Abstract
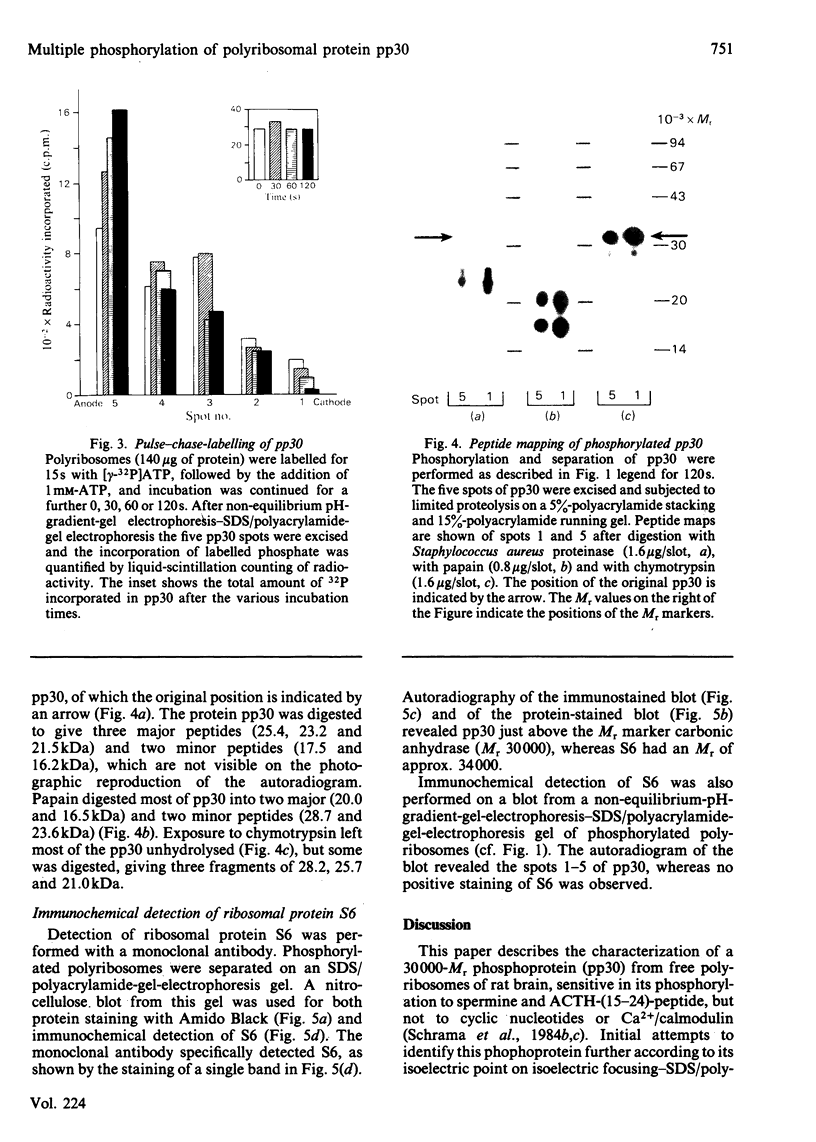
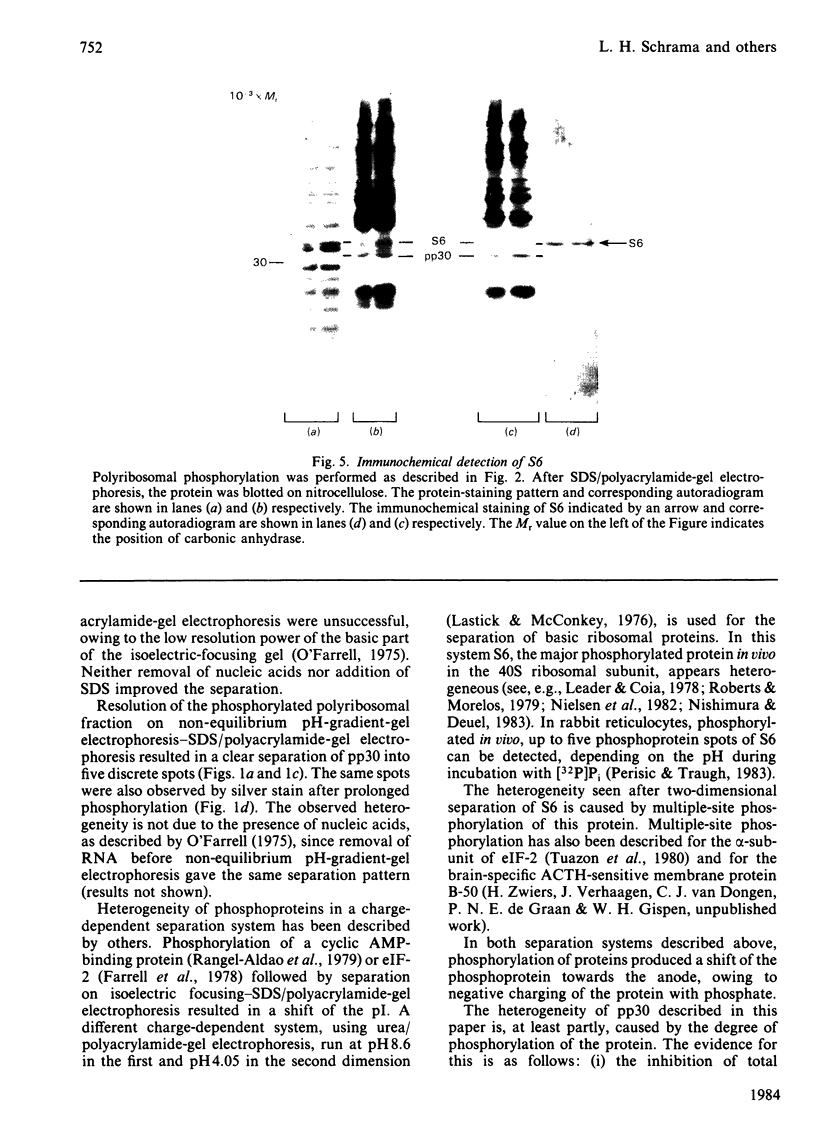
A rat brain polyribosomal protein with an apparent Mr of 30 000, designated pp30, was further characterized. The protein was identified by its phosphorylation by an endogenous protein kinase sensitive to both corticotropin and spermine. Two-dimensional separation of a polyribosomal fraction was applied, combining non-equilibrium pH-gradient-gel electrophoresis in the first and sodium dodecyl sulphate/polyacrylamide-gel electrophoresis in the second dimension. In this system, pp30 was separated into at least five defined phosphoprotein spots. Pulse-labelling with [gamma-32P]ATP followed by a chase for various time periods with excess unlabelled ATP resulted in a shift of the distribution of radioactivity and protein staining along the spots towards the anode. This suggests that the various spots of pp30 may represent multiple phosphorylation states. Limited proteolysis of the five spots with three different proteinases resulted in the same one-dimensional peptide maps with a given proteinase, indicating that all five spots represent different forms of a single phosphoprotein. Inhibition of the overall phosphorylation of pp30 by corticotropin or spermine was accompanied by a shift in the recovery of labelled phosphate towards spots nearer the cathode. Immunoblotting with monoclonal antibodies directed against ribosomal protein S6 stained only one band, a protein that had an apparent Mr of 34 000 and was clearly distinct from pp30.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckel P., Zehelein E. Expression of Pseudomonas fluorescens D-galactose dehydrogenase in E. coli. Gene. 1981 Dec;16(1-3):149–159. doi: 10.1016/0378-1119(81)90071-8. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Farrell P. J., Hunt T., Jackson R. J. Analysis of phosphorylation of protein synthesis initiation factor eIF-2 by two-dimensional gel electrophoresis. Eur J Biochem. 1978 Sep 1;89(2):517–521. doi: 10.1111/j.1432-1033.1978.tb12556.x. [DOI] [PubMed] [Google Scholar]
- Lastick S. M., McConkey E. H. Exchange and stability of HeLa ribosomal proteins in vivo. J Biol Chem. 1976 May 25;251(10):2867–2875. [PubMed] [Google Scholar]
- Leader D. P., Coia A. A. The phosphorylation of ribosomal protein S6 on the monoribosomes and polyribosomes of baby hamster kidney fibroblasts. FEBS Lett. 1978 Jun 15;90(2):270–274. doi: 10.1016/0014-5793(78)80383-4. [DOI] [PubMed] [Google Scholar]
- Martin-Pérez J., Thomas G. Ordered phosphorylation of 40S ribosomal protein S6 after serum stimulation of quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Feb;80(4):926–930. doi: 10.1073/pnas.80.4.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen P. J., Manchester K. L., Towbin H., Gordon J., Thomas G. The phosphorylation of ribosomal protein S6 in rat tissues following cycloheximide injection, in diabetes, and after denervation of diaphragm. A simple immunological determination of the extent of S6 phosphorylation on protein blots. J Biol Chem. 1982 Oct 25;257(20):12316–12321. [PubMed] [Google Scholar]
- Nishimura J., Deuel T. F. Platelet-derived growth factor stimulates the phosphorylation of ribosomal protein S6. FEBS Lett. 1983 May 30;156(1):130–134. doi: 10.1016/0014-5793(83)80263-4. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Perisic O., Traugh J. A. Protease-activated kinase II mediates multiple phosphorylation of ribosomal protein S6 in reticulocytes. J Biol Chem. 1983 Nov 25;258(22):13998–14002. [PubMed] [Google Scholar]
- Rangel-Aldao R., Kupiec J. W., Rosen O. M. Resolution of the phosphorylated and dephosphorylated cAMP-binding proteins of bovine cardiac muscle by affinity labeling and two-dimensional electrophoresis. J Biol Chem. 1979 Apr 10;254(7):2499–2508. [PubMed] [Google Scholar]
- Roberts S., Ashby D. Ribosomal protein phosphorylation in rat cerebral cortex in vitro. Influence of cyclic adenosine 3':5'-monophosphate. J Biol Chem. 1978 Jan 10;253(1):288–296. [PubMed] [Google Scholar]
- Roberts S., Morelos B. S. Phosphorylation of multiple proteins of both ribosomal subunits in rat cerebral cortex in vivo. Effect of adenosine 3':5'-cyclic monophosphate. Biochem J. 1979 Nov 15;184(2):233–244. doi: 10.1042/bj1840233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrama L. H., Edwards P. M., Schotman P. Modulation of protein synthesis in a cell-free system derived from rat brain by corticotropin (ACTH), magnesium, and spermine. J Neurosci Res. 1984;11(1):67–77. doi: 10.1002/jnr.490110108. [DOI] [PubMed] [Google Scholar]
- Tuazon P. T., Merrick W. C., Traugh J. A. Site-specific phosphorylation if initiation factor 2 by three cyclic nucleotide-independent protein kinases. J Biol Chem. 1980 Nov 25;255(22):10954–10958. [PubMed] [Google Scholar]
- Van Dijk A. M., King G. B., Schotman P., Gispen W. H. GTP-sensitive phosphorylation of proteins in a postmitochondrial supernatant from rat brainstem affected by ACTH1-24. Neurochem Res. 1981 Aug;6(8):847–861. doi: 10.1007/BF00965043. [DOI] [PubMed] [Google Scholar]
- Zwiers H., Schotman P., Gispen W. H. Purification and some characteristics of an ACTH-sensitive protein kinase and its substrate protein in rat brain membranes. J Neurochem. 1980 Jun;34(6):1689–1699. doi: 10.1111/j.1471-4159.1980.tb11262.x. [DOI] [PubMed] [Google Scholar]





