Abstract
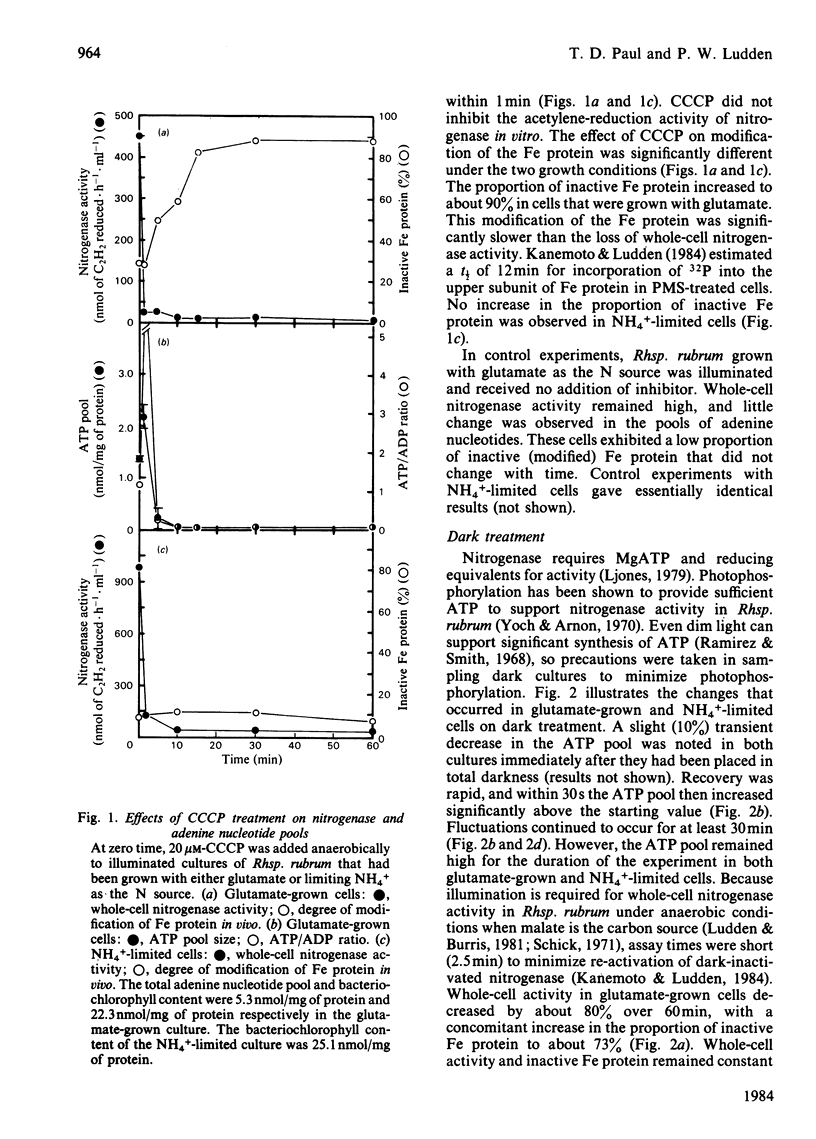
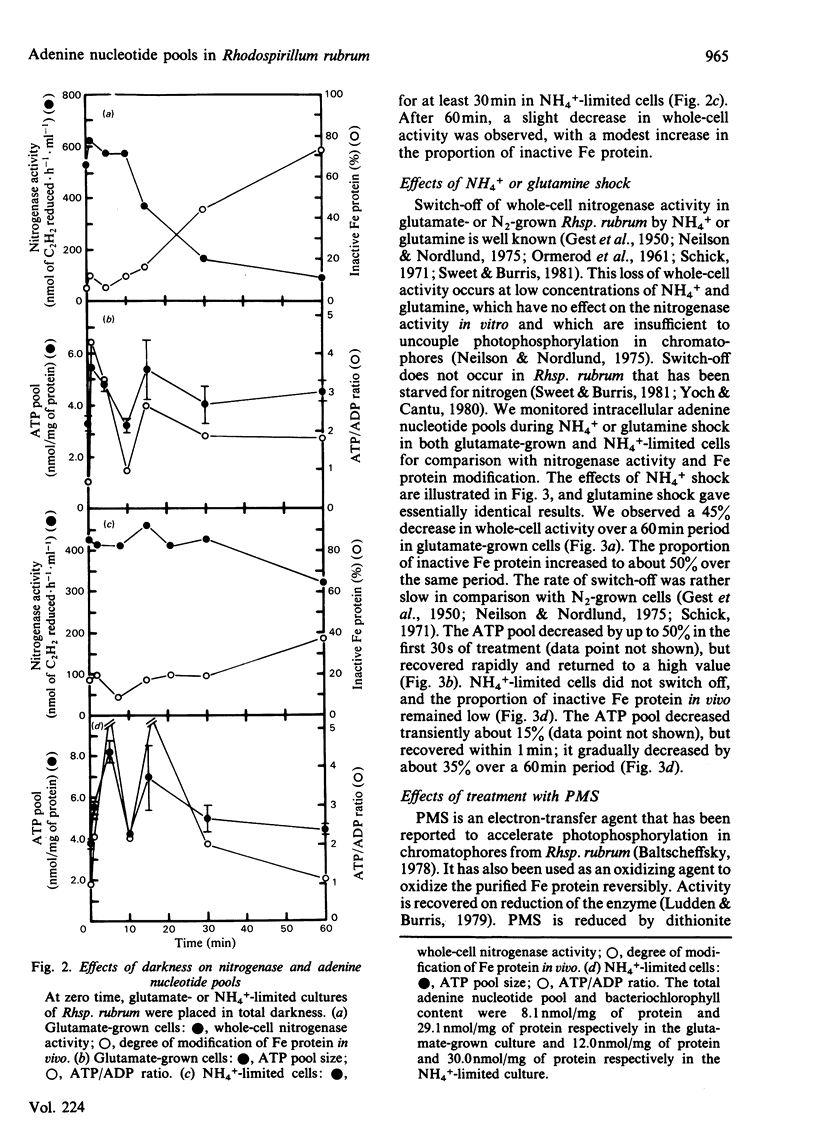
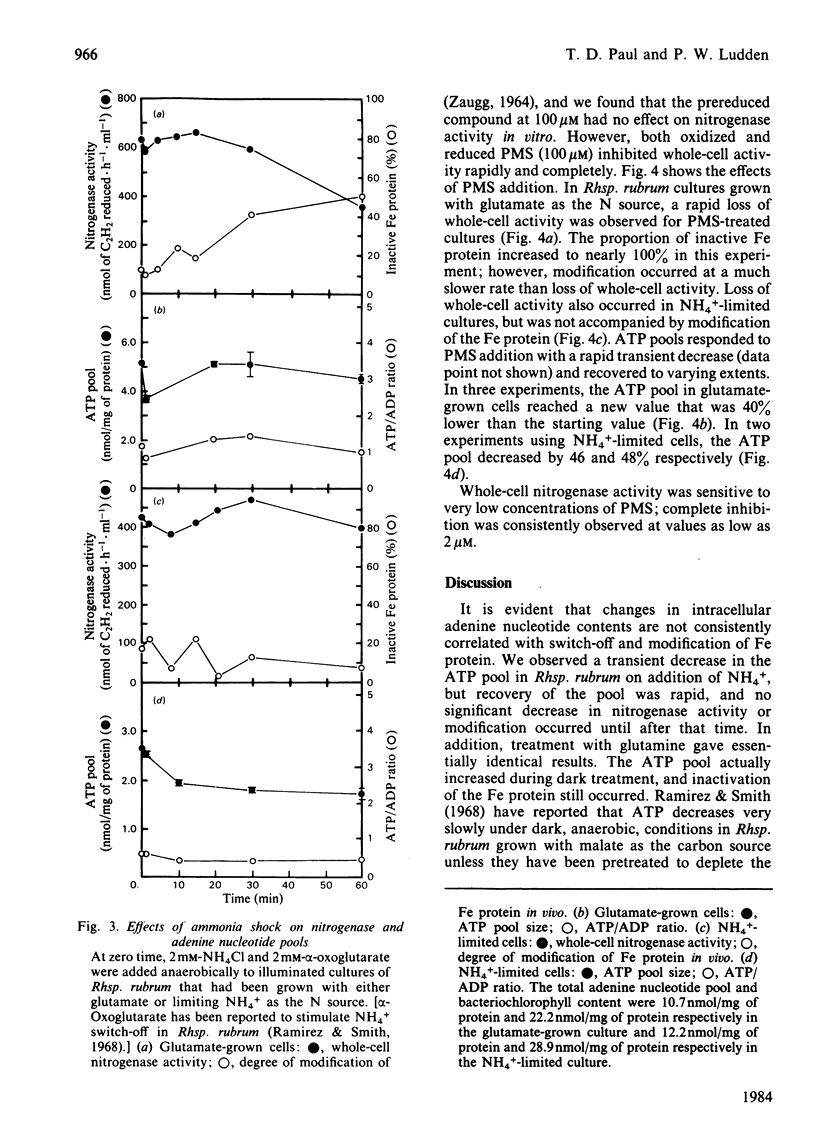
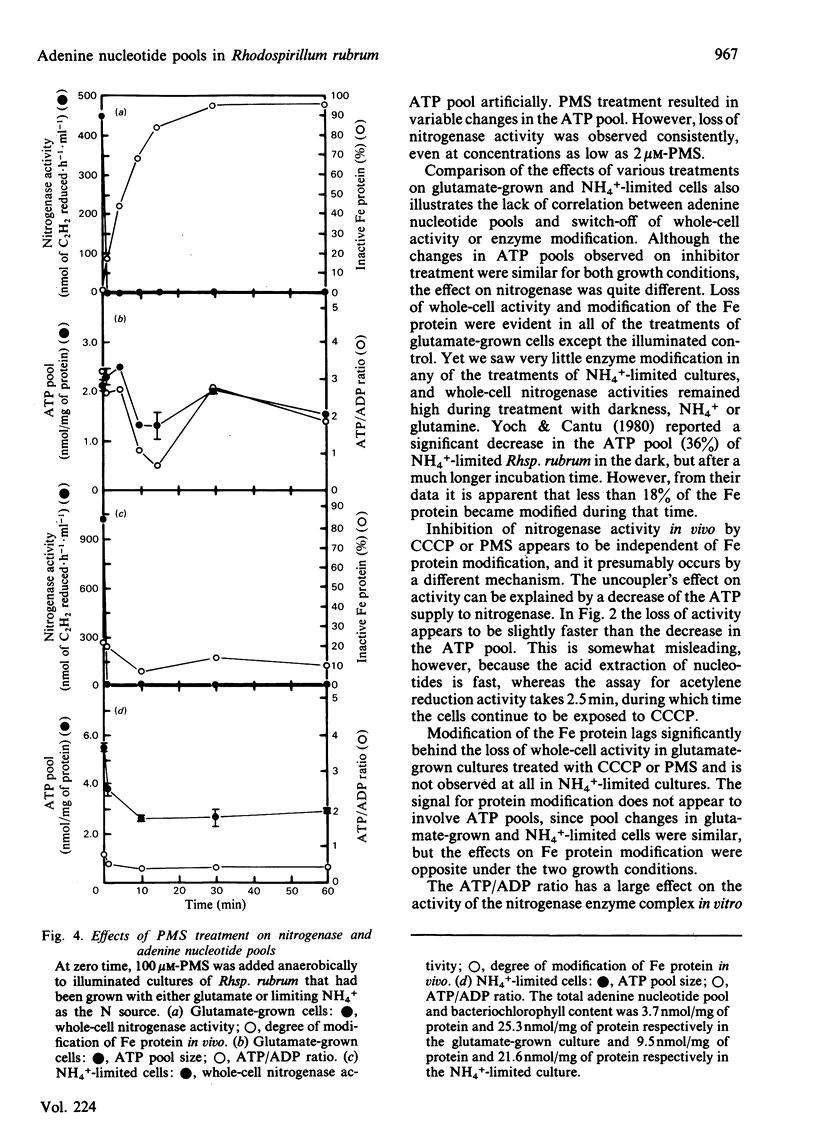
Adenine nucleotide pools were measured in Rhodospirillum rubrum cultures that contained nitrogenase. The average energy charge [([ATP] + 1/2[ADP])/([ATP] + [ADP] + [AMP])] was found to be 0.66 and 0.62 in glutamate-grown and N-limited cultures respectively. Treatment of glutamate-grown cells with darkness, ammonia, glutamine, carbonyl cyanide m-chlorophenylhydrazone, or phenazine methosulphate resulted in perturbations in the adenine nucleotide pools, and led to loss of whole-cell nitrogenase activity and modification in vivo of the Fe protein. Treatment of N-limited cells resulted in similar changes in adenine nucleotide pools but not enzyme modification. No correlations were found between changes in adenine nucleotide pools or ratios of these pools and switch-off of nitrogenase activity by Fe protein modification in vivo. Phenazine methosulphate inhibited whole-cell activity at low concentrations. The effect on nitrogenase activity was apparently independent of Fe protein modification.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bognar A., Desrosiers L., Libman M., Newman E. B. Control of nitrogenase in a photosynthetic autotrophic bacterium, Ectothiorhodospira sp. J Bacteriol. 1982 Nov;152(2):706–713. doi: 10.1128/jb.152.2.706-713.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill W. J. Biochemical genetics of nitrogen fixation. Microbiol Rev. 1980 Sep;44(3):449–467. doi: 10.1128/mr.44.3.449-467.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L. C., Kotake S. Regulation of nitrogenase activity in aerobes by Mg2+ availability: an hypothesis. Biochem Biophys Res Commun. 1980 Apr 14;93(3):934–940. doi: 10.1016/0006-291x(80)91165-1. [DOI] [PubMed] [Google Scholar]
- Davis L. C., Orhme-Johnson W. H. Nitrogenase IX. Effect of the MgATP generator on the catalytic and EPR properties of the enzyme in vitro. Biochim Biophys Acta. 1976 Nov 8;452(1):42–58. doi: 10.1016/0005-2744(76)90056-5. [DOI] [PubMed] [Google Scholar]
- GOA J. A micro biuret method for protein determination; determination of total protein in cerebrospinal fluid. Scand J Clin Lab Invest. 1953;5(3):218–222. doi: 10.3109/00365515309094189. [DOI] [PubMed] [Google Scholar]
- Gotto J. W., Yoch D. C. Regulation of Rhodospirillum rubrum nitrogenase activity. Properties and interconversion of active and inactive Fe protein. J Biol Chem. 1982 Mar 25;257(6):2868–2873. [PubMed] [Google Scholar]
- Haaker H., Laane C., Hellingwerf K., Houwer B., Konings W. N., Veeger C. Short-term regulation of the nitrogenase activity in Rhodopseudomonas sphaeroides. Eur J Biochem. 1982 Oct;127(3):639–645. doi: 10.1111/j.1432-1033.1982.tb06920.x. [DOI] [PubMed] [Google Scholar]
- Haaker H., de Kok A., Veeger C. Regulation of dinitrogen fixation in intact Azotobacter vinelandii. Biochim Biophys Acta. 1974 Sep 20;357(3):344–357. doi: 10.1016/0005-2728(74)90024-3. [DOI] [PubMed] [Google Scholar]
- Hallenbeck P. C., Meyer C. M., Vignais P. M. Nitrogenase from the photosynthetic bacterium Rhodopseudomonas capsulata: purification and molecular properties. J Bacteriol. 1982 Feb;149(2):708–717. doi: 10.1128/jb.149.2.708-717.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkesford M. J., Reed R. H., Rowell P., Stewart W. D. Nitrogenase activity and membrane electrogenesis in the cyanobacterium Plectonema boryanum. Eur J Biochem. 1982 Sep;127(1):63–66. doi: 10.1111/j.1432-1033.1982.tb06837.x. [DOI] [PubMed] [Google Scholar]
- Hillmer P., Gest H. H2 metabolism in the photosynthetic bacterium Rhodopseudomonas capsulata: production and utilization of H2 by resting cells. J Bacteriol. 1977 Feb;129(2):732–739. doi: 10.1128/jb.129.2.732-739.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard K. S., Hales B. J., Socolofsky M. D. Nitrogen fixation and ammonia switch-off in the photosynthetic bacterium Rhodopseudomonas viridis. J Bacteriol. 1983 Jul;155(1):107–112. doi: 10.1128/jb.155.1.107-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. L., Monty K. J. Glutamine as a feedback inhibitor of the Rhodopseudomonas sphaeroides nitrogenase system. J Bacteriol. 1979 Sep;139(3):1007–1013. doi: 10.1128/jb.139.3.1007-1013.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemoto R. H., Ludden P. W. Effect of ammonia, darkness, and phenazine methosulfate on whole-cell nitrogenase activity and Fe protein modification in Rhodospirillum rubrum. J Bacteriol. 1984 May;158(2):713–720. doi: 10.1128/jb.158.2.713-720.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmich G. A., Randles J., Brand J. S. Assay of picomole amounts of ATP, ADP, and AMP using the luciferase enzyme system. Anal Biochem. 1975 Nov;69(1):187–206. doi: 10.1016/0003-2697(75)90580-1. [DOI] [PubMed] [Google Scholar]
- Koch B., Evans H. J. Reduction of acetylene to ethylene by soybean root nodules. Plant Physiol. 1966 Dec;41(10):1748–1750. doi: 10.1104/pp.41.10.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ljones T. Nitrogen fixation and bioenergetics: the role of ATP in nitrogenase catalysis. FEBS Lett. 1979 Feb 1;98(1):1–8. doi: 10.1016/0014-5793(79)80138-6. [DOI] [PubMed] [Google Scholar]
- Ludden P. W., Burris R. H. Activating factor for the iron protein of nitrogenase from Rhodospirillum rubrum. Science. 1976 Oct 22;194(4263):424–426. doi: 10.1126/science.824729. [DOI] [PubMed] [Google Scholar]
- Ludden P. W., Burris R. H. Purification and properties of nitrogenase from Rhodospirillum rubrum, and evidence for phosphate, ribose and an adenine-like unit covalently bound to the iron protein. Biochem J. 1978 Oct 1;175(1):251–259. doi: 10.1042/bj1750251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludden P. W., Burris R. H. Removal of an adenine-like molecule during activation of dinitrogenase reductase from Rhodospirillum rubrum. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6201–6205. doi: 10.1073/pnas.76.12.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludden P. W., Preston G. G., Dowling T. E. Comparison of active and inactive forms of iron protein from Rhodospirillum rubrum. Biochem J. 1982 Jun 1;203(3):663–668. doi: 10.1042/bj2030663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson A. H., Nordlund S. Regulation of nitrogenase synthesis in intact cells of Rhodospirillum rubrum: inactivation of nitrogen fixation by ammonia, L-glutamine and L-asparagine. J Gen Microbiol. 1975 Nov;91(1):53–62. doi: 10.1099/00221287-91-1-53. [DOI] [PubMed] [Google Scholar]
- Nordlund S., Eriksson U., Baltscheffsky H. Necessity of a membrane component for nitrogenase activity in Rhodospirillum rubrum. Biochim Biophys Acta. 1977 Oct 12;462(1):187–195. doi: 10.1016/0005-2728(77)90201-8. [DOI] [PubMed] [Google Scholar]
- Nordlund S., Ludden P. W. Incorporation of adenine into the modifying group of inactive iron protein of nitrogenase from Rhodospirillum rubrum. Biochem J. 1983 Mar 1;209(3):881–884. doi: 10.1042/bj2090881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORMEROD J. G., ORMEROD K. S., GEST H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochem Biophys. 1961 Sep;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- Preston G. G., Ludden P. W. Change in subunit composition of the iron protein of nitrogenase from Rhodospirillum rubrum during activation and inactivation of iron protein. Biochem J. 1982 Sep 1;205(3):489–494. doi: 10.1042/bj2050489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalle L. S., Burris R. H. Adenine nucleotide levels in and nitrogen fixation by the cyanobacterium Anabaena sp. strain 7120. J Bacteriol. 1983 Apr;154(1):351–355. doi: 10.1128/jb.154.1.351-355.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez J., Smith L. Synthesis of adenosine triphosphate in intact cells of Rhodospirillum rubrum and Rhodopseudomonas spheroides on oxygenation or illumination. Biochim Biophys Acta. 1968 Feb 12;153(2):466–475. doi: 10.1016/0005-2728(68)90088-1. [DOI] [PubMed] [Google Scholar]
- Schick H. J. Substrate and light dependent fixation of molecular nitrogen in Rhodospirillum rubrum. Arch Mikrobiol. 1971;75(2):89–101. doi: 10.1007/BF00407997. [DOI] [PubMed] [Google Scholar]
- Schön G. Der Einfluss der Kulturbedingungen auf den ATP-, ADP- und AMP-spiegel bei Rhodospirillum rubrum. Arch Mikrobiol. 1969;66(4):348–364. [PubMed] [Google Scholar]
- Stewart W. D., Fitzgerald G. P., Burris R. H. In situ studies on N2 fixation using the acetylene reduction technique. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2071–2078. doi: 10.1073/pnas.58.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strehler B. L. Bioluminescence assay: principles and practice. Methods Biochem Anal. 1968;16:99–181. doi: 10.1002/9780470110348.ch2. [DOI] [PubMed] [Google Scholar]
- Sweet W. J., Burris R. H. Inhibition of nitrogenase activity by NH+4 in Rhodospirillum rubrum. J Bacteriol. 1981 Feb;145(2):824–831. doi: 10.1128/jb.145.2.824-831.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upchurch R. G., Mortenson L. E. In vivo energetics and control of nitrogen fixation: changes in the adenylate energy charge and adenosine 5'-diphosphate/adenosine 5'-triphosphate ratio of cells during growth on dinitrogen versus growth on ammonia. J Bacteriol. 1980 Jul;143(1):274–284. doi: 10.1128/jb.143.1.274-284.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoch D. C., Arnon D. I. The nitrogen fixation system of photosynthetic bacteria. II. Chromatium nitrogenase activity linked to photochemically generated assimilatory power. Biochim Biophys Acta. 1970 Mar 3;197(2):180–184. doi: 10.1016/0005-2728(70)90029-0. [DOI] [PubMed] [Google Scholar]
- Yoch D. C., Cantu M. Changes in the regulatory form of Rhodospirillum rubrum nitrogenase as influenced by nutritional and environmental factors. J Bacteriol. 1980 Jun;142(3):899–907. doi: 10.1128/jb.142.3.899-907.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoch D. C., Gotto J. W. Effect of light intensity and inhibitors of nitrogen assimilation on NH4+ inhibition of nitrogenase activity in Rhodospirillum rubrum and Anabaena sp. J Bacteriol. 1982 Aug;151(2):800–806. doi: 10.1128/jb.151.2.800-806.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAUGG W. S. SPECTROSCOPIC CHARACTERISTICS AND SOME CHEMICAL PROPERTIES OF N-METHYLPHENAZINIUM METHYL SULFATE (PHENAZINE METHOSULFATE) AND PYOCYANINE AT THE SEMIQUIDNOID OXIDATION LEVEL. J Biol Chem. 1964 Nov;239:3964–3970. [PubMed] [Google Scholar]
- Zumft W. G., Castillo F. Regulatory properties of the nitrogenase from Rhodopseudomonas palustris. Arch Microbiol. 1978 Apr 27;117(1):53–60. doi: 10.1007/BF00689351. [DOI] [PubMed] [Google Scholar]


