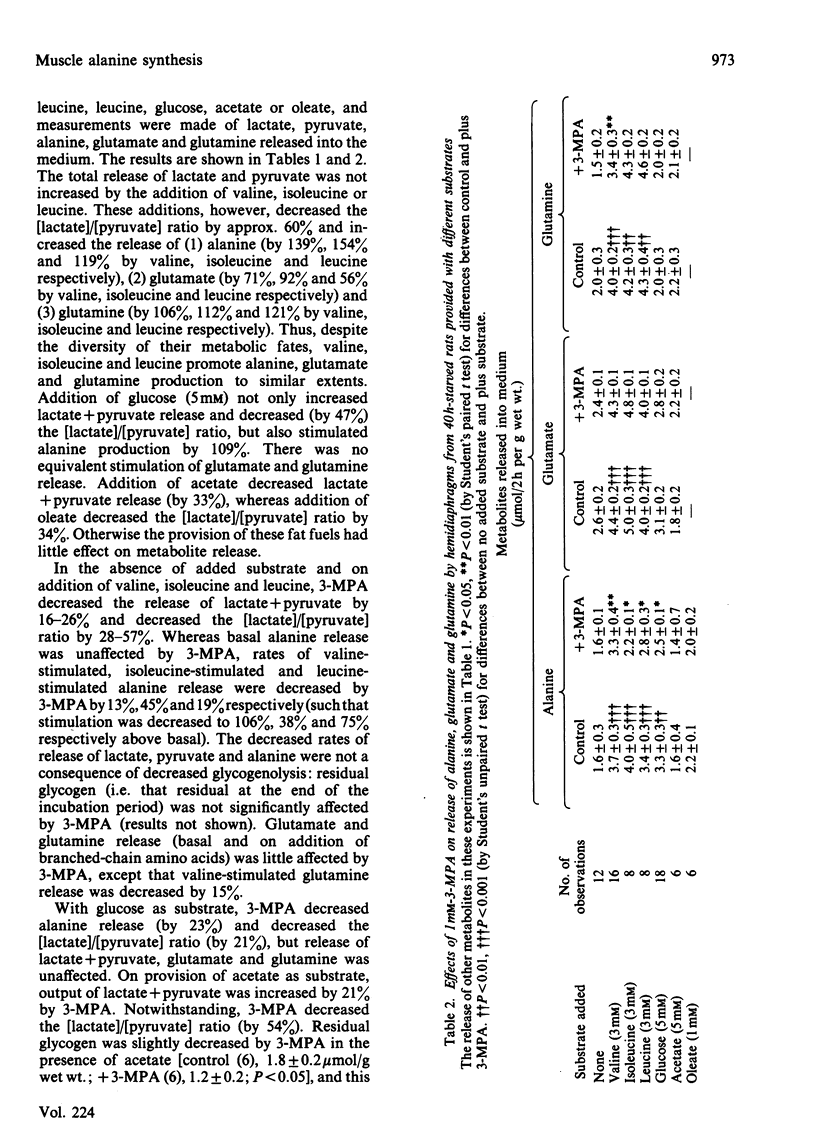
Abstract
3-Mercaptopicolinic acid (3-MPA) is reportedly a specific inhibitor of phosphoenolpyruvate (PEP) carboxykinase and has hitherto been used accordingly to elucidate the metabolic role of PEP carboxykinase in vitro and in vivo. We show that 3-MPA has multiple effects on intermediary metabolism in hemidiaphragms from 40 h-starved rats. It decreases the release of lactate + pyruvate and alanine in hemidiaphragms provided with no added substrate or with valine, leucine or isoleucine. Moreover, irrespective of the substrate provided (none, valine, leucine, isoleucine, glucose, acetate, oleate), 3-MPA decreases the [lactate]/[pyruvate] ratio. 3-MPA is without effect on 14CO2 production from [U-14C]valine, [1-14C]valine, [1-14C]leucine, [U-14C]isoleucine or [1-14C]oleate, but stimulates 14CO2 production from [U-14C]glucose and [1-14C]pyruvate and inhibits 14CO2 production from [1-14C]acetate. Glycolytic flux (measured as 3H2O formation from [5-3H]glucose) is stimulated by 3-MPA. It is concluded that 3-MPA has site(s) of actions other than PEP carboxykinase and that the putative role of PEP carboxykinase in alanine synthesis de novo in skeletal muscle from tricarboxylic acid-cycle intermediates and related amino acids requires reappraisal.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J., Weerasinghe L. C., Bassett J. M., Randle P. J. The pentose cycle and insulin release in mouse pancreatic islets. Biochem J. 1972 Feb;126(3):525–532. doi: 10.1042/bj1260525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear P. J., Holloway P. A., Aberti K. G. The effects of inhibition of gluconeogenesis on ketogenesis in starved and diabetic rats. Biochem J. 1975 Jun;148(3):353–362. doi: 10.1042/bj1480353b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. W., Goldberg A. L. The origin of alanine produced in skeletal muscle. J Biol Chem. 1978 May 25;253(10):3677–3684. [PubMed] [Google Scholar]
- DiTullio N. W., Berkoff C. E., Blank B., Kostos V., Stack E. J., Saunders H. L. 3-mercaptopicolinic acid, an inhibitor of gluconeogenesis. Biochem J. 1974 Mar;138(3):387–394. doi: 10.1042/bj1380387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff D. A., Snell K. Limitations of commonly used spectrophotometric assay methods for phosphoenolypyruvate carboxykinase activity in crude extracts of muscle. Biochem J. 1982 Jul 15;206(1):147–152. doi: 10.1042/bj2060147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P. Amino acid metabolism in man. Annu Rev Biochem. 1975;44:933–955. doi: 10.1146/annurev.bi.44.070175.004441. [DOI] [PubMed] [Google Scholar]
- Garber A. J., Karl I. E., Kipnis D. M. Alanine and glutamine synthesis and release from skeletal muscle. IV. beta-Adrenergic inhibition of amino acid release. J Biol Chem. 1976 Feb 10;251(3):851–857. [PubMed] [Google Scholar]
- Goldstein L., Newsholme E. A. The formation of alanine from amino acids in diaphragm muscle of the rat. Biochem J. 1976 Feb 15;154(2):555–558. doi: 10.1042/bj1540555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M. N. Effect of 3-mercaptopicolinic acid on gluconeogenesis and gluconeogenic metabolite concentrations in the isolated perfused rat liver. Biochem J. 1975 Jul;150(1):137–139. doi: 10.1042/bj1500137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostos V., DiTullio N. W., Rush J., Cieslinski L., Saunders H. L. The effect of 3-mercaptopicolinic acid on phosphoenolpyruvate carboxykinase (GTP) in the rat and guinea pig. Arch Biochem Biophys. 1975 Dec;171(2):459–465. doi: 10.1016/0003-9861(75)90054-5. [DOI] [PubMed] [Google Scholar]
- Nordlie R. C., Alvares F. L., Sukalski K. A. Stimulation by 3-mercaptopicolinate of net glucose uptake by perfused livers from diabetic rats. Biochim Biophys Acta. 1982 Nov 24;719(2):244–250. doi: 10.1016/0304-4165(82)90095-2. [DOI] [PubMed] [Google Scholar]
- Palmer T. N., Caldecourt M. A., Slavin J. P. Pyruvate kinase and alanine synthesis in skeletal muscle. Biosci Rep. 1982 Nov;2(11):941–948. doi: 10.1007/BF01114901. [DOI] [PubMed] [Google Scholar]
- Palmer T. N., Caldecourt M. A., Sugden M. C. Adrenergic inhibition of branched-chain 2-oxo acid dehydrogenase in rat diaphragm muscle in vitro. Biochem J. 1983 Oct 15;216(1):63–70. doi: 10.1042/bj2160063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston J. M. The relative carbon flux through the alpha- and the beta-keto pathways of leucine metabolism. J Biol Chem. 1984 Feb 25;259(4):2059–2061. [PubMed] [Google Scholar]
- Robinson B. H., Oei J. 3-Mercaptopicolinic acid, a preferential inhibitor of the cytosolic phosphoenolpyruvate carboxykinase. FEBS Lett. 1975 Oct 15;58(1):12–15. doi: 10.1016/0014-5793(75)80214-6. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B. Muscle amino acid metabolism and gluconeogenesis. Annu Rev Med. 1975;26:245–258. doi: 10.1146/annurev.me.26.020175.001333. [DOI] [PubMed] [Google Scholar]
- Snell K., Duff D. A. The hepato-muscular metabolic axis and gluconeogenesis. Prog Clin Biol Res. 1982;102(Pt 100):279–291. [PubMed] [Google Scholar]
- Snell K., Duff D. A. The release of alanine by rat diaphragm muscle in vitro. Biochem J. 1977 Feb 15;162(2):399–403. doi: 10.1042/bj1620399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K. Muscle alanine synthesis and hepatic gluconeogenesis. Biochem Soc Trans. 1980 Apr;8(2):205–213. doi: 10.1042/bst0080205. [DOI] [PubMed] [Google Scholar]


