis (193). Moreover, hevin has been identified as a key regulator of corneal wound healing and fibrosis processes intimately tied to tissue homeostasis (194).
CCN family proteins can affect cell-matrix interactions, directly influencing cell behavior and extracellular matrix assembly (195). For instance, CCN1 (CYR61) regulates cell adhesion and migration in a tissue-dependent manner, facilitating wound healing and tissue repair and inducing fibroblast senescence by binding to integrin α6β1 and heparin sulphate proteoglycans (196). CCN2 (CTGF), another member of the CCN family, has been well-studied. It is known to play a central role in tissue repair and fibrosis. CCN2 is essential for connective tissue formation and wound healing, mediating recruitment of Sox2-expressing progenitor cells and myofibroblast differentiation (197). CCN3 (NOV) has been shown to regulate skin fibroblast adhesion and chemotaxis through integrin receptors, which are critical to wound healing and tissue repair processes (198). CCN4 (WISP-1) also contributes to maintaining tissue homeostasis. CCN4 has been found to be upregulated during tissue injury. Previous studies have suggested that CCN4 plays a role in promoting wound healing by modulating proliferation, migration, and ECM expression in dermal fibroblast (199).
The fibulin family proteins contribute to an array of biological functions that underpin tissue integrity, including cell adhesion, migration, proliferation, and differentiation (200). Fibulin-1 facilitates cell adhesion by binding to other ECM components such as fibronectin, thereby enabling structuring of tissues (201). Fibulin-1 has been considered to play a pivotal role in aortic stiffness with aggrecan that modulate the vascular and cardiac remodeling (202). Fibulin-2 contributes significantly to tissue homeostasis. Its influence is particularly prominent within the skeletal system, where it supports tissue development and homeostasis. Genetic deletion of fibulin-2 in murine models leads to enhanced mineralized bone formation and decreased bone resorption (203). Fibulin-3 has a role in the maintenance of vascular tissues. In a hypertensive vascular model, fibluin-3 is considered as a growth factor for arteries. It inhibits MMP-2, MMP-9, and oxidative stress (204). Fibulin-4 play crucial roles in maintaining tissue elasticity by interaction with elastin-cross-linking enzyme (133).
Collectively, matricellular proteins play essential roles in tissue homeostasis across different tissue types (Fig. 2). They contribute to maintaining structural integrity and cellular functions. Dysregulations in their expression or function often result in tissue-specific pathologies, including cancers and fibrosis (205, 206).
Fig. 2.
The role of matricellular proteins in tissue homeostasis. Matricellular proteins maintain tissue homeostasis across various tissue types. The schematic diagram represents the impact of matricellular proteins on essential processes in different tissue types, such as blood vessels, eyes, skin, heart, bone, and brain. These processes include tissue remodeling, tissue repair, angiogenesis, and wound healing, all of which significantly contribute to maintaining tissue homeostasis. The matricellular proteins play different roles in various tissues and contribute to maintaining the equilibrium of the body’s internal environment.
CONCLUSION
The role of matricellular proteins in regulating cell function, tissue homeostasis, and immunometabolism is becoming increasingly evident. These diverse groups of matricellular proteins, including TSPs, TNs, OPN, SPARC family, CCN family, and the fibulin family, play crucial roles in maintaining dynamic interactions between cells and their microenvironment.
Our understanding of the crucial role of matricellular proteins in immunometabolism has grown significantly. They contribute to complex regulation of immune cell behavior and function, including recruitment, activation, and differentiation. Matricellular proteins also exert direct and indirect effects on systemic and cellular metabolism, influencing energy homeostasis and contributing to the fine-tuning of immune responses.
In addition, matricellular proteins play key roles in maintaining tissue homeostasis, influencing processes such as cell adhesion, proliferation, migration, and ECM organization. Any dysregulation in these processes can lead to a wide range of pathologies, further emphasizing the importance of understanding roles of matricellular proteins in tissue homeostasis.
The involvement of matricellular proteins in various pathological conditions such as metabolic disorders, cancer, fibrosis, and inflammatory diseases makes them promising therapeutic targets. Strategies aiming for modulating the activity of these proteins could be beneficial in treating a range of diseases.
ACKNOWLEDGEMENTS
This research was supported by Hallym University Research Fund, 2022 (HRF-202204-008).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
References
- 1.Murphy-Ullrich JE, Sage EH. Revisiting the matricellular concept. Matrix Biol. 2014;37:1–14. doi: 10.1016/j.matbio.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14:608–616. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 3.Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol. 1995;130:503–506. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiodoni C, Colombo MP, Sangaletti S. Matricellular proteins: from homeostasis to inflammation, cancer, and metastasis. Cancer Metastasis Rev. 2010;29:295–307. doi: 10.1007/s10555-010-9221-8. [DOI] [PubMed] [Google Scholar]
- 5.O’Neill LAJ, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16:553–565. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 7.Schellings MWM, Vanhoutte D, Swinnen M, et al. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J Exp Med. 2008;206:113–123. doi: 10.3410/f.1145065.602193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiefer FW, Zeyda M, Gollinger K, et al. Neutralization of osteopontin inhibits obesity-induced inflammation and insulin resistance. Diabetes. 2010;59:935–946. doi: 10.2337/db09-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuijpers MJE, Witt Sd, Nergiz-Unal R, et al. Supporting roles of platelet thrombospondin-1 and CD36 in thrombus formation on collagen. Arterioscler Thromb Vasc Biol. 2014;34:1187–1192. doi: 10.1161/ATVBAHA.113.302917. [DOI] [PubMed] [Google Scholar]
- 10.Phelan MW, Forman LW, Perrine SP, Faller DV. Hypoxia increases thrombospondin-1 transcript and protein in cultured endothelial cells. J Lab Clin Med. 1998;132:519–529. doi: 10.1016/s0022-2143(98)90131-7. [DOI] [PubMed] [Google Scholar]
- 11.DiPietro LA, Nissen NN, Gamelli RL, Koch AE, Pyle JM, Polverini PJ. Thrombospondin 1 synthesis and function in wound repair. Am J Pathol. 1996;148:1851–1860. [PMC free article] [PubMed] [Google Scholar]
- 12.Kyriakides TR, Tam JWY, Bornstein P. Accelerated wound healing in mice with a disruption of the thrombospondin 2 gene. J Invest Dermatol. 1999;113:782–787. doi: 10.1046/j.1523-1747.1999.00755.x. [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa T, Li JH, Garcia G, et al. TGF-β induces proangiogenic and antiangiogenic factorsvia parallel but distinct Smad pathways. Kidney Int. 2004;66:605–613. doi: 10.1111/j.1523-1755.2004.00780.x. [DOI] [PubMed] [Google Scholar]
- 14.Morgan-Rowe L, Nikitorowicz J, Shiwen X, et al. Thrombospondin 1 in hypoxia-conditioned media blocks the growth of human microvascular endothelial cells and is increased in systemic sclerosis tissues. Fibrogenesis Tissue Repair. 2011;4:13. doi: 10.1186/1755-1536-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bae ON, Wang JM, Baek SH, Wang Q, Yuan H, Chen AF. Oxidative stress-mediated thrombospondin-2 upregulation impairs bone marrow-derived angiogenic cell function in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2013;33:1920–1927. doi: 10.1161/ATVBAHA.113.301609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiménez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- 17.Chandrasekaran L, He CZ, Al-Barazi H, Krutzsch HC, Iruela-Arispe ML, Roberts DD. Cell contact-dependent activation of α3β1 integrin modulates endothelial cell responses to thrombospondin-1. Mol Biol Cell. 2000;11:2885–2900. doi: 10.1091/mbc.11.9.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao AG, Lindberg FP, Dimitry JM, Brown EJ, Frazier WA. Thrombospondin modulates alpha v beta 3 function through integrin-associated protein. J Cell Biol. 1996;135:533–544. doi: 10.1083/jcb.135.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nunes SS, Outeiro-Bernstein MA, Juliano L, et al. Syndecan-4 contributes to endothelial tubulogenesis through interactions with two motifs inside the pro-angiogenic N-terminal domain of thrombospondin-1. J Cell Physiol. 2008;214:828–837. doi: 10.1002/jcp.21281. [DOI] [PubMed] [Google Scholar]
- 20.Crawford SE, Stellmach V, Murphy-Ullrich JE, et al. Thrombospondin-1 is a major activator of TGF-β1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 21.Adams JC, Lawler J. The thrombospondins. Cold Spring Harbor Perspect Biol. 2011;3:a009712–a009712. doi: 10.1101/cshperspect.a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao AG, Lindberg FP, Finn MB, Blystone SD, Brown EJ, Frazier WA. Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J Biol Chem. 1996;271:21–24. doi: 10.1074/jbc.271.1.21. [DOI] [PubMed] [Google Scholar]
- 23.Qian X, Wang TN, Rothman VL, Nicosia RF, Tuszynski GP. Thrombospondin-1 modulates angiogenesisin vitroby up-regulation of matrix metalloproteinase-9 in endothelial cells. Exp Cell Res. 1997;235:403–412. doi: 10.1006/excr.1997.3681. [DOI] [PubMed] [Google Scholar]
- 24.Calabro NE, Kristofik NJ, Kyriakides TR. Thrombospondin-2 and extracellular matrix assembly. Biochim Biophys Acta Gen Subj. 2014;1840:2396–2402. doi: 10.1016/j.bbagen.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amend SR, Uluckan O, Hurchla M, et al. Thrombospondin-1 regulates bone homeostasis through effects on bone matrix integrity and nitric oxide signaling in osteoclasts. J Bone Miner Res. 2015;30:106–115. doi: 10.1002/jbmr.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oskarsson T, Acharyya S, Zhang XHF, et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med. 2011;17:867–874. doi: 10.1038/nm.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joester A, Faissner A. The structure and function of tenascins in the nervous system. Matrix Biol. 2001;20:13–22. doi: 10.1016/s0945-053x(00)00136-0. [DOI] [PubMed] [Google Scholar]
- 28.Apostolova I, Irintchev A, Schachner M. Tenascin-R restricts posttraumatic remodeling of motoneuron innervation and functional recovery after spinal cord injury in adult mice. J Neurosci. 2006;26:7849–7859. doi: 10.1523/JNEUROSCI.1526-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakai T, Furukawa Y, Chiquet-Ehrismann R, et al. Tenascin-X expression in tumor cells and fibroblasts: glucocorticoids as negative regulators in fibroblasts. J Cell Sci. 1996;109:2069–2077. doi: 10.1242/jcs.109.8.2069. [DOI] [PubMed] [Google Scholar]
- 30.Martina E, Degen M, Ruegg C, et al. Tenascin-W is a specific marker of glioma-associated blood vessels and stimulates angiogenesis in vitro. FASEB J. 2010;24:778–787. doi: 10.1096/fj.09-140491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berking C, Takemoto R, Schaider H, et al. Transforming growth factor-β1 increases survival of human melanoma through stroma remodeling1. Cancer Res. 2001;61:8306–8316. [PubMed] [Google Scholar]
- 32.Nakamura Y, Esnault Sp, Maeda T, Kelly EAB, Malter JS, Jarjour NN. Ets-1 regulates TNF-α-induced matrix metalloproteinase-9 and tenascin expression in primary bronchial fibroblasts1. J Immunol. 2004;172:1945–1952. doi: 10.4049/jimmunol.172.3.1945. [DOI] [PubMed] [Google Scholar]
- 33.Chiquet M, Gelman L, Lutz R, Maier S. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim Biophys Acta Mol Cell Res. 2009;1793:911–920. doi: 10.1016/j.bbamcr.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Derr LB, McKae LA, Tucker RP. The distribution of tenascin-R in the developing avian nervous system. J Exp Zool. 1998;280:152–164. doi: 10.1002/(sici)1097-010x(19980201)280:2<152::aid-jez6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 35.Takeda K, Shiba H, Mizuno N, et al. Brain-derived neurotrophic factor enhances periodontal tissue regeneration. Tissue Eng. 2005;11:1618–1629. doi: 10.1089/ten.2005.11.1618. [DOI] [PubMed] [Google Scholar]
- 36.Scherberich A, Tucker RP, Degen M, Brown-Luedi M, Andres AC, Chiquet-Ehrismann R. Tenascin-W is found in malignant mammary tumors, promotes alpha8 integrin-dependent motility and requires p38MAPK activity for BMP-2 and TNF-alpha induced expression in vitro. Oncogene. 2005;24:1525–1532. doi: 10.1038/sj.onc.1208342. [DOI] [PubMed] [Google Scholar]
- 37.Huang W, Chiquet-Ehrismann R, Moyano JV, Garcia-Pardo A, Orend G. Interference of tenascin-C with syndecan-4 binding to fibronectin blocks cell adhesion and stimulates tumor cell proliferation1. Cancer Res. 2001;61:8586–8594. [PubMed] [Google Scholar]
- 38.Midwood KS, Valenick LV, Hsia HC, Schwarzbauer JE. Coregulation of fibronectin signaling and matrix contraction by tenascin-c and syndecan-4. Mol Biol Cell. 2004;15:5670–5677. doi: 10.1091/mbc.E04-08-0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zacharias U, Rauch U. Competition and cooperation between tenascin-R, lecticans and contactin 1 regulate neurite growth and morphology. J Cell Sci. 2006;119:3456–3466. doi: 10.1242/jcs.03094. [DOI] [PubMed] [Google Scholar]
- 40.Elefteriou F, Exposito JY, Garrone R, Lethias C. Cell adhesion to tenascin-X. Eur J Biochem. 1999;263:840–848. doi: 10.1046/j.1432-1327.1999.00563.x. [DOI] [PubMed] [Google Scholar]
- 41.Chung CY, Murphy-Ullrich JE, Erickson HP. Mitogenesis, cell migration, and loss of focal adhesions induced by tenascin-C interacting with its cell surface receptor, annexin II. Mol Biol Cell. 1996;7:883–892. doi: 10.1091/mbc.7.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishio T, Kawaguchi S, Yamamoto M, Iseda T, Kawasaki T, Hase T. Tenascin-C regulates proliferation and migration of cultured astrocytes in a scratch wound assay. Neuroscience. 2005;132:87–102. doi: 10.1016/j.neuroscience.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 43.Sun Z, Schwenzer A, Rupp T, et al. Tenascin-C promotes tumor cell migration and metastasis through integrin α9β1-mediated YAP inhibition. Cancer Res. 2018;78:950–961. doi: 10.1158/0008-5472.CAN-17-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faissner A, Roll L, Theocharidis U. Tenascin-C in the matrisome of neural stem and progenitor cells. Mol Cell Neurosci. 2017;81:22–31. doi: 10.1016/j.mcn.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Imanaka-Yoshida K, Aoki H. Tenascin-C and mechanotransduction in the development and diseases of cardiovascular system. Front Physiol. 2014;5:283. doi: 10.3389/fphys.2014.00283.edf2b8c83a904ea98a6d35f3fe286b6b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elefteriou F, Exposito JY, Garrone R, Lethias C. Binding of tenascin-X to decorin. FEBS Lett. 2001;495:44–47. doi: 10.1016/s0014-5793(01)02361-4. [DOI] [PubMed] [Google Scholar]
- 47.Yamate T, Mocharla H, Taguchi Y, Igietseme JU, Manolagas SC, Abe E. Osteopontin expression by osteoclast and osteoblast progenitors in the murine bone marrow: demonstration of its requirement for osteoclastogenesis and its increase after ovariectomy. Endocrinology. 1997;138:3047–3055. doi: 10.1210/endo.138.7.5285. [DOI] [PubMed] [Google Scholar]
- 48.Ashkar S, Weber GF, Panoutsakopoulou V, et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–864. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 49.Shinohara ML, Lu L, Bu J, et al. Osteopontin expression is essential for interferon-α production by plasmacytoid dendritic cells. Nat Immunol. 2006;7:498–506. doi: 10.1038/ni1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mori R, Shaw TJ, Martin P. Molecular mechanisms linking wound inflammation and fibrosis: knockdown of osteopontin leads to rapid repair and reduced scarring. J Exp Med. 2008;205:43–51. doi: 10.1084/jem.20071412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao H, Chen Q, Alam A, et al. The role of osteopontin in the progression of solid organ tumour. Cell Death Dis. 2018;9:356. doi: 10.1038/s41419-018-0391-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schulz G, Renkl AC, Seier A, Liaw L, Weiss JM. Regulated osteopontin expression by dendritic cells decisively affects their migratory capacity. J Invest Dermatol. 2008;128:2541–2544. doi: 10.1038/jid.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prince CW, Butler WT. 1,25-Dihydroxyvitamin D3 regulates the biosynthesis of osteopontin, a bone-derived cell attachment protein, in clonal osteoblast-like osteosarcoma cells. Coll Relat Res. 1987;7:305–313. doi: 10.1016/s0174-173x(87)80036-5. [DOI] [PubMed] [Google Scholar]
- 54.Hullinger TG, Pan Q, Viswanathan HL, Somerman MJ. TGFβ and BMP-2 activation of the OPN promoter: roles of smad- and hox-binding elements. Exp Cell Res. 2001;262:69–74. doi: 10.1006/excr.2000.5074. [DOI] [PubMed] [Google Scholar]
- 55.Maeda N, Ohashi T, Chagan-Yasutan H, et al. Osteopontin-integrin interaction as a novel molecular target for antibody-mediated immunotherapy in adult T-cell leukemia. Retrovirology. 2015;12:99. doi: 10.1186/s12977-015-0225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katagiri YU, Sleeman J, Fujii H, et al. CD44 variants but not CD44s cooperate with β1-containing integrins to permit cells to bind to osteopontin independently of arginine-glycine-aspartic acid, thereby stimulating cell motility and chemotaxis1. Cancer Res. 1999;59:219–226. [PubMed] [Google Scholar]
- 57.Kläning E, Christensen B, Sørensen ES, Vorup-Jensen T, Jensen JK. Osteopontin binds multiple calcium ions with high affinity and independently of phosphorylation status. Bone. 2014;66:90–95. doi: 10.1016/j.bone.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 58.Senger DR, Perruzzi CA, Papadopoulos-Sergiou A, Water LVd. Adhesive properties of osteopontin: regulation by a naturally occurring thrombin-cleavage in close proximity to the GRGDS cell-binding domain. Mol Biol Cell. 1994;5:565–574. doi: 10.1091/mbc.5.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chellaiah MA, Hruska KA. The integrin alpha(v)beta(3) and CD44 regulate the actions of osteopontin on osteoclast motility. Calcif Tissue Int. 2003;72:197–205. doi: 10.1007/s00223-002-1025-6. [DOI] [PubMed] [Google Scholar]
- 60.Zhang H, Guo M, Chen JH, et al. Osteopontin knockdown inhibits αv,β3 integrin-induced cell migration and invasion and promotes apoptosis of breast cancer cells by inducing autophagy and inactivating the PI3K/Akt/mTOR pathway. Cell Physiol Biochem. 2014;33:991–1002. doi: 10.1159/000358670.40983ab2bf704af1a00d34209e6b6160 [DOI] [PubMed] [Google Scholar]
- 61.Zhu B, Suzuki K, Goldberg HA, et al. Osteopontin modulates CD44-dependent chemotaxis of peritoneal macrophages through G-protein-coupled receptors: evidence of a role for an intracellular form of osteopontin. J Cell Physiol. 2004;198:155–167. doi: 10.1002/jcp.10394. [DOI] [PubMed] [Google Scholar]
- 62.Liu J, Liu Q, Wan Y, et al. Osteopontin promotes the progression of gastric cancer through the NF-κB pathway regulated by the MAPK and PI3K. Int J Oncol. 2014;45:282–290. doi: 10.3892/ijo.2014.2393. [DOI] [PubMed] [Google Scholar]
- 63.Weber GF, Ashkar S, Glimcher MJ, Cantor H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1) Science. 1996;271:509–512. doi: 10.1126/science.271.5248.509. [DOI] [PubMed] [Google Scholar]
- 64.Ge Q, Ruan CC, Ma Y, et al. Osteopontin regulates macrophage activation and osteoclast formation in hypertensive patients with vascular calcification. Sci Rep. 2017;7:40253. doi: 10.1038/srep40253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moreno-Viedma V, Tardelli M, Zeyda M, Sibilia M, Burks JD, Stulnig TM. Osteopontin-deficient progenitor cells display enhanced differentiation to adipocytes. Obes Res Clin Pract. 2018;12:277–285. doi: 10.1016/j.orcp.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 66.Bradshaw AD. The role of SPARC in extracellular matrix assembly. J Cell Commun Signaling. 2009;3:239–246. doi: 10.1007/s12079-009-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Termine JD, Kleinman HK, Whitson SW, Conn KM, McGarvey ML, Martin GR. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981;26:99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- 68.Arnold SA, Brekken RA. SPARC: a matricellular regulator of tumorigenesis. J Cell Commun Signaling. 2009;3:255–273. doi: 10.1007/s12079-009-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gan KJ, Südhof TC. SPARCL1 promotes excitatory but not inhibitory synapse formation and function independent of neurexins and neuroligins. J Neurosci. 2020;40:8088–8102. doi: 10.1523/jneurosci.0454-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reed MJ, Vernon RB, Abrass IB, Sage EH. TGF-β1 induces the expression of type I collagen and SPARC, and enhances contraction of collagen gels, by fibroblasts from young and aged donors. J Cell Physiol. 1994;158:169–179. doi: 10.1002/jcp.1041580121. [DOI] [PubMed] [Google Scholar]
- 71.Kato Y, Lewalle JM, Baba Y, et al. Induction of SPARC by VEGF in human vascular endothelial cells. Biochem Biophys Res Commun. 2001;287:422–426. doi: 10.1006/bbrc.2001.5622. [DOI] [PubMed] [Google Scholar]
- 72.McKinnon PJ, Margolskee RF. SC1: a marker for astrocytes in the adult rodent brain is upregulated during reactive astrocytosis. Brain Res. 1996;709:27–36. doi: 10.1016/0006-8993(95)01224-9. [DOI] [PubMed] [Google Scholar]
- 73.Shin M, Mizokami A, Kim J, et al. Exogenous SPARC suppresses proliferation and migration of prostate cancer by interacting with integrin β1. Prostate. 2013;73:1159–1170. doi: 10.1002/pros.22664. [DOI] [PubMed] [Google Scholar]
- 74.Kupprion C, Motamed K, Sage EH. SPARC (BM-40, Osteonectin) inhibits the mitogenic effect of vascular endothelial growth factor on microvascular endothelial cells. J Biol Chem. 1998;273:29635–29640. doi: 10.1074/jbc.273.45.29635. [DOI] [PubMed] [Google Scholar]
- 75.Sullivan MM, Barker TH, Funk SE, et al. Matricellular hevin regulates decorin production and collagen assembly. J Biol Chem. 2006;281:27621–27632. doi: 10.1074/jbc.M510507200. [DOI] [PubMed] [Google Scholar]
- 76.Martinek N, Shahab J, Saathoff M, Ringuette M. Haemocyte-derived SPARC is required for collagen-IV-dependent stability of basal laminae in Drosophila embryos. J Cell Sci. 2008;121:1671–1680. doi: 10.1242/jcs.021931. [DOI] [PubMed] [Google Scholar]
- 77.Wang H, Workman G, Chen S, et al. Secreted protein acidic and rich in cysteine (SPARC/osteonectin/BM-40) binds to fibrinogen fragments D and E, but not to native fibrinogen. Matrix Biol. 2006;25:20–26. doi: 10.1016/j.matbio.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 78.Girard JP, Springer TA. Modulation of endothelial cell adhesion by hevin, an acidic protein associated with high endothelial venules. J Biol Chem. 1996;271:4511–4517. doi: 10.1074/jbc.271.8.4511. [DOI] [PubMed] [Google Scholar]
- 79.McClung HM, Thomas SL, Osenkowski P, et al. SPARC upregulates MT1-MMP expression, MMP-2 activation, and the secretion and cleavage of galectin-3 in U87MG glioma cells. Neurosci Lett. 2007;419:172–177. doi: 10.1016/j.neulet.2007.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y, Liu S, Yan Y, Li S, Tong H. SPARCL1 influences bovine skeletal muscle-derived satellite cell migration and differentiation through an ITGB1-mediated signaling pathway. Animals. 2020;10:1361. doi: 10.3390/ani10081361.cd92af7e6f0845f5b12e18b886aea7db [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Delany AM, Amling M, Priemel M, Howe C, Baron R, Canalis E. Osteopenia and decreased bone formation in osteonectin-deficient mice. J Clin Invest. 2000;105:915–923. doi: 10.1172/JCI7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perbal B. CCN proteins: a centralized communication network. J Cell Commun Signal. 2013;7:169–177. doi: 10.1007/s12079-013-0193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hutchenreuther J, Nguyen J, Quesnel K, et al. Cancer-associated fibroblast-specific expression of the matricellular protein CCN1 coordinates neovascularization and stroma deposition in melanoma metastasis. Cancer Res Commun. 2024;4:556–570. doi: 10.1158/2767-9764.CRC-23-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee S, Elaskandrany M, Lau LF, Lazzaro D, Grant MB, Chaqour B. Interplay between CCN1 and Wnt5a in endothelial cells and pericytes determines the angiogenic outcome in a model of ischemic retinopathy. Sci Rep. 2017;7:1405. doi: 10.1038/s41598-017-01585-8.081b1e69b3a246369c5c4a860c3ee750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takigawa M. CCN2: a master regulator of the genesis of bone and cartilage. J Cell Commun Signal. 2013;7:191–201. doi: 10.1007/s12079-013-0204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaasbøll OJ, Gadicherla AK, Wang JH, et al. Connective tissue growth factor (CCN2) is a matricellular preproprotein controlled by proteolytic activation. J Biol Chem. 2018;293:17953–17970. doi: 10.1074/jbc.RA118.004559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hall-Glenn F, De Young RA, Huang BL, et al. CCN2/connective tissue growth factor is essential for pericyte adhesion and endothelial basement membrane formation during angiogenesis. PLoS One. 2012;7:e30562. doi: 10.1371/journal.pone.0030562.c9188197a81f442bb161d3ab4a6bcd26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ellis PD, Chen Q, Barker PJ, Metcalfe JC, Kemp PR. Nov gene encodes adhesion factor for vascular smooth muscle cells and is dynamically regulated in response to vascular injury. Arterioscler Thromb Vasc Biol. 2000;20:1912–1919. doi: 10.1161/01.atv.20.8.1912. [DOI] [PubMed] [Google Scholar]
- 89.Son S, Kim H, Lim H, Lee Jh, Lee Km, Shin I. CCN3/NOV promotes metastasis and tumor progression via GPNMB-induced EGFR activation in triple-negative breast cancer. Cell Death Dis. 2023;14:81. doi: 10.1038/s41419-023-05608-3.c30e8cd80dff4de691d30fa1d220bb31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Giusti V, Scotlandi K. CCN proteins in the musculoskeletal system: current understanding and challenges in physiology and pathology. J Cell Commun Signal. 2021;15:545–566. doi: 10.1007/s12079-021-00631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Banerjee S, Dhar G, Haque I, et al. CCN5/WISP-2 expression in breast adenocarcinoma is associated with less frequent progression of the disease and suppresses the invasive phenotypes of tumor cells. Cancer Res. 2008;68:7606–7612. doi: 10.1158/0008-5472.CAN-08-1461. [DOI] [PubMed] [Google Scholar]
- 92.Pennica D, Swanson TA, Welsh JW, et al. WISP genes are members of the connective tissue growth factor family that are up-regulated in Wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc Natl Acad Sci U S A. 1998;95:14717–14722. doi: 10.1073/pnas.95.25.14717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang F, Hao F, An D, et al. The matricellular protein Cyr61 is a key mediator of platelet-derived growth factor-induced cell migration. J Biol Chem. 2015;290:8232–8242. doi: 10.1074/jbc.M114.623074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Athanasopoulos AN, Schneider D, Keiper T, et al. Vascular endothelial growth factor (VEGF)-induced Up-regulation of CCN1 in osteoblasts mediates proangiogenic activities in endothelial cells and promotes fracture healing. J Biol Chem. 2007;282:26746–26753. doi: 10.1074/jbc.M705200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grotendorst GR, Okochi H, Hayashi N. A novel transforming growth factor beta response element controls the expression of the connective tissue growth factor gene. Cell Growth Differ. 1996;7:469–480. doi: 10.1007/springerreference_39918. [DOI] [PubMed] [Google Scholar]
- 96.van Roeyen CRC, Eitner F, Scholl T, et al. CCN3 is a novel endogenous PDGF-regulated inhibitor of glomerular cell proliferation. Kidney Int. 2008;73:86–94. doi: 10.1038/sj.ki.5002584. [DOI] [PubMed] [Google Scholar]
- 97.Liu Y, Song Y, Ye M, Hu X, Wang ZP, Zhu X. The emerging role of WISP proteins in tumorigenesis and cancer therapy. J Transl Med. 2019;17:28. doi: 10.1186/s12967-019-1769-7.6c1d38e88b2e401ea589322f6a5ac70e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leu SJ, Lam SCT, Lau LF. Pro-angiogenic activities of CYR61 (CCN1) mediated through Integrins αvβ3 and α6β1 in human umbilical vein endothelial cells. J Biol Chem. 2002;277:46248–46255. doi: 10.1074/jbc.m209288200. [DOI] [PubMed] [Google Scholar]
- 99.Inoki I, Shiomi T, Hashimoto G, et al. Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. FASEB J. 2002;16:1–27. doi: 10.1096/fj.01-0332fje. [DOI] [PubMed] [Google Scholar]
- 100.Gao R, Brigstock DR. Connective tissue growth factor (CCN2) induces adhesion of rat activated hepatic stellate cells by binding of its C-terminal domain to integrin αvβ3 and heparan sulfate proteoglycan. J Biol Chem. 2004;279:8848–8855. doi: 10.1074/jbc.m313204200. [DOI] [PubMed] [Google Scholar]
- 101.Lin CG, Leu SJ, Chen N, et al. CCN3 (NOV) Is a novel angiogenic regulator of the CCN protein family. J Biol Chem. 2003;278:24200–24208. doi: 10.1074/jbc.M302028200. [DOI] [PubMed] [Google Scholar]
- 102.Desnoyers L, Arnott D, Pennica D. WISP-1 binds to decorin and biglycan. J Biol Chem. 2001;276:47599–47607. doi: 10.1074/jbc.M108339200. [DOI] [PubMed] [Google Scholar]
- 103.Myers RB, Wei L, Castellot JJ. The matricellular protein CCN5 regulates podosome function via interaction with integrin αvβ3. J Cell Commun Signal. 2014;8:135–146. doi: 10.1007/s12079-013-0218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen CC, Chen N, Lau LF. The angiogenic factors Cyr61 and connective tissue growth factor induce adhesive signaling in primary human skin fibroblasts. J Biol Chem. 2001;276:10443–10452. doi: 10.1074/jbc.M008087200. [DOI] [PubMed] [Google Scholar]
- 105.Kiwanuka E, Andersson L, Caterson EJ, Junker JPE, Gerdin B, Eriksson E. CCN2 promotes keratinocyte adhesion and migration via integrin α5β1. Exp Cell Res. 2013;319:2938–2946. doi: 10.1016/j.yexcr.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 106.Ivkovic S, Yoon BS, Popoff SN, et al. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130:2779–2791. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Safadi FF, Xu J, Smock SL, et al. Expression of connective tissue growth factor in bone: its role in osteoblast proliferation and differentiation in vitro and bone formation in vivo. J Cell Physiol. 2003;196:51–62. doi: 10.1002/jcp.10319. [DOI] [PubMed] [Google Scholar]
- 108.de Vega S, Iwamoto T, Nakamura T, et al. TM14 is a new member of the fibulin family (fibulin-7) that interacts with extracellular matrix molecules and is active for cell binding. J Biol Chem. 2007;282:30878–30888. doi: 10.1074/jbc.M705847200. [DOI] [PubMed] [Google Scholar]
- 109.Godyna S, Diaz-Ricart M, Argraves WS. Fibulin-1 mediates platelet adhesion via a bridge of fibrinogen. Blood. 1996;88:2569–2577. doi: 10.1182/blood.v88.7.2569.bloodjournal8872569. [DOI] [PubMed] [Google Scholar]
- 110.Ibrahim AM, Sabet S, El-Ghor AA, et al. Fibulin-2 is required for basement membrane integrity of mammary epithelium. Sci Rep. 2018;8:14139. doi: 10.1038/s41598-018-32507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsuda T, Wang H, Timpl R, Chu ML. Fibulin-2 expression marks transformed mesenchymal cells in developing cardiac valves, aortic arch vessels, and coronary vessels. Dev Dynam. 2001;222:89–100. doi: 10.1002/dvdy.1172. [DOI] [PubMed] [Google Scholar]
- 112.McLaughlin PJ, Bakall B, Choi J, et al. Lack of fibulin-3 causes early aging and herniation, but not macular degeneration in mice. Hum Mol Genet. 2007;16:3059–3070. doi: 10.1093/hmg/ddm264. [DOI] [PubMed] [Google Scholar]
- 113.Kowal RC, Richardson JA, Miano JM, Olson EN. EVEC, a novel epidermal growth factor-like repeat-containing protein upregulated in embryonic and diseased adult vasculature. Circ Res. 1999;84:1166–1176. doi: 10.1161/01.res.84.10.1166. [DOI] [PubMed] [Google Scholar]
- 114.Ramnath NWM, Hawinkels LJAC, van Heijningen PM, et al. Fibulin-4 deficiency increases TGF-β signalling in aortic smooth muscle cells due to elevated TGF-β2 levels. Sci Rep. 2015;5:16872. doi: 10.1038/srep16872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chowdhury A, Herzog C, Hasselbach L, et al. Expression of fibulin-6 in failing hearts and its role for cardiac fibroblast migration. Cardiovasc Res. 2014;103:509–520. doi: 10.1093/cvr/cvu161. [DOI] [PubMed] [Google Scholar]
- 116.Chakraborty P, Dash SP, Sarangi PP. The role of adhesion protein fibulin7 in development and diseases. Mol Med. 2020;26:47. doi: 10.1186/s10020-020-00169-z.ed7f6db6ad2743fd8f916ca683f4c143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen L, Ge Q, Black JL, Deng L, Burgess JK, Oliver BGG. Differential regulation of extracellular matrix and soluble fibulin-1 levels by TGF-β1 in airway smooth muscle cells. PLoS One. 2013;8:e65544. doi: 10.1371/journal.pone.0065544.c676cf90d28b4c5ea686dcd152ddcea5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang H, Wu J, Dong H, Khan Shaukat A, Chu ML, Tsuda T. Fibulin-2 deficiency attenuates angiotensin II-induced cardiac hypertrophy by reducing transforming growth factor-β signalling. Clin Sci. 2013;126:275–288. doi: 10.1042/cs20120636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hu B, Thirtamara-Rajamani KK, Sim H, Viapiano MS. Fibulin-3 is uniquely upregulated in malignant gliomas and promotes tumor cell motility and invasion. Mol Cancer Res. 2009;7:1756–1770. doi: 10.1158/1541-7786.MCR-09-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Murtha LA, Hardy SA, Mabotuwana NS, et al. Fibulin-3 is necessary to prevent cardiac rupture following myocardial infarction. Sci Rep. 2023;13:14995. doi: 10.1038/s41598-023-41894-9.a7e2f43b7c7844a0a23cc1ccd8d00927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gallagher WM, Greene LM, Ryan MP, et al. Human fibulin-4: analysis of its biosynthetic processing and mRNA expression in normal and tumour tissues. FEBS Lett. 2001;489:59–66. doi: 10.1016/s0014-5793(00)02389-9. [DOI] [PubMed] [Google Scholar]
- 122.Guadall A, Orriols M, Rodríguez-Calvo R, et al. Fibulin-5 is up-regulated by hypoxia in endothelial cells through a hypoxia-inducible factor-1 (HIF-1α)-dependent mechanism. J Biol Chem. 2011;286:7093–7103. doi: 10.1074/jbc.M110.162917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.de Vega S, Kondo A, Suzuki M, et al. Fibulin-7 is overexpressed in glioblastomas and modulates glioblastoma neovascularization through interaction with angiopoietin-1. Int J Cancer. 2019;145:2157–2169. doi: 10.1002/ijc.32306. [DOI] [PubMed] [Google Scholar]
- 124.Tran H, VanDusen WJ, Argraves WS. The self-association and fibronectin-binding sites of fibulin-1 map to calcium-binding epidermal growth factor-like domains. J Biol Chem. 1997;272:22600–22606. doi: 10.1074/jbc.272.36.22600. [DOI] [PubMed] [Google Scholar]
- 125.Olin AI, Mörgelin M, Sasaki T, Timpl R, Heinegård D, Aspberg A. The proteoglycans aggrecan and versican form networks with fibulin-2 through their lectin domain binding. J Biol Chem. 2001;276:1253–1261. doi: 10.1074/jbc.M006783200. [DOI] [PubMed] [Google Scholar]
- 126.Reinhardt DP, Sasaki T, Dzamba BJ, et al. Fibrillin-1 and fibulin-2 interact and are colocalized in some tissues. J Biol Chem. 1996;271:19489–19496. doi: 10.1074/jbc.271.32.19489. [DOI] [PubMed] [Google Scholar]
- 127.Kobayashi N, Kostka G, Garbe JHO, et al. A comparative analysis of the fibulin protein family: biochemical characterization, binding interactions, and tissue localization. J Biol Chem. 2007;282:11805–11816. doi: 10.1074/jbc.M611029200. [DOI] [PubMed] [Google Scholar]
- 128.Papke CL, Yanagisawa H. Fibulin-4 and fibulin-5 in elastogenesis and beyond: insights from mouse and human studies. Matrix Biol. 2014;37:142–149. doi: 10.1016/j.matbio.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yanagisawa H, Schluterman MK, Brekken RA. Fibulin-5, an integrin-binding matricellular protein: its function in development and disease. J Cell Comm Signal. 2009;3:337–347. doi: 10.1007/s12079-009-0065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang JL, Richetti S, Ramezani T, et al. Vertebrate extracellular matrix protein hemicentin-1 interacts physically and genetically with basement membrane protein nidogen-2. Matrix Biol. 2022;112:132–154. doi: 10.1016/j.matbio.2022.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Albig AR, Neil JR, Schiemann WP. Fibulins 3 and 5 antagonize tumor angiogenesis in vivo. Cancer Res. 2006;66:2621–2629. doi: 10.1158/0008-5472.CAN-04-4096. [DOI] [PubMed] [Google Scholar]
- 132.Cooley MA, Kern CB, Fresco VM, et al. Fibulin-1 is required for morphogenesis of neural crest-derived structures. Dev Biol. 2008;319:336–345. doi: 10.1016/j.ydbio.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Horiguchi M, Inoue T, Ohbayashi T, et al. Fibulin-4 conducts proper elastogenesis via interaction with cross-linking enzyme lysyl oxidase. Proc Natl Acad Sci U S A. 2009;106:19029–19034. doi: 10.1073/pnas.0908268106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stein EV, Miller TW, Ivins-O’Keefe K, Kaur S, Roberts DD. Secreted thrombospondin-1 regulates macrophage interleukin-1β production and activation through CD47. Sci Rep. 2016;6:19684. doi: 10.1038/srep19684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kong P, Gonzalez-Quesada C, Li N, Cavalera M, Lee DW, Frangogiannis NG. Thrombospondin-1 regulates adiposity and metabolic dysfunction in diet-induced obesity enhancing adipose inflammation and stimulating adipocyte proliferation. Am J Physiol Endocrinol Metab. 2013;305:E439–E450. doi: 10.1152/ajpendo.00006.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shitaye HS, Terkhorn SP, Combs JA, Hankenson KD. Thrombospondin-2 is an endogenous adipocyte inhibitor. Matrix Biol. 2010;29:549–556. doi: 10.1016/j.matbio.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Manna PP, Frazier WA. The mechanism of CD47-dependent killing of t cells: heterotrimeric Gi-dependent inhibition of protein kinase A1. J Immun. 2003;170:3544–3553. doi: 10.4049/jimmunol.170.7.3544. [DOI] [PubMed] [Google Scholar]
- 138.Liu Z, Morgan S, Ren J, et al. Thrombospondin-1 (TSP1) contributes to the development of vascular inflammation by regulating monocytic cell motility in mouse models of abdominal aortic aneurysm. Circ Res. 2015;117:129–141. doi: 10.1161/circresaha.117.305262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bergström SE, Bergdahl E, Sundqvist KG. A cytokine-controlled mechanism for integrated regulation of T-lymphocyte motility, adhesion and activation. Immunology. 2013;140:441–455. doi: 10.1111/imm.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Doyen V, Rubio M, Braun D, et al. Thrombospondin 1 is an autocrine negative regulator of human dendritic cell activation. J Exp Med. 2003;198:1277–1283. doi: 10.1084/jem.20030705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Catalán V, Gómez-Ambrosi J, Rodríguez A, et al. Increased tenascin C and toll-like receptor 4 levels in visceral adipose tissue as a link between inflammation and extracellular matrix remodeling in obesity. J Clin Endocrinol Metab. 2012;97:E1880–E1889. doi: 10.1210/jc.2012-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Midwood K, Sacre S, Piccinini AM, et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15:774–780. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- 143.Murdamoothoo D, Sun Z, Yilmaz A, et al. Tenascin-C immobilizes infiltrating T lymphocytes through CXCL12 promoting breast cancer progression. EMBO Mol Med. 2021;13:e13270. doi: 10.15252/emmm.202013270.b3b3bbcf9e5c4240a68764406229c8d2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Koguchi Y, Kawakami K, Uezu K, et al. High plasma osteopontin level and its relationship with interleukin-12-mediated type 1 T helper cell response in tuberculosis. Am J Respir Crit Care Med. 2003;167:1355–1359. doi: 10.1164/rccm.200209-1113oc. [DOI] [PubMed] [Google Scholar]
- 145.Weber GF, Zawaideh S, Hikita S, Kumar VA, Cantor H, Ashkar S. Phosphorylation-dependent interaction of osteopontin with its receptors regulates macrophage migration and activation. J Leukoc Biol. 2002;72:752–761. doi: 10.1189/jlb.72.4.752. [DOI] [PubMed] [Google Scholar]
- 146.Nomiyama T, Perez-Tilve D, Ogawa D, et al. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J Clin Invest. 2007;117:2877–2888. doi: 10.1172/JCI31986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zeyda M, Gollinger K, Todoric J, et al. Osteopontin is an activator of human adipose tissue macrophages and directly affects adipocyte function. Endocrinology. 2011;152:2219–2227. doi: 10.1210/en.2010-1328. [DOI] [PubMed] [Google Scholar]
- 148.Ikeda T, Shirasawa T, Esaki Y, Yoshiki S, Hirokawa K. Osteopontin mRNA is expressed by smooth muscle-derived foam cells in human atherosclerotic lesions of the aorta. J Clin Invest. 1993;92:2814–2820. doi: 10.1172/JCI116901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Syn WK, Choi SS, Liaskou E, et al. Osteopontin is induced by hedgehog pathway activation and promotes fibrosis progression in nonalcoholic steatohepatitis. Hepatology. 2011;53:106–115. doi: 10.1002/hep.23998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tartare-Deckert S, Chavey C, Monthouel MN, Gautier N, Van Obberghen E. The matricellular protein SPARC/osteonectin as a newly identified factor up-regulated in obesity. J Biol Chem. 2001;276:22231–22237. doi: 10.1074/jbc.M010634200. [DOI] [PubMed] [Google Scholar]
- 151.Kos K, Wilding JPH. SPARC: a key player in the pathologies associated with obesity and diabetes. Nat Rev Endocrinol. 2010;6:225–235. doi: 10.1038/nrendo.2010.18. [DOI] [PubMed] [Google Scholar]
- 152.Shen Y, Zhao Y, Yuan L, et al. SPARC is over-expressed in adipose tissues of diet-induced obese rats and causes insulin resistance in 3T3-L1 adipocytes. Acta Histochem. 2014;116:158–166. doi: 10.1016/j.acthis.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 153.Nie J, Sage EH. SPARC inhibits adipogenesis by its enhancement of β-catenin signaling. J Biol Chem. 2009;284:1279–1290. doi: 10.1074/jbc.M808285200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bradshaw AD, Graves DC, Motamed K, Sage EH. SPARC-null mice exhibit increased adiposity without significant differences in overall body weight. Proc Natl Acad Sci U S A. 2003;100:6045–6050. doi: 10.1073/pnas.1030790100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ryu S, Sidorov S, Ravussin E, et al. The matricellular protein SPARC induces inflammatory interferon-response in macrophages during aging. Immunity. 2022;55:1609–1626.e7. doi: 10.1016/j.immuni.2022.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ryu S, Spadaro O, Sidorov S, et al. Reduction of SPARC protects mice against NLRP3 inflammasome activation and obesity. J Clin Invest. 2023;133:e169173. doi: 10.1172/JCI169173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Toba H, Brás LEdC, Baicu CF, Zile MR, Lindsey ML, Bradshaw AD. Secreted protein acidic and rich in cysteine facilitates age-related cardiac inflammation and macrophage M1 polarization. Am J Physiol Cell Physiol. 2015;308:C972–C982. doi: 10.1152/ajpcell.00402.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ju L, Sun Y, Xue H, et al. CCN1 promotes hepatic steatosis and inflammation in non-alcoholic steatohepatitis. Sci Rep. 2020;10:3201. doi: 10.1038/s41598-020-60138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bai T, Chen CC, Lau LF. Matricellular protein CCN1 activates a proinflammatory genetic program in murine macrophages. J Immunol. 2010;184:3223–3232. doi: 10.4049/jimmunol.0902792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Imhof BA, Jemelin S, Ballet R, et al. CCN1/CYR61-mediated meticulous patrolling by Ly6Clow monocytes fuels vascular inflammation. Proc Natl Acad Sci U S A. 2016;113:E4847–4856. doi: 10.1073/pnas.1607710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tan JTM, McLennan SV, Williams PF, et al. Connective tissue growth factor/CCN-2 is upregulated in epididymal and subcutaneous fat depots in a dietary-induced obesity model. Am J Physiol Endocrinol Metab. 2013;304:E1291–E1302. doi: 10.1152/ajpendo.00654.2012. [DOI] [PubMed] [Google Scholar]
- 162.Spencer M, Yao-Borengasser A, Unal R, et al. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am J Physiol Endocrinol Metab. 2010;299:E1016–E1027. doi: 10.1152/ajpendo.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rodrigues-Díez R, Rodrigues-Díez RR, Rayego-Mateos S, et al. The C-terminal module IV of connective tissue growth factor is a novel immune modulator of the Th17 response. Lab Invest. 2013;93:812–824. doi: 10.1038/labinvest.2013.67. [DOI] [PubMed] [Google Scholar]
- 164.Pakradouni J, Le Goff W, Calmel C, et al. Plasma NOV/CCN3 levels are closely associated with obesity in patients with metabolic disorders. PLoS One. 2013;8:e66788. doi: 10.1371/journal.pone.0066788.b2e4f187d15a43ef969468e840ebe89c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Martinerie C, Garcia M, Do TTH, et al. NOV/CCN3: a new adipocytokine involved in obesity-associated insulin resistance. Diabetes. 2016;65:2502–2515. doi: 10.2337/db15-0617. [DOI] [PubMed] [Google Scholar]
- 166.Akashi S, Nishida T, El-Seoudi A, Takigawa M, Iida S, Kubota S. Metabolic regulation of the CCN family genes by glycolysis in chondrocytes. J Cell Commun Signal. 2018;12:245–252. doi: 10.1007/s12079-017-0420-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ferrand N, Béreziat V, Moldes M, Zaoui M, Larsen AK, Sabbah M. WISP1/CCN4 inhibits adipocyte differentiation through repression of PPARγ activity. Sci Rep. 2017;7:1749. doi: 10.1038/s41598-017-01866-2.5b0ca59d5a4540bd99b82b8a5908c4f0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Holmager P, Egstrup M, Gustafsson I, et al. Galectin-3 and fibulin-1 in systolic heart failure - relation to glucose metabolism and left ventricular contractile reserve. BMC Cardiovasc Disord. 2017;17:22. doi: 10.1186/s12872-016-0437-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Skov V, Cangemi C, Gram J, et al. Metformin, but not rosiglitazone, attenuates the increasing plasma levels of a new cardiovascular marker, fibulin-1, in patients with type 2 diabetes. Diabetes Care. 2014;37:760–766. doi: 10.2337/dc13-1022. [DOI] [PubMed] [Google Scholar]
- 170.Nadler ST, Stoehr JP, Schueler KL, Tanimoto G, Yandell BS, Attie AD. The expression of adipogenic genes is decreased in obesity and diabetes mellitus. Proc Natl Acad Sci U S A. 2000;97:11371–11376. doi: 10.1073/pnas.97.21.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li S, Jiang H, Wang S, et al. Fibulin-2: a potential regulator of immune dysfunction after bone trauma. Immun Inflamm Dis. 2023;11:e846. doi: 10.1002/iid3.846.b45c42bd31b44491a2bf35895ab1aa20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Runhaar J, Sanchez C, Taralla S, Henrotin Y, Bierma-Zeinstra SMA. Fibulin-3 fragments are prognostic biomarkers of osteoarthritis incidence in overweight and obese women. Osteoarthr Cartil. 2016;24:672–678. doi: 10.1016/j.joca.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 173.Lau ES, Paniagua SM, Zarbafian S, et al. Cardiovascular biomarkers of obesity and overlap with cardiometabolic dysfunction. J Am Heart Assoc. 2021;10:e020215. doi: 10.1161/JAHA.120.020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Okuyama T, Shirakawa J, Yanagisawa H, et al. Identification of the matricellular protein Fibulin-5 as a target molecule of glucokinase-mediated calcineurin/NFAT signaling in pancreatic islets. Sci Rep. 2017;7:2364. doi: 10.1038/s41598-017-02535-0.2101103c43844a20b6c04abcf16cd35b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chu LY, Ramakrishnan DP, Silverstein RL. Thrombospondin-1 modulates VEGF signaling via CD36 by recruiting SHP-1 to VEGFR2 complex in microvascular endothelial cells. Blood. 2013;122:1822–1832. doi: 10.1182/blood-2013-01-482315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rodríguez-Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela-Arispe ML. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci U S A. 2001;98:12485–12490. doi: 10.1073/pnas.171460498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Streit M, Velasco P, Riccardi L, et al. Thrombospondin-1 suppresses wound healing and granulation tissue formation in the skin of transgenic mice. EMBO J. 2000;19:3272–3282. doi: 10.1093/emboj/19.13.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kyriakides TR, Zhu YH, Smith LT, et al. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J Cell Biol. 1998;140:419–430. doi: 10.1083/jcb.140.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hecht JT, Nelson LD, Crowder E, et al. Mutations in exon 17B of cartilage oligomeric matrix protein (COMP) cause pseudoachondroplasia. Nat Genet. 1995;10:325–329. doi: 10.1038/ng0795-325. [DOI] [PubMed] [Google Scholar]
- 180.Midwood KS, Hussenet T, Langlois B, Orend G. Advances in tenascin-C biology. Cell Mol Life Sci. 2011;68:3175–3199. doi: 10.1007/s00018-011-0783-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tamaoki M, Imanaka-Yoshida K, Yokoyama K, et al. Tenascin-C regulates recruitment of myofibroblasts during tissue repair after myocardial injury. Am J Pathol. 2005;167:71–80. doi: 10.1016/S0002-9440(10)62954-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Matsuda A, Yoshiki A, Tagawa Y, Matsuda H, Kusakabe M. Corneal wound healing in tenascin knockout mouse. Invest Ophthalmol Vis Sci. 1999;40:1071–1080. [PubMed] [Google Scholar]
- 183.Imanaka-Yoshida K, Yoshida T, Miyagawa-Tomita S. Tenascin-C in development and disease of blood vessels. Anat Rec. 2014;297:1747–1757. doi: 10.1002/ar.22985. [DOI] [PubMed] [Google Scholar]
- 184.Zweers MC, Bristow J, Steijlen PM, et al. Haploinsufficiency of TNXB is associated with hypermobility type of ehlers-danlos syndrome. Am J Hum Genet. 2003;73:214–217. doi: 10.1086/376564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Icer MA, Gezmen-Karadag M. The multiple functions and mechanisms of osteopontin. Clin Biochem. 2018;59:17–24. doi: 10.1016/j.clinbiochem.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 186.Ross FP, Chappel J, Alvarez JI, et al. Interactions between the bone matrix proteins osteopontin and bone sialoprotein and the osteoclast integrin alpha v beta 3 potentiate bone resorption. J Biol Chem. 1993;268:9901–9907. doi: 10.1016/s0021-9258(18)98430-9. [DOI] [PubMed] [Google Scholar]
- 187.Wang W, Li P, Li W, et al. Osteopontin activates mesenchymal stem cells to repair skin wound. PLoS One. 2017;12:e0185346. doi: 10.1371/journal.pone.0185346.b14f33ed3ab04d3aaf53f56ce09595cd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gao H, Steffen MC, Ramos KS. Osteopontin regulates α-smooth muscle actin and calponin in vascular smooth muscle cells. Cell Biol Int. 2012;36:155–161. doi: 10.1042/CBI20100240. [DOI] [PubMed] [Google Scholar]
- 189.Riew TR, Kim S, Jin X, Kim HL, Lee JH, Lee MY. Osteopontin and its spatiotemporal relationship with glial cells in the striatum of rats treated with mitochondrial toxin 3-nitropropionic acid: possible involvement in phagocytosis. J Neuroinflamm. 2019;16:99. doi: 10.1186/s12974-019-1489-1.21cc3440ba5e4526ab0e3eac029c28a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Norose K, Clark JI, Syed NA, et al. SPARC deficiency leads to early-onset cataractogenesis. Invest Ophthalmol Vis Sci. 1998;39:2674–2680. [PubMed] [Google Scholar]
- 191.Rentz TJ, Poobalarahi F, Bornstein P, Sage EH, Bradshaw AD. SPARC regulates processing of procollagen I and collagen fibrillogenesis in dermal fibroblasts. J Biol Chem. 2007;282:22062–22071. doi: 10.1074/jbc.M700167200. [DOI] [PubMed] [Google Scholar]
- 192.Reed MJ, Puolakkainen P, Lane TF, Dickerson D, Bornstein P, Sage EH. Differential expression of SPARC and thrombospondin 1 in wound repair: immunolocalization and in situ hybridization. J Histochem Cytochem. 1993;41:1467–1477. doi: 10.1177/41.10.8245406. [DOI] [PubMed] [Google Scholar]
- 193.Kucukdereli H, Allen NJ, Lee AT, et al. Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc Natl Acad Sci U S A. 2011;108:E440–E449. doi: 10.1073/pnas.1104977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chaurasia SS, Perera PR, Poh R, Lim RR, Wong TT, Mehta JS. Hevin plays a pivotal role in corneal wound healing. PLoS One. 2013;8:e81544. doi: 10.1371/journal.pone.0081544.0b4d4b55138a4dc7ae983419ece8b199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chen CC, Lau LF. Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol. 2009;41:771–783. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010;12:676–685. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tsang M, Leask A. CCN2 is required for recruitment of Sox2-expressing cells during cutaneous tissue repair. J Cell Commun Signal. 2015;9:341–346. doi: 10.1007/s12079-014-0245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lin CG, Chen CC, Leu SJ, Grzeszkiewicz TM, Lau LF. Integrin-dependent functions of the angiogenic inducer NOV (CCN3): implication in wound healing. J Biol Chem. 2005;280:8229–8237. doi: 10.1074/jbc.M404903200. [DOI] [PubMed] [Google Scholar]
- 199.Ono M, Masaki A, Maeda A, et al. CCN4/WISP1 controls cutaneous wound healing by modulating proliferation, migration and ECM expression in dermal fibroblasts via α5β1 and TNFα. Matrix Biol 68- 2018;69:533–546. doi: 10.1016/j.matbio.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Argraves WS, Tran H, Burgess WH, Dickerson K. Fibulin is an extracellular matrix and plasma glycoprotein with repeated domain structure. J Cell Biol. 1990;111:3155–3164. doi: 10.1083/jcb.111.6.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Balbona K, Tran H, Godyna S, Ingham KC, Strickland DK, Argraves WS. Fibulin binds to itself and to the carboxyl-terminal heparin-binding region of fibronectin. J Biol Chem. 1992;267:20120–20125. doi: 10.1016/s0021-9258(19)88674-x. [DOI] [PubMed] [Google Scholar]
- 202.Yasmin, Maskari RA, McEniery CM, et al. The matrix proteins aggrecan and fibulin-1 play a key role in determining aortic stiffness. Sci Rep. 2018;8:8550. doi: 10.1038/s41598-018-25851-5.f4f9722e6a394997bed126349eb17c39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Li SD, Xing W, Wang SC, et al. Fibulin2: a negative regulator of BMSC osteogenic differentiation in infected bone fracture healing. Exp Mol Med. 2023;55:443–456. doi: 10.1038/s12276-023-00942-0.0d39ed4ab10545e6b03e4cf73e4a7980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lin Z, Wang Z, Li G, Li B, Xie W, Xiang D. Fibulin-3 may improve vascular health through inhibition of MMP-2/9 and oxidative stress in spontaneously hypertensive rats. Mol Med Rep. 2016;13:3805–3812. doi: 10.3892/mmr.2016.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gerarduzzi C, Hartmann U, Leask A, Drobetsky E. The matrix revolution: matricellular proteins and restructuring of the cancer microenvironment. Cancer Res. 2020;80:2705–2717. doi: 10.1158/0008-5472.CAN-18-2098. [DOI] [PubMed] [Google Scholar]
- 206.Prakoura N, Chatziantoniou C. Matricellular proteins and organ fibrosis. Curr Pathobiol Rep. 2017;5:111–121. doi: 10.1007/s40139-017-0138-6. [DOI] [Google Scholar]