Abstract
Background
Advanced cell therapies emerged as promising candidates for treatment of knee articular diseases, but robust evidence regarding their clinical applicability is still lacking.
Objective
To assess the efficacy and safety of advanced mesenchymal stromal cells (MSC) therapy for knee osteoarthritis (OA) and chondral lesions.
Methods
Systematic review of randomized controlled trials conducted in accordance with Cochrane Handbook and reported following PRISMA checklist. GRADE approach was used for assessing the evidence certainty.
Results
25 randomized controlled trials that enrolled 1048 participants were included. Meta-analyses data showed that, compared to viscosupplementation (VS), advanced MSC therapy resulted in a 1.91 lower pain VAS score (95 % CI ‐3.23 to −0.59; p < 0.00001) for the treatment of knee OA after 12 months. Compared to placebo, the difference was 0.99 lower pain VAS points (95 % CI ‐1.94 to −0.03; p = 0.76). According to the GRADE approach, the evidence was very uncertain for both comparisons. By excluding studies with high risk of bias, there was a similar size of effect (VAS MD ‐1.54, 95 % CI ‐2.09 to −0.98; p = 0.70) with improved (moderate) certainty of evidence, suggesting that MSC therapy probably reduces pain slightly better than VS. Regarding serious adverse events, there was no difference from advanced MSC therapy to placebo or to VS, with very uncertain evidence.
Conclusion
Advanced MSC therapy resulted in lower pain compared to placebo or VS for the treatment of knee OA after 12 months, with no difference in adverse events. However, the evidence was considered uncertain.
The Translational Potential of this Article
Currently, there is a lack of studies with good methodological structure aiming to evaluate the real clinical impact of advanced cell therapy for knee OA. The present study was well structured and conducted, with Risk of Bias, GRADE certainty assessment and sensitivity analysis. It explores the translational aspect of the benefits and safety of MSC compared with placebo and gold-standard therapy to give practitioners and researchers support to expand this therapy in their practice.
PROSPERO registration number
CRD42020158173. Access at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=158173.
Keywords: Articular Cartilage, Cell and tissue-based therapy, Knee Osteoarthritis, Mesenchymal stem cell, Regenerative medicine, Stem cells
Graphical abstract
1. Introduction
Osteoarthritis (OA) and chondral injuries are a major health problem and financial burden for health and social welfare systems around the world [[1], [2], [3], [4], [5]].
OA is a highly prevalent degenerative musculoskeletal disorder, involving all tissues of the joint, that symptomatically affects more than 10 % of the world's population aged 60 years or older and represents one of the major causes of disability worldwide [1,6]. Pain management, activity modification, and weight loss are prescribed in the early stages, but the demand for knee arthroplasty surgeries remains high and is continuously growing. However, while the surgical approach can provide a high rate of success and satisfaction for older patients, in young patients the high functional demand and longer life expectancy constitute an obstacle to arthroplasty [[4], [5], [6]].
Chondral lesions are a challenge for physicians in different areas, as the ability to repair the joint cartilage is very limited. Conservative treatment is limited mainly to pain control and physical therapy [[7], [8], [9], [10], [11]]. Currently available invasive therapies only show better results in small lesions and require expensive surgeries, such as osteochondral allograft transplantation and matrix-associated autologous chondrocyte implantation, with a long period away from activities for rehabilitation. They present a return to sport rate that still needs to be improved [[7], [8], [9], [10], [11], [12], [13], [14], [15], [16]].
Regenerative medicine emerged as an optimistic promise of a more effective conservative treatment for these conditions. It is based on the employment of components of our own biology enhanced to provide the healing of the original tissue. The effort in this field runs towards the use of precursor cells and immunologic molecules as therapies [2,17]. The mesenchymal stromal cells (MSC) are the main active agent of most of these therapies. These cells originate in the mesoderm and have the ability to self-renew and to differentiate into several cell types (bone (osteocytes), cartilage (chondrocytes), fat (adipocytes), blood cell precursors and fibroblasts). The origin of the cells can be either from the patient himself (autologous) or from a donor (allogeneic). Historically, the most used source of MSCs has been the bone marrow. Another well-explored source is the adipose tissue. With the advancement of allogeneic techniques, bank tissue from the umbilical cord and placenta were also used [2,5,[17], [18], [19], [20], [21], [22]].
According to mechanistic, animal and pilot studies, culture-expanded MSC populations have paracrine effects in the articular microenvironment that works via trophic, immunomodulatory and chemoattractant properties [5,[18], [19], [20], [21]]. Locally targeted MSC could work through cell differentiation and this paracrine stimulation to inhibit the articular injury, induce immunomodulation, reduce scarring, promote actual chondral repair and slow down the OA process [5,7,10,[18], [19], [20], [21],23].
The literature presents ample evidence supporting the ability of MSC therapy to stimulate cartilage repair in large animals [13,23]. Human studies less frequently include histological assessment, but there are many studies reporting the repair tissue formation on MRI scans [3,5,10,13,20]. Human clinical trials are, ultimately, the sole means by which symptomatic and functional outcomes can be assessed, and these outcomes are paramount for both patients and healthcare providers. Therefore, these studies have the power to direct clinical practice [2,3,13,18,20].
Some minimally manipulated autogenous techniques, like bone marrow aspirate and microfragmented adipose tissue, are already in clinical use. They involve harvesting the respective donor tissue and applying it to the injured area in a single procedure, without laboratory manipulation. In this way, it would be possible to take advantage of the benefits of MSC naturally available in these tissues [5,13,24,25]. However, these therapies still present conflicting and heterogeneous results. One hypothesis is that despite having MSC, their concentration would be very low and with great variation depending on the patient's health profile and harvesting technique and location [5,13,24,25].
The demand to enhance the regenerative properties of progenitor cells and, consequently, their clinical benefits, has brought up the advanced cell therapy modalities, in which the progenitor MSC populations pass through enzymatic digestion and are culture-expanded in laboratory, without genetic modifications, to achieve much higher concentrations after some passages [[2], [3], [4], [5],13,[24], [25], [26], [27], [28], [29], [30], [31]], In theory, it is possible to use the expansion as a means of multiplying and activating the effector cells, with depletion of inhibitory cells and contaminants in the tissue obtained, aiming to preserve the desirable intrinsic biological characteristics of the effector cells in the final product [2,5,[24], [25], [26], [27], [28], [29], [30], [31]]. These techniques are the study object of this paper.
These clinical benefits are not yet well established, particularly the translational aspect to pain and functional improvement [18,[31], [32], [33], [34], [35], [36], [37], [38], [39]]. Also, although the literature points them to be very rare, there are potential risk factors evolved in the therapy such as the differentiation into undesired cell types and ectopic tissue formation that still needs to be properly evaluated [[2], [3], [4], [5],28,[40], [41], [42], [43], [44], [45], [46], [47]].
In this study, our objective was to assess, through a systematic review of randomized clinical trials, the efficacy and safety of advanced therapy with mesenchymal stromal cells for the treatment of knee osteoarthritis or chondral lesions.
2. Methods
2.1. Study design and setting
This was a systematic review of randomized controlled trials (RCT) carried out in Hospital Sírio-Libanês, São Paulo– SP, Brazil, and conducted in accordance with The Cochrane Collaboration Handbook for Systematic Reviews of Interventions [48].
This review was prospectively registered in the PROSPERO database under number CRD42020158173 (available from: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=158173).
The reporting of this review followed the PRISMA 2020 Statement checklist [49].
2.2. Criteria for including studies
2.2.1. Types of studies
Only parallel RCTs (with cluster or individual randomization) were included. Cross-over trials were not included.
2.2.2. Types of participants
Adults with symptomatic or asymptomatic knee OA or chondral lesions, at any stage of disease. RCTs assessing these participants as a subgroup of a wider population were considered only if the authors presented subgroup analyses and results including only them.
2.2.3. Types of interventions
Any advanced MSC therapy (autologous or allogeneic, combined or not with biocompatible materials). RCTs that assessed MSC therapy combined with other techniques were considered only if the same technique was also administered in the control group.
The following interventions, which don't comprise advanced MSC therapy, were not considered: micro-fragmented adipose tissue, implant of chondrocytes, bone marrow aspirate concentrate (BMAC) and peripheral blood stem cells.
2.3. Outcomes of interest
We considered the following outcomes based on recommendations for outcomes in OA trials [50].
2.3.1. Primary outcomes
-
1.
Pain;
-
2.
Physical function and
-
3.
Number of participants experiencing any serious adverse events (those that are life-threatening; cause death, require treatment in emergency room, hospitalization, disability or permanent damage, or congenital anomaly/birth defect [51].
For studies using more than one pain scale, we used a hierarchy of pain‐related outcomes [52], extracting data on the pain scale that was highest on this list: 1. global pain measured using the visual analog scale (VAS- from 0 to 10, being 0 equivalent to no pain and 10 to the worst pain possible); 2. pain on walking; 3. Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain subscore; 4. composite pain scores other than WOMAC; 5. pain on activities other than walking; 6. rest pain or pain during the night; 7. WOMAC Global Algofunctional score; 8. Lequesne Osteoarthritis Index global score; 9 other algofunctional scale; 10. participant's global assessment; 11. physician's global assessment.
For studies with more than one physical function scale, we used a hierarchy, extracting data on the pain scale that was highest [52]: 1. global disability score; 2. walking disability; 3. WOMAC Disability subscore; 4. composite disability scores other than WOMAC; 5. disability other than walking; 6. WOMAC Global Scale; 7. Lequesne Osteoarthritis Index global score; 8. other algofunctional scale; 9. participant's global assessment; 10. physician's global assessment. The WOMAC score range from 0 to 96 for the total WOMAC, in which 0 represents the best health status and 96 the worst possible status.
When a study reported pain or function outcomes at several time points, we considered only the last measure.
2.3.2. Secondary outcomes
The secondary outcomes were:
-
1.
Health‐related quality of life measures;
-
2.
Number of participants experiencing any adverse event and
-
3.
Need of a ‘second look’ intervention.
We assessed all outcomes listed above at any time point and only pooled similar time points together, considering the longest measurement.
2.4. Search for studies
A broad search of the literature was performed using electronic and hand search. There was no restriction regarding date, language or status of publication. The date of the last search was on October 31, 2022.
Sensitive search strategies (Supplementary file 1) were developed for the following databases:
Cochrane Central Register of Controlled Trials - CENTRAL (via Wiley);
EMBASE (via Elsevier);
Literatura Latino Americana em Ciências da Saúde e do Caribe - LILACS (via Biblioteca Virtual em Saúde - BVS);
MEDLINE (via Pubmed);
Physiotherapy Evidence Database (PEDro);
SPORTDiscus (via EBSCO).
Additional electronic searches for ongoing studies or grey literature were conducted in: ClinicalTrials.gov (www.clinicaltrials.gov); Open Grey (http://www.opengrey.eu/).
2.5. Selection of studies
The selection was performed in a two-stage process aided by Rayyan reference management software [53]. In the first stage, two groups of authors (CGT/TLF and RLP/ALCM) independently assessed all titles and abstracts. Studies marked as ‘potentially eligible’ were then screened at the second stage by reading the full text. Disagreements were solved consulting a third reviewer (RR).
2.6. Data extraction and management
Data extraction was performed by two independent reviewers (CGT and RLP, consulting RR if disagreements) using a pre-established protocol to collect data referring to study identification, eligibility criteria, methodological aspects (design, allocation method and concealment, masking, risk of bias and type of analysis), participants (sample number, age, severity of disease), interventions, comparisons, outcomes, follow-up time and results.
2.7. Risk of bias assessment
The risk of bias (RoB) assessment was independently performed by two groups of reviewers (group 1: CGT. and TLF.; group 2: RLP. and ALCM) using Cochrane RoB table, in accordance with the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions [48]. Disagreements were solved with a more experienced reviewer (RR).
2.8. Data synthesis
When possible, the results of the studies were grouped and summarized in the form of meta-analyses forest plots graphics, generated by Review Manager version 5.4.1 [54].
Studies that compared more than two intervention or control groups had the shared group split into two or more groups with smaller sample size, and were included as two or more comparisons [48].
2.9. Measures of treatment effect and analysis procedures
We extracted dichotomous data and calculated risk ratio (RR) with 95 % confidence intervals (CI) for the number of participants experiencing any serious adverse events, any adverse event and need of a ‘second look’.
We extracted continuous data and calculated mean difference (MD) with 95 % CI for the outcomes of pain, physical function and health‐related quality of life measures. Most studies reported the mean differences of the studied outcomes. When the studies didn't report it directly, but provided other statistical resources as statistical dispersion measures and graphic data, it was possible to calculate the mean and standard deviation (SD) following Cochrane recommendations using Review Manager software to standardize the outcome reporting method and include the data in the meta-analyses [48,54,55]. If studies used different scales, we expressed the treatment effect as standardized mean difference (SMD) and 95 % CI. When not available at all, that study results were analyzed only qualitatively.
Most studies reported the change in VAS from baseline. When the studies reported the absolute values and the baseline value, it was possible to calculate the change from baseline following Cochrane recommendations and using Review Manager, to standardize the outcome reporting method and enhance the data in the meta-analysis [48,54,55].
Random-effects meta-analyses were performed considering the heterogeneity and the availability of data.
2.10. Unit of analysis
We considered the unit of analysis included in each RCT. Both participant or knee were considered, but analyses were performed separately for each unit of analysis.
2.11. Dealing with missing data
RCT authors were contacted for requesting missing data when relevant. Missing means or standard deviations were calculated using available statistics when possible. Data available in graphs without the accurate value were estimated using a ruler positioned over the graph line.
2.12. Assessment of heterogeneity
We assessed the studies clinical and methodological differences and performed visual inspection of the forest plot along with statistical consideration of the Chi-squared and I2 tests [48,55].
2.13. Additional analysis
Sensitivity analysis: 1. Fixed-effect versus random effects model meta-analysis; 2. Excluding from analysis those RCTs at high RoB on at least one domain of RoB table and; 3. Excluding RCTs with industry sponsorship.
Subgroup analysis: 1. Autologous versus or allogeneic MSC; 2. MSC combined versus not combined with biocompatible materials.
2.14. Publication bias assessment
Publication bias assessment was planned to be performed by funnel plots if more than 10 RCTs were included in a single meta-analysis [48].
2.15. Summary of findings and assessment of the certainty of the evidence
The certainty of evidence was independently assessed by two authors (CGT, RLP) using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach [56,57], which consists of five domains (imprecision, inconsistency, indirectness, risk of bias and publication bias).
There were four levels of evidence quality: 'high', 'moderate', 'low' or 'very low'. Quality may be downgraded due to study limitations (risk of bias), imprecision, indirectness, inconsistency or publication bias.
The certainty of evidence was assessed for all primary outcomes. The results were incorporated in a summary of findings table using GRADEpro GDT software [58].
3. Results
3.1. Overview of the search
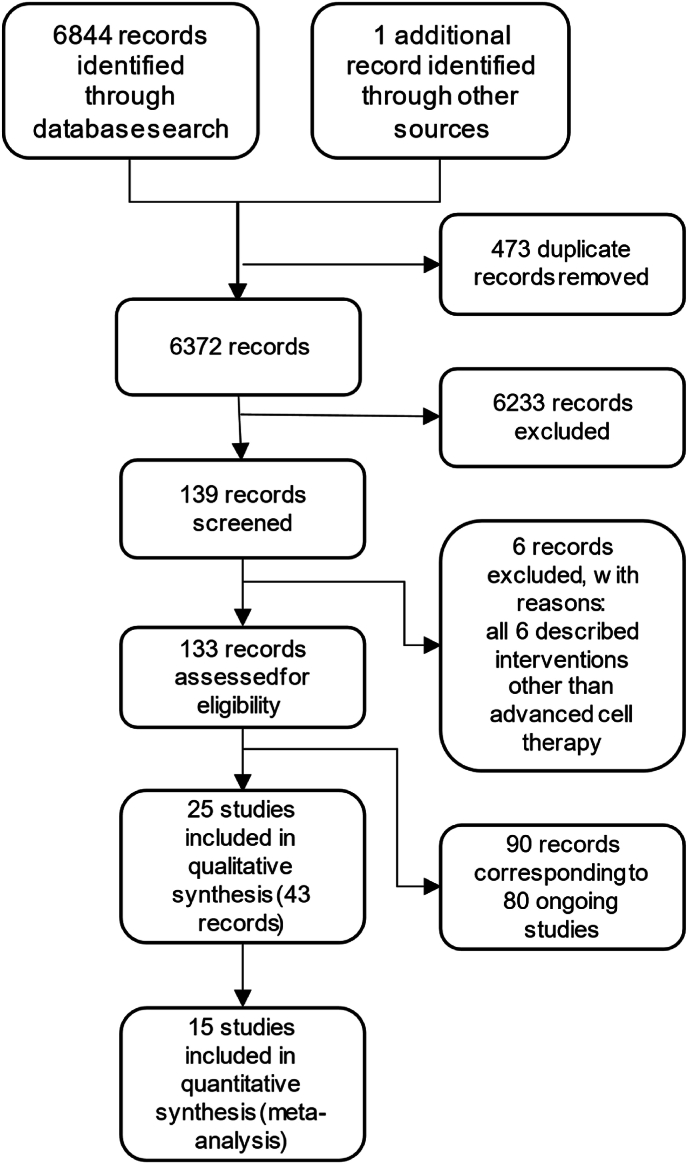
The electronic searches retrieved 6,844 records, of which 473 were duplicates and were eliminated. 1 record was identified from manual sources. The reading of 6,372 titles and abstracts in the first phase resulted in the exclusion of 6,233 records that did not meet the eligibility criteria.
After reading the full text of the remaining 139 records (second phase), 6 records were excluded and 133 records were included: 43 records corresponding to 25 complete studies and the remaining 90 corresponding to ongoing studies. The flowchart of the selection process is shown in Fig. 1: The flowchart of the selection process. Studies excluded in the second phase and its reasons were referenced in the ‘Supplementary file 2: Characteristics of excluded studies table’. Ongoing studies are presented in the ‘Supplementary file 3: Characteristics of ongoing studies table’.
Figure 1.
The flowchart of the selection process.
3.2. Included studies
We included 25 studies that enrolled 1048 adult patients, published between 2002 and 2022. Participants were adults from both sexes, aged between 18 and 75 years.
The main characteristics of the included studies are available at ‘Table 1: Main characteristics of the included clinical trials’.
Table 1.
Main characteristics of the included clinical trials.
| Id | Study | Country | Population | Intervention | Comparison | Outcomes | Follow-up | Funding and Registry |
|---|---|---|---|---|---|---|---|---|
| 1 | Akgun 2015 [59] | Turkey | N = 14 Mean age between 32.3 ± 7.9 (18.0–41.0), 32.7 ± 10.4 (18.0–46.0), p = 0.898 years Cartilage defects >2 cm2 |
Autologous S-MSC (8 × 106 cells) N = 7 |
m-ACI N = 7 |
KOOS VAS Tegner ROM MOCART |
Up to 24 months | No funding supports mentioned Protocol registration not reported |
| 2 | Bastos 2020 [60] | Portugal | N = 47 Mean age between 55.7 ± 7.8; 60.8 ± 9.9; 55.9 ± 13.4 years KL grade I-IV KOA |
Group A: Autologous BM-MSC (40 × 106 cells) Group B: Autologous BM-MSC (40 × 106 cells) + PRP N = 17 |
Group C: Corticosteroid N = 16 |
KOOS ROM |
Up to 12 months | No funding supports mentioned Protocol registration not reported |
| 3 | Chen 2021 [61] |
Taiwan | N = 57 Mean age of 67.6 ± 6.6 years KL grade I-III KOA |
Allogenic ADMSC 3 groups: 64 × 106 cells, 64 M; 32 × 106 cells, 32 M; 16 × 106 cells, 16 M. N = 49 |
VS N = 8 |
Adverse events Serious adverse events WOMAC VAS Physical function KSCRS |
Up to 96 weeks | UnicoCell Biomed CO. LTD Protocol registration reported (NCT02784964) |
| 4 | Emadedin 2018 [62] | Iran | N = 47 Mean age 53 years KL grade II-III-IV KOA |
Autologous BM-MSC (40 × 106 cells) N = 24 |
Placebo N = 19 |
VAS WOMAC Adverse events Serious adverse events |
Up to 6 months | Royan Institute Protocol registration reported (NCT01504464) |
| 5 | Fiolin 2020 [63] | Indonesia | N = 15 Mean age not reported KL grade I-II KOA |
UCBMSC (1 × 106 cells) + VS N = 5 |
Group B: VS + Somatotropin N = 5 Group C: Conservative management N = 5 |
VAS WOMAC IKDC |
Up to 12 months | No funding supports mentioned Protocol registration not reported |
| 6 | Freitag 2019 [64] | Australia | n = 30 Mean age between 51.5 and 54.7 years KL grade II-III KOA |
Autologous ADMSC (one-injection group and two-injection group of 100 × 106 cells) N = 10 |
Conservative management N = 10 |
NPRS KOOS WOMAC MRI analysis MOAKS Adverse events Serious adverse events |
Up to 12 months | Magellan Stem Cells and Melbourne Stem Cell Centre Protocol registration reported (ACTRN12614000814673) |
| 7 | Gupta 2016 [65] | India | N = 60 Mean age between 54.00 ± 6.73 and 58.10 ± 8.2 years KL grade II-III KOA |
Allogeneic BM-MSC (four groups with 5, 50, 75, or 150 × 106 cells) + VS N = 10 |
Placebo N = 5 |
Adverse events Serious adverse events VAS WOMAC ICOAP WORMS X-ray |
Up to 2 years | Stempeutics Research Pvt. Ltd. Protocol registration reported (NCT01453738) |
| 8 | Gupta 2022 [66] |
India | N = 146 Mean age of 40–60 years KL grade II-III KOA |
Allogenic BM-MSC (25 × 106 cells) + VS N = 73 |
VS N = 73 |
Adverse events Serious adverse events WOMAC VAS MRI |
Up to 12 months | Stempeutics Research Pvt LtdProtocol registration reported (CTRI/2018/09/015785) |
| 9 | Hashimoto 2019 [67] |
Japan | N = 11 Mean age 44.1 years ICRS ≥3 cartilage defect ≥2cm2 |
Autologous BM-MSC + MFX N = 4 |
MFX N = 7 |
Adverse events Serious adverse events IKDC KOOS MOCART |
Up to 48 weeks | Grant by apanese Ministry of Health, Labourand Welfare Protocol registration not reported |
| 10 | Ho 2022 [68] |
Hong Kong | N = 20 Mean age of 58.00 ± 4.51 years KL grade II-III KOA |
Autologous BM-MSC (1 × 106 cells) N = 10 |
VS N = 10 |
Adverse events Serious adverse events VAS WOMAC SF-36 KSS KSFS MRI |
Up to 12 months | The Chinese University of Hong Kong Protocol registration reported (CUHK_CCT00469) |
| 11 | Kuah 2018 [69] | Australia | N = 20 Mean age of groups between 55.0 ± 10.42; 50.8 ± 7.29; 55.0 ± 5.15 years KL grade I-III KOA |
Allogenic ADMSC Cohort 1: 3.9 × 106 cells Cohort 2: 6.7 × 106 cells N = 8 |
Placebo N = 2 |
Adverse events Serious adverse events VAS WOMAC AQoL-4D MOAKS MRI cartilage volume |
Up to 12 months | Regeneus Ltd Protocol registration reported (ACTRN12615000439549) |
| 12 | Lamo-Espinosa 2016 [70] | Spain | N = 30 Mean age of groups A,B and C between 60.3 (55.1, 61.1)65.9 (59.5, 70.6)57.8 (55.0, 60.8) (median and interquartile) years KL grade II-IV KOA |
Group A: Autologous BM-MSC (100 × 106 cells) + VS Group B: Autologous BM-MSC (10 × 106 cells) + VS N = 10 |
Group C: VS N = 10 |
Adverse events Serious adverse events VAS WOMAC ROM X-ray WORMS |
Up to 4 years | Clínica Universidad de Navarra Protocol registration reported (NCT02123368) |
| 13 | Lamo-Espinosa 2020 [44] | Spain | N = 60 Mean age between 54.6 (33,70) and 56 (40, 62) years KL grade II-IV KOA |
Autologous BM-MSC (100 × 106 cells) + PRP N = 30 |
PRP N = 30 |
Adverse events Serious adverse events VAS WOMAC ROM X-ray WORMS |
Up to 12 months | Clínica Universidad de Navarra FEDER Funds Spanish Ministry of Health Protocol registration reported (NCT02365142) |
| 14 | Lee 2019 [71] |
South Korea | N = 24 Mean age between 62.2 ± 6.5 and 63.2 ± 4.2 years KL grade II-IV KOA |
Autologous ADMSC (1 × 108 cells) N = 12 |
Placebo N = 12 |
Adverse events Serious adverse events VAS WOMAC KOOS X-ray MRI scan |
Up to 6 months | R-Bio Co., Ltd. Protocol registration reported (NCT02658344) |
| 15 | Lim 2021 [28] |
South Korea | n = 114 Mean age 55.9 years ICRS grade 4 cartilage defect 2–9 cm2 |
UCBMSC (7.x106 cells per 1.5 mL) + VS N = 43 |
MFX N = 43 |
Cartilage restoration (ICRS) Histological evaluation VAS WOMAC IKDC Adverse events Serious adverse events |
Up to 5 years | Medipost Co Ltd. Protocol registration reported (NCT01041001, NCT01626677) |
| 16 | Lu 2020 [72] |
China | N = 53 Mean age between 55.03 (9.19) and 59.64 (5.97) (p = 0.0375) years KL grade I-III KOA |
Autologous ADMSC (5.0 × 107 cells) N = 26 |
VS N = 26 |
Adverse events Serious adverse events VAS WOMAC SF-36 MRI cartilage volume |
Up to 12 months | Cellular Biomedicine Group Ltd. Protocol registration reported (NCT02162693) |
| 17 | Matas 2019 [73] | Chile | N = 26 Mean age between 54.8 ± 4; 556.1 ± 6.8; 56.7 ± 4.1; (p = 0.7) years KL grade I-III KOA |
Group A: UCBMSC (two doses of 20 × 106 cells) Group B: UCBMSC (one doses of 20 × 106 cells) N = 8 |
Group C: VS N = 9 |
Adverse events Serious adverse events VAS WOMAC SF-36 OMERACT/OARSI WORMS |
Up to 12 months | Cells for Cells Protocol registration reported (NCT02580695) |
| 18 | Sadat-Ali 2021 [74] |
Saudi Arabia | N = 60 Mean age between 45 and 70 KL grade II-III KOA |
Autologous BM-MSC (5 × 106 cells) N = 30 |
VS N = 30 |
Adverse events Serious adverse events VAS MKSSSF QOL MRI |
Up to 24 months | No funding supports mentioned Protocol registration not reported |
| 19 | Shadmanfar 2018 [69] |
Iran | N = 30 Mean age of 48.9 ± 1.7 years KL grade II-III KOA RA |
Autologous BM-MSC (40 × 106 cells) N = 15 |
Placebo N = 15 |
Adverse events Serious adverse events VAS WOMAC MRI |
Up to 12 months | Royan Institute Protocol registration reported (NCT01873625). |
| 20 | Soltani 2019 [75] | Iran | N = 20 Mean age between 35 and 75 years KL grade II-IV KOA |
Allogenic PLMSC (0.5–0.6 × 108 cells) N = 10 |
Placebo N = 10 |
Adverse events Serious adverse events VAS KOOS ROM MRI |
Up to 24 weeks | Grant from National Institute or Medical Research Development (NIMAD) Protocol registration reported (RCT2015101823298N) |
| 21 | Vega 2015 [76] | Spain | N = 30 Mean age 57±9years KL grade II-IV KOA |
Allogenic BM-MSC (40 × 106 cells) N = 15 |
VS N = 15 |
Adverse events Serious adverse events VAS WOMAC Lequesne MRI T2 mapping |
Up to 1 year | Red de Terapia Celular Protocol registration reported (NCT01586312) |
| 22 | Wakitani 2002 [77] |
Japan | N = 24 Mean age of 63 (49–70) years Ahlback grade I-II medial KOA |
Autologous BM-MSC (1 × 107 cells) + HTO N = 12 |
HTO N = 12 |
HSSKRS Arthroscopic assessment Histological evaluation |
Up to 16 months | Japan Orthopaedics and Traumatology Foundation Protocol registration not reported |
| 23 | Wang 2016 [78] | China | n = 36 Mean age intervention group: 54.28 years and control group: 52.37 years Moderate or Severe KOA |
UCBMSC (3 × 107 cells, two infiltrations) N = 18 |
VS N = 18 |
SF-36 Lysholm WOMAC Adverse events |
Up to 6 months | No funding supports mentioned Protocol registration not reported |
| 24 | Wong 2013 [79] | Singapore | N = 56 Mean age 51 years KOA with genu varum |
Autologous BM-MSC (1.46 ± 0.29 × 107) + VS + MFX + HTO N = 28 |
VS + MFX + HTO N = 28 |
IKDC Tegner Lysholm MOCART Serious adverse events |
Up to 2 years | No funding supports mentioned Protocol registration not reported |
| 25 | Zhao 2019 [80] | China | N = 18 Mean age between 52.05 ± 11.64, 59.58 ± 10.24, 52.69 ± 8.72, p = 0.41 KL grade II-III KOA |
Group A: Allogenic ADMSC (5.0 × 107 cells) Group B: Allogenic ADMSC 1.0 × 107 cells) Group C: Allogenic ADMSC 2.0 × 107 cells) N = 6 |
Comparison between doses | VAS WOMAC SF-36 Composition MRI animations WORMS |
Up to 48 weeks | Cellular Biomedicine Group, National Key Research and Development Program of China and National Natural Science Foundation of China Protocol registration reported (NCT02641860.) |
ACTRN: Australian and New Zealand Clinic Trial Registry; ADMSC: Adipose-derived mesenchymal stromal cells; AQoL-4D: assessment of quality of life 4D questionnaire; BM-MSC: bone marrow-derived mesenchymal stromal cells; ICRS: International Cartilage Repair Society; HA: hyaluronic acid; HSSKRS: Hospital for Special Surgery knee-rating scale; HTO: high tibial osteotomy; ICOAP: Intermittent and Constant Osteoarthritis Pain; IKDC: International Knee Documentation Committee; KL: Kellgren–Lawrence classification; KOA: Knee Osteoarthritis; KOOS: Knee Injury and Osteoarthritis Outcome Score; KSCRS: Knee Society Clinical Rating System; KSFS: Knee Society Function Score; m-ACI: matrix-induced autologous chondrocyte implantation; MKSSSF: Modified Knee Society Score-Short Form; MOAKS: MOCART: Magnetic Resonance Observation of Cartilage Repair Tissue; MRI Osteoarthritis Knee Score; MFX: microfracture; MRI: Magnetic Resonance Image; NCT: National Clinical Trial number; NPRS: Numeric Pain Rating Scale; OMERACT/OARSI: Outcome Measures in Rheumatology Committee/Osteoarthritis Research Society International Responder Index Criteria; PLMSC: Placenta mesenchymal stromal cells; PRP: Platelet Rich Plasma; RA: Rheumatoid arthritis; ROM: range of motion; SF-36: Short Form Health Survey; S-MSC: Synovial-mesenchymal stromal cells; UCBMSC: Umbilical Cord Blood–Derived Mesenchymal Stromal Cell; VAS: visual analog scale for pain; VS: viscossuplementation with hyaluronic acid; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index; WORMS: Whole-Organ Magnetic Resonance Imaging Score.
3.3. Risk of bias assessment
The RoB for all 25 studies are presented in Supplementary file 4: RoB summary and graphic. Full justifications of the assessment were included in ‘Supplementary file 5: Risk of Bias support for judgement table’. The performance and detection bias domains were evaluated in an outcome-level assessment.
3.4. Effects of interventions
We evaluated separately the studies assessing osteoarthritis and chondral lesions.
For each medical condition, we evaluated together studies comparing MSC versus the same comparator. Studies that assessed MSC combined with other techniques were considered for meta-analysis only if the same technique was also administered in the control group (which allowed us to investigate the additional effect of advanced MSC therapy).
3.4.1. Condition 1: Knee osteoarthritis
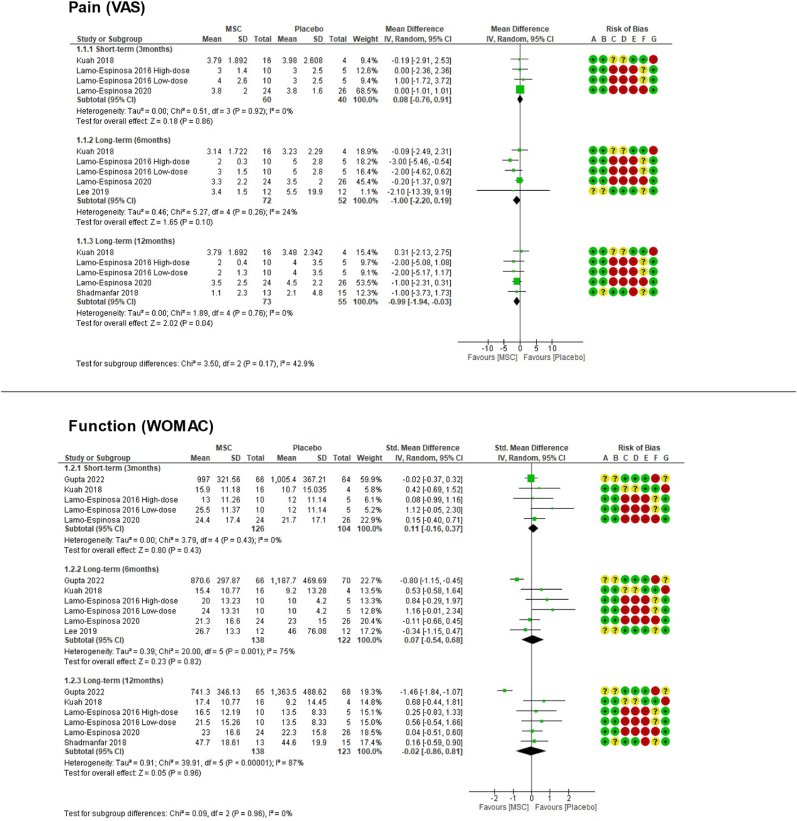
3.4.1.1. Comparison 1: MSC compared with placebo
(See Fig. 2)
Figure 2.
Comparison 1 (MSC therapy versus placebo) forest plot graphs.
This comparison was evaluated by eight RCT [43,44], [62,75] Lamo-Espinosa et al., 2016 [70] reported two different intervention groups (High and Low dose) with separate data of the effect. According to Cochrane protocols, to avoid overweighting a single group or study, studies that compared more than two intervention or control groups had the shared group split into two or more groups with smaller sample size and were included as two or more comparisons reasonably independent, using the Review Manager software [48,54,55].
3.4.1.1.1. Primary outcomes
3.4.1.1.1.1. Pain
This outcome was evaluated using the VAS in seven RCTs, however, only five provided the data [43,44,[69], [70], [71]].
In the longer-term available (period of 12 months), pooled results showed MSC was associated with lower pain VAS scores: MD ‐0.99 (95 % CI ‐1.94 to −0.03).
3.4.1.1.1.2. Physical function
This outcome was evaluated using the WOMAC by seven RCT, however, only six provided the data [43,44,[66], [69], [70], [71]].
In the longer-term available (12 months), pooled results showed a MD ‐0.02 (95 % CI ‐0.86 to 0.81) [81].
3.4.1.1.1.3. Serious adverse events
This outcome was evaluated by seven RCTs, with zero serious adverse events in both groups up to the longer term available (12 months), precluding meta-analysis.
3.4.1.1.2. Secondary outcomes
3.4.1.1.2.1. Any adverse event
This outcome was evaluated using the VAS in six RCTs [43,44,[69], [70], [71], [75]].
In the longer-term available (period of 12 months), pooled results showed no differences regarding any adverse events: RR 1.20 (95 % CI 0.81 to 1.79).
3.4.1.1.2.2. Health-related quality of life and need of a ‘second look’ intervention
No studies objectively reported these outcomes.
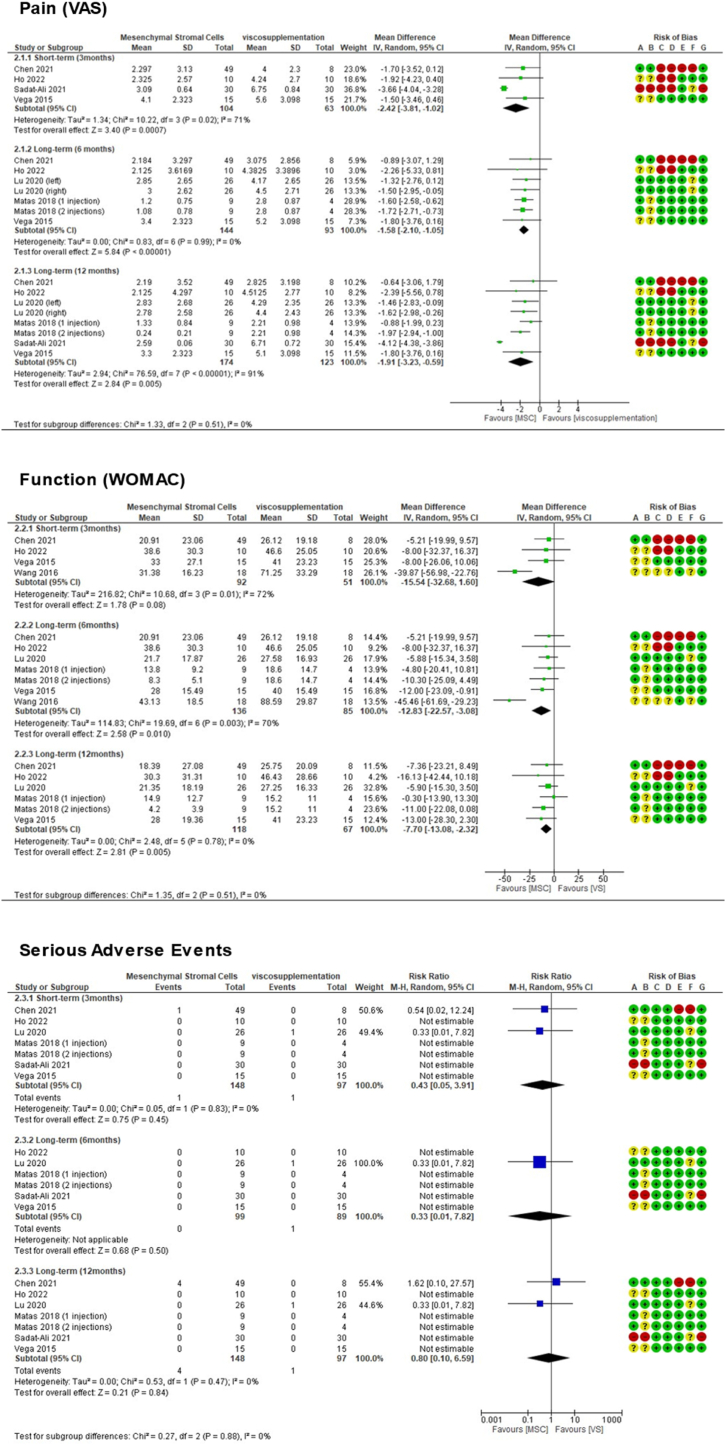
3.4.1.2. Comparison 2: MSC compared with viscosupplementation (VS)
(See Fig. 3)
Figure 3.
Comparison 2 (MSC therapy versus viscosupplementation) forest plot graphs.
This comparison was done by seven RCT [[61], [68], [72], [73], [74], [76], [78]]. Matas et al., 2019 [73] reported two different intervention groups (one or two doses) with separate data of the effect. There was no overlap of patients in the groups and there is homogeneity among the comparatives. According to Cochrane protocols, to avoid overweighting a single group or study, studies that compared more than two intervention or control groups had the shared group split into two or more groups with smaller sample size and were included as two or more comparisons reasonably independent, using the Review Manager software [48,54,55].
3.4.1.2.1. Primary outcomes
3.4.1.2.1.1. Pain
This outcome was evaluated using the VAS in six RCTs [[61], [68], [72], [73], [74], [76]]. In this specific outcome, Lu et al. [72] was described twice considering each knee as the unit of analysis for the intervention and the control groups, as reported in the study itself. There was no overlap of patients in the groups.
In the longer-term available (period of 12 months), pooled results showed MSC was associated with lower pain VAS scores: MD ‐1.91 (95 % CI ‐3.23 to −0.59).
3.4.1.2.1.2. Physical function
This outcome was assessed using the global WOMAC by six RCTs [[61], [68], [72], [73], [74], [76], [78]].
In the longer-term available (period of 12 months), pooled results showed MSC was associated with lower WOMAC scores: MD ‐7.70 (95 % CI ‐13.08 to −2.32).
3.4.1.2.1.3. Serious adverse events
This outcome was assessed by six RCTs [[61], [68], [72], [73], [74], [76]].
In the longer-term available (period of 12 months), pooled results showed no differences regarding serious adverse events: RR 0.80 (95 % CI 0.10 to 6.59).
3.4.1.2.2. Secondary outcomes
3.4.1.2.2.1. Any adverse event
This outcome was assessed by five RCTs.68, 69, 70, 72, 73
In the longer-term available (period of 12 months), pooled results showed no differences regarding any adverse events: RR 0.85, 95 % (CI 0.48 to 1.49).
3.4.1.2.2.2. Health-related quality of life
This outcome was evaluated using the SF-36 by two RCTs [72,78], but at different time points, precluding meta-analysis.
3.4.1.2.2.3. Need of a ‘second look’ intervention
No studies objectively reported this outcome.
3.4.1.3. Osteoarthritis studies not included in meta-analyses
Some included studies regarding OA employed other comparators without the possibility of pooling the results in the meta-analyses [[60], [63], [64], [65], [77], [79], [80]]. Therefore, they were analyzed only qualitatively. Bastos et al., 2020 [60], Freitag et al., 2019 [64], Gupta et al., 2016 [65] and Wong et al., 2013 [79] reported results favorable to advanced cell therapy, regarding pain and function. Fiolin et al., 2020 [63] and Wakitani et al., 2002 [77] reported no differences between groups. Zhao et al., 2019 [80] performed a comparison between different doses, ith significant improvement in higher doses.
3.4.2. Condition 2: Knee chondral lesions
As the review only identified 3 studies evaluating focal chondral lesions [59,67,28], and each of them employed a different technique as comparator, it was not possible to perform a meta-analysis of this section, as it was planned in our protocol.
Akgun et al., 2015 [59] compared matrix-induced MSC versus matrix-induced autologous chondrocyte implantation, with significantly better scores for the MSC group. Hashimoto et al., 2019 [67] compared MSC + MFX versus MFX alone. There were no serious adverse events. KOOS quality of life was higher in the intervention group (p = 0.07). Lim et al., 2021 [28] compared MSC + HA versus MFX. Improvement in VAS pain, WOMAC, and IKDC scores were significantly better in the intervention group at 5 years follow-up (P < 0.05) and greater than the MCID.
3.5. Certainty of evidence assessment through GRADE approach
3.5.1. Certainty of evidence assessment- question 1: advanced therapy with MSC compared to placebo for knee OA
In the ‘MSC versus placebo’ comparison, the certainty of evidence was 'very low’ for the 12-month pain (VAS) reduction of 0.99 (−1.94 to −0.03) with the intervention. All included studies had at least one high RoB domain. Regarding inconsistency, it presented low heterogeneity (I2 of 0 %) and overlapping CI. The evidence was sufficiently direct for all domains. Regarding imprecision downgrade, the studies included few patients (128), but the confidence interval (CI) did not cross the null effect. Publication bias was not assessable due to the low number of studies.
For the 12-month function (WOMAC) evidence of a 0.02 reduction (−0.86 to 0.81), the certainty was 'very low'. All included studies presented crucial limitations for one or more RoB criteria. It downgraded the inconsistency due to CI not overlapping and high heterogeneity (I2 = 87 %). For the imprecision downgrade, the studies included few patients (261) and presented a wide CI crossing the null effect. Publication bias was not assessable due to the low number of studies.
Regarding serious adverse events, there were no events in both arms in this comparison, making meta-analysis impossible.(See Table 2)
Table 2.
Summary of findings.
| Comparison 1 (MSC therapy versus placebo) Patient or population: knee osteoarthritis. Setting: Advanced therapy with mesenchymal stromal cells for knee osteoarthritis or chondral lesions: a systematic review. Intervention: Advanced therapy with mesenchymal stromal cells. Comparison: placebo | ||||||
|---|---|---|---|---|---|---|
| Outcomes | Anticipated absolute effects* (95 % CI) | Relative effect (95 % CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Pain (VAS) - 12 months | The mean pain (VAS) - 12 months ranged from 2.1 to 4.5 | MD 0.99 lower (1.94 lower to 0.03 lower) | — | 128 (5 RCTs) | ⨁◯◯◯ Very lowa,b,c,d,e,g,h |
Advanced therapy with mesenchymal stromal cells may reduce/have little to no effect on pain (VAS) - 12 months but the evidence is very uncertain. |
| Function (WOMAC) - 12 months | The mean function (WOMAC) - 12 months ranged from 9.2 to 44.6 | MD 0.02 lower (0.86 lower to 0.81 higher) |
— | 261 (6 RCTs) | ⨁◯◯◯ Very lowa,d,e,f,g,i,j,k |
The evidence is very uncertain about the effect of advanced therapy with mesenchymal stromal cells on function (WOMAC) - 12 months. |
| Serious Adverse Events - 12 months | not pooled | not pooled | not pooled | 128 (5 RCTs) | ⨁◯◯◯ Very lowd,i,l,m |
Advanced therapy with mesenchymal stromal cells likely does not increase/reduce serious Adverse Events - 12months. |
| Comparison 2 (MSC X Viscosupplementation) Patient or population: knee osteoarthritis. Setting: Advanced therapy with mesenchymal stromal cell for knee osteoarthritis or chondral lesions: a systematic review. Intervention: Advanced therapy with MSC. Comparison: viscosupplementation | ||||||
|---|---|---|---|---|---|---|
| Outcomes | Anticipated absolute effects* (95 % CI) |
Relative effect (95 % CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with VS | Risk with MSC | |||||
| Pain - Long-term (12 months) | The mean pain - Long-term (12 months) ranged from 2.21 to 6.71 | MD 1.91 lower (3.23 lower to 0.59 lower) |
— | 297 (8 RCTs) | ⨁◯◯◯ Very lowb,d,e,f,g,h,j |
Advanced therapy with mesenchymal stromal cells may reduce/have little to no effect on pain - Long-term (12 months) but the evidence is very uncertain. |
| Function - Long-term (12 months) | The mean function - Long-term (12 months) ranged from 15.2 to 46.43 | MD 7.7 lower (13.08 lower to 2.32 lower) |
— | 185 (6 RCTs) | ⨁⨁◯◯ Lowc,d,e,f,g,h,i |
Advanced therapy with mesenchymal stromal cells may result in little to no difference in function - Long-term (12 months). |
| Serious Adverse Events - Long-term (12 months) | 10 per 1.000 |
8 per 1.000 (1–68) |
RR 0.80 (0.10–6.59) |
245 (7 RCTs) | ⨁◯◯◯ Very lowc,d,e,g,h,i,k,l |
The evidence is very uncertain about the effect of advanced therapy with mesenchymal stromal cells on serious Adverse Events - Long-term (12 months). |
CI: confidence interval; MD: mean difference; RR: risk ratio.Explanations: a. Crucial limitation for one or more risk of bias criteria sufficient to substantially lower confidence in the estimate of effect. b. High I2 (>50 %). c. Overlapping CI. d. The evidence is sufficiently direct for all domains. e. Studies include relatively few patients. f. Confidence interval not crossing null effect. g. Publication bias not assessable due to low number of studies. h. Potential limitations are likely to lower confidence in the estimate of effect. i. Low I2. j. CI not overlapping. k. Confidence interval crossing null effect. l. Wide confidence interval. m. Potential limitations are unlikely to lower confidence in the estimate of effect.
3.5.2. Certainty of evidence assessment- question 2: advanced therapy with MSC compared to VS for knee OA
In the MSC versus VS comparison, the evidence presented 'very low' certainty for the 12-month pain (VAS) reduction of 1.91 (−3.23 to −0.59) with MSC. The meta-analysis included a high proportion of information from three studies at high RoB. Also, there was an inconsistency downgrade due to high heterogeneity (I2 of 91 %) and CI not overlapping. Regarding imprecision, the studies included few patients (297). The CI did not cross the null effect, but it ranged from a clinically important effect to an effect value that did not reach the MCID. Publication bias was not assessable due to the low number of studies. Advanced MSC therapy may reduce this outcome, but the evidence is very uncertain.
In this comparison, the evidence for 12-month function (WOMAC) improvement of 7.70 (−13.08 to −2.32) was of 'low' certainty of evidence. Potential RoB limitations were likely to lower confidence in the estimate of effect. There was no inconsistency downgrade, once the studies’ CI were overlapping and the analysis presented low I2 (o%). The evidence was sufficiently direct for all domains. Regarding imprecision, the studies included few patients (185). The CI did not cross the null effect, but it ranged from a clinically important effect to an effect value that did not reach the MCID. Publication bias was not assessable due to the low number of studies. MSC therapy may result in little to no effect on this outcome.
Regarding serious adverse events, the MSC group showed an RR of 0.80 (0.10–6.59) compared with the VS group. This evidence presented 'very low' certainty. Regarding RoB, potential limitations were likely to lower confidence in the estimate of effect. It was not downgraded in inconsistency because it presented low heterogeneity (I2 of 0 %) and overlapping CI among studies. We downgraded the imprecision due to small sample sizes and a wide CI crossing null effect. Publication bias was not assessable due to the low number of studies.(See Table 2)
3.6. Subgroup and sensitivity analyses
3.6.1. Sensitivity analysis
There was insufficient data to perform analyses of autologous versus allogeneic sources of cell and of MSC combined versus not combined with biocompatible materials. The other proposed subgroup analyses are displayed next.
3.6.1.1. Sensitivity analyses of meta-analysis excluding RCTs at high risk of bias on at least one domain
3.6.1.1.1. Comparison 1: MSC compared with placebo
All of the six included studies that compared MSC with placebo had at least one domain classified as high risk, and, therefore, there was insufficient data to perform this analysis.
3.6.1.1.2. Comparison 2: MSC therapy versus VS
Pain (VAS): 12 months period: MD ‐1.54 (95 % CI ‐2.09 to −0.98).
Function (WOMAC): In the 12 months period: MD ‐7.33 (95 % CI ‐13.18 to −1.47).
Serious Adverse Events: Only Lu et al., [72] reported any events (1/26 in intervention and 0/26 in control groups), precluding the meta-analysis.
3.6.1.1.2.1. Certainty of evidence assessment for sensitivity analysis excluding RCTs at high risk of bias- summary of findings
The evidence for advanced therapy with MSC compared to VS for knee OA presents 'moderate' certainty for the 12-month pain (VAS) reduction of 1.54 (−2.09 to −0.98), suggesting that MSC therapy probably results in a slight reduction in this outcome.
There is ‘moderate’ certainty of evidence for the 12-month function improvement of WOMAC 7.33 lower (−13.18 to −1.47), suggesting that MSC therapy probably results in little to no effect on this outcome.
The certainty of evidence improvement was due to exclusion of studies with high RoB, to the low heterogeneity (I2 of 0 %) and to the overlapping of studies’ CI for both pain and function.
3.6.2. Sensitivity analyses of meta-analysis excluding from analysis RCTs with industry sponsorship
3.6.2.1. Comparison 1: MSC compared with placebo
Pain (VAS): In the 12 months period: MD ‐1.22 (95 % CI ‐2.26 to −0.18).
Function (WOMAC): In the 12 months period: MD 0.16 (95 % CI ‐0.22 to −0.55).
3.6.2.2. Comparison 2: MSC compared with VS
Pain (VAS): In the 12 months period: MD -4.00 (95 % CI -4.85 to −3.15).
Function (WOMAC): In the 12 months period: MD -16.13 (95 % CI -42.44 to 10.18).
4. Discussion
The current review has the merit of bringing up a robust and well-developed methodology with evidence certainty analysis and it highlights the current highest level of evidence on clinical outcomes of advanced MSC therapy for knee OA and chondral injuries.
Advanced MSC therapy was better than viscosupplementation to reduce pain and improve function of knee OA after 12 months. The difference of VAS pain (1.91 lower) is considered to be clinically perceptible, as it achieved the aimed minimal clinically important difference (MCID from 1.1 to 2/10) [82]. The same wasn't observed with WOMAC [83,81].
To refine these results, our protocol planned a sensitivity analysis excluding RCTs with high risk of bias, which showed similar size of effect for these comparisons, but with improved certainty. GRADE evaluation here suggests, with moderate certainty, that MSC probably reduces pain slightly better than VS.
When excluding RCTs with industry sponsorship, the effect size favoring MSC for pain and function improvement was even larger (VAS MD -4.00 (−4.85 to −3.15) and WOMAC MD -16.13 (−42.44 to 10.18), ratifying that the findings were probably not influenced by these factors.
When compared to placebo, the advanced MSC therapy resulted in lower pain, although the difference observed did not reach MCID [82] and could be clinically imperceptible. According to GRADE, this evidence was considered to be very uncertain.
When we performed the sensitivity analysis excluding RCTs with industry sponsorship, MSC therapy resulted again in better pain and function improvement than placebo.
Regarding serious adverse events, there was no difference from MSC advanced therapy to placebo or VS, but there were too few people in the combined analysis to properly detect an alteration and the evidence is very uncertain.
The safety profile of this intervention is a crucial aspect [84]. While the present analysis suggests potential benefits without added adverse events, clinicians must be aware of the low number of participants and relatively short follow-up [85,86]. Furthermore, there is added concern about publication bias involved with this type of therapy and underreport of possible effects.
The few studies that evaluated chondral lesions showed no difference in serious adverse events and, in general, no significant differences in clinical outcome scores, all three with high risk of performance and detection bias.
Previous review articles that attempted to investigate clinical outcomes lack a good methodological structure and a well-directed clinical question to have an impact on medical practice [2,3,5,7,10,12,13,[24], [25], [26], [27], [28], [29],32,34,35,47]. The present study was well structured and conducted, with Risk of Bias (RoB), GRADE certainty assessment and sensitivity analysis, to answer a specific question by pooling the most current and highest-level evidence and, thus, expanding the field of research in this area.
It is wise to look carefully at the data and to consider the quality of evidence influenced by within-study biases and by the methodological variation across studies. For example, it is interesting to observe that MSC therapy had a greater effect when compared to VS than when compared to placebo, which, at first, could suggest that VS itself would be worse than placebo. But we can notice that the studies with placebo were studies with worse methodology and higher risk of bias scores in our analyses. All of them had at least 1 domain with high RoB, unlike those studies with VS. Also, there were far fewer included patients for pain evaluation in the placebo studies.
Another interesting observation is the consistent increase in the effect size for pain improvement accompanied by better certainty of evidence when we exclude highly biased and sponsored studies. In other words, when filtering the most reliable studies, the impact of MSC therapy effect was even better. This mitigates the hypothesis that the positive effect could be influenced by lower quality studies. We believe that the quality of the evidence was not higher due to the heterogeneity of both techniques and outcome assessment design.
Our observations confirms and improves previous results presented in most other human studies and in reviews with different objectives or methodologies [3,35,[87], [88], [89], [90], [91], [92], [93], [94]], suggesting that MSC treatment can lead to pain relief in both short-term and long-term assessments and also tends to improve physical function in patients with knee osteoarthritis. Although it is adequate to consider all the differences of design and methodology weakness of each one, collectively these findings complement each other to suggest that MSC treatment could be an effective and safety alternative for OA and chondral injuries.
This therapy has shown great promise for the treatment of osteoarthritis and can benefit a huge number of people and healthcare systems, being a less costly, less morbid and more effective option.
The positive results found here should stimulate approval of MSC studies in regulatory centers and encourage major research institutes around the world to invest in larger RCTs, as it appears to be safe and better than current conservative options. The medical world needs standardization of MSC sources, doses and manufacturing to facilitate its implementation.
In conclusion, although the evidence was considered by GRADE assessment to be uncertain, the present review shows that advanced MSC therapy resulted in lower pain than placebo or viscosupplementation for the treatment of knee OA after 12 months.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors, and no material support of any kind was received.
Declaration of competing interest
The authors declare that they have no conflicts of interest related to this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2024.07.012.
Contributor Information
Caio Gomes Tabet, Email: caiotabet@hotmail.com, caiotabet9@gmail.com.
Rafael Leite Pacheco, Email: rleitepacheco@hotmail.com.
Ana Luiza Cabrera Martimbianco, Email: analuizacabrera@hotmail.com.
Rachel Riera, Email: rachelriera@hotmail.com.
Arnaldo José Hernandez, Email: ajhernandez@uol.com.br.
Daniela Franco Bueno, Email: dbuenousp@gmail.com.
Tiago Lazzaretti Fernandes, Email: tiagot86@hotmail.com.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.James S.L.G., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chu C.R., Rodeo S., Bhutani N., Goodrich L.R., Huard J., Irrgang J., et al. Optimizing clinical use of biologics in orthopaedic surgery: consensus recommendations from the 2018 AAOS/NIH U-13 conference. J Am Acad Orthop Surg. 2019;27(2):e50–e63. doi: 10.5435/JAAOS-D-18-00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang R., Li W., Zhao Y., Yang F., Xu M. Clinical efficacy and safety of stem cell therapy for knee osteoarthritis: a meta-analysis. Medicine (Baltim) 2020;99(11) doi: 10.1097/MD.0000000000019434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filardo G., Kon E., Longo U.G., Madry H., Marchettini P., Marmotti A., et al. Non-surgical treatments for the management of early osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1775–1785. doi: 10.1007/s00167-016-4089-y. [DOI] [PubMed] [Google Scholar]
- 5.Thoene M., Bejer-Olenska E., Wojtkiewicz J. The current state of osteoarthritis treatment options using stem cells for regenerative therapy: a review. Int J Mol Sci. 2023;24(10) doi: 10.3390/ijms24108925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glyn-Jones S., Palmer A.J., Agricola R., Price A.J., Vincent T.L., Weinans H., et al. Osteoarthritis. Lancet. 2015;386(9991):376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 7.Magnussen R.A., Dunn W.R., Carey J.L., Spindler K.P. Treatment of focal articular cartilage defects in the knee: a systematic review. Clin Orthop Relat Res. 2008;466(4):952–962. doi: 10.1007/s11999-007-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krych A.J., Pareek A., King A.H., Johnson N.R., Stuart M.J., Williams R.J. Return to sport after the surgical management of articular cartilage lesions in the knee: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2017;25(10):3186–3196. doi: 10.1007/s00167-016-4262-3. [DOI] [PubMed] [Google Scholar]
- 9.Shimomura K., Ando W., Moriguchi Y., Sugita N., Yasui Y., Koizumi K., et al. Next generation mesenchymal stem cell (MSC)-Based cartilage repair using scaffold-free tissue engineered constructs generated with synovial mesenchymal stem cells. Cartilage. 2015;6(2 Suppl):13S–29S. doi: 10.1177/1947603515571002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brambilla L., Scotti C., Gobbi A., Peretti G.M. In: Bio-orthopaedics. Gobbi A., Espregueira-Mendes J., Lane J.G., Karahan M., editors. Springer; Berlin Heidelberg: 2017. Evolving perspectives in orthobiologic approaches to articular cartilage regeneration; pp. 637–649. [DOI] [Google Scholar]
- 11.Seo S.-S., Kim C.-W., Jung D.-W. Management of focal chondral lesion in the knee joint. Knee Surg Relat Res. 2011;23(4):185–196. doi: 10.5792/ksrr.2011.23.4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandes T.L., Cortez de SantAnna J.P., Frisene I., Gazarini J.P., Gomes Pinheiro C.C., Gomoll A.H., et al. Systematic review of human dental pulp stem cells for cartilage regeneration. Tissue Eng Part B. 2020;26(1):1–12. doi: 10.1089/ten.TEB.2019.0140. [DOI] [PubMed] [Google Scholar]
- 13.Fernandes T.L., Shimomura K., Hart D.A., Boffa A., Nakamura N. In: Orthobiologics: injectable therapies for the musculoskeletal system. Filardo G., Mandelbaum B.R., Muschler G.F., Rodeo S.A., Nakamura N., editors. Springer International Publishing; 2022. Cartilage lesions and osteoarthritis: cell therapy; pp. 301–314. [DOI] [Google Scholar]
- 14.Flanigan D.C., Harris J.D., Trinh T.Q., Siston R.A., Brophy R.H. Prevalence of chondral defects in athletes' knees: a systematic review. Med Sci Sports Exerc. 2010;42(10):1795–1801. doi: 10.1249/MSS.0b013e3181d9eea0. [DOI] [PubMed] [Google Scholar]
- 15.Goldring M.B., Marcu K.B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther. 2009;11(3) doi: 10.1186/ar2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demoor M., Ollitrault D., Gomez-Leduc T., Bouyoucef M., Hervieu M., Fabre H., et al. Cartilage tissue engineering: molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochim Biophys Acta. 2014;1840(8):2414–2440. doi: 10.1016/j.bbagen.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 17.Caplan A.I. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 18.Caplan A.I. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217(2):318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fakunle E.S., Lane J.G. In: Bio-orthopaedics. Gobbi A., Espregueira-Mendes J., Lane J.G., Karahan M., editors. Springer; Berlin Heidelberg: 2017. Cell culture approaches for articular cartilage: repair and regeneration; pp. 161–172. [DOI] [Google Scholar]
- 20.Caplan A.I. Mesenchymal stem cells: time to change the name. Stem Cells Transl Med. 2017;6(6):1445–1451. doi: 10.1002/sctm.17-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muschler G.F., Simmons H., Mantripragada V., Piuzzi N.S. In: Orthobiologics: injectable therapies for the musculoskeletal system. Filardo G., Mandelbaum B.R., Muschler G.F., Rodeo S.A., Nakamura N., editors. Springer International Publishing; 2022. The stem and progenitor cell paradigms and engineering principles guiding the clinical use of cells or cell-derived products for regenerative medicine; pp. 3–28. [DOI] [Google Scholar]
- 22.Wagner W., Horn P., Castoldi M., Diehlmann A., Bork S., Saffrich R., et al. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 2008;3(5) doi: 10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee E., Epanomeritakis I.E., Lu V., Khan W. Bone marrow-derived mesenchymal stem cell implants for the treatment of focal chondral defects of the knee in animal models: a systematic review and meta-analysis. Int J Mol Sci. 2023 Feb 6;24(4):3227. doi: 10.3390/ijms24043227. PMID: 36834639; PMCID: PMC9958893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belk J.W., Kraeutler M.J., Houck D.A., Goodrich J.A., Dragoo J.L., McCarty E.C. Platelet-Rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am J Sports Med. 2021;49(1):249–260. doi: 10.1177/0363546520909397. [DOI] [PubMed] [Google Scholar]
- 25.Chahla J., Dean C.S., Moatshe G., Pascual-Garrido C., Serra Cruz R., LaPrade R.F. Concentrated bone marrow aspirate for the treatment of chondral injuries and osteoarthritis of the knee: a systematic review of outcomes. Orthop J Sports Med. 2016;4(1) doi: 10.1177/2325967115625481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salem H.K., Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cell. 2010;28(3):585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slynarski K., Stevens H.P., van Dongen J.A., Baszczeski F., Lipinski L. In: Bio-orthopaedics. Gobbi A., Espregueira-Mendes J., Lane J.G., Karahan M., editors. Springer; Berlin Heidelberg: 2017. Use of stem cells in orthopaedics; pp. 197–204. [DOI] [Google Scholar]
- 28.Lim H.C., Park Y.B., Ha C.W., Cole B.J., Lee B.K., Jeong H.J., et al. Allogeneic umbilical cord blood-derived mesenchymal stem cell implantation versus microfracture for large, full-thickness cartilage defects in older patients: a multicenter randomized clinical trial and extended 5-year clinical follow-up. Orthop J Sports Med. 2021;9(1) doi: 10.1177/2325967120973052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scotti C., Koizumi K., Nakamura N. In: Bio-orthopaedics. Gobbi A., Espregueira-Mendes J., Lane J.G., Karahan M., editors. Springer; Berlin Heidelberg: 2017. Stem cells in joint repair; pp. 205–211. [DOI] [Google Scholar]
- 30.Gobbi A., de Girolamo L., Whyte G.P., Sciarretta F.V. In: Bio-orthopaedics. Gobbi A., Espregueira-Mendes J., Lane J.G., Karahan M., editors. Springer; Berlin Heidelberg: 2017. Clinical applications of adipose tissue-derived stem cells; pp. 553–559. [DOI] [Google Scholar]
- 31.Sekiya I., Ozeki N. In: Orthobiologics: injectable therapies for the musculoskeletal system. Filardo G., Mandelbaum B.R., Muschler G.F., Rodeo S.A., Nakamura N., editors. Springer International Publishing; 2022. Injections of synovial mesenchymal stromal cells; pp. 63–74. [DOI] [Google Scholar]
- 32.Robinson P.G., Murray I.R., West C.C., Goudie E.B., Yong L.Y., White T.O., et al. Reporting of mesenchymal stem cell preparation protocols and composition: a systematic review of the clinical orthopaedic literature. Am J Sports Med. 2019;47(4):991–1000. doi: 10.1177/0363546518758667. [DOI] [PubMed] [Google Scholar]
- 33.Agung M., Ochi M., Yanada S., Adachi N., Izuta Y., Yamasaki T., et al. Mobilization of bone marrow-derived mesenchymal stem cells into the injured tissues after intraarticular injection and their contribution to tissue regeneration. Knee Surg Sports Traumatol Arthrosc. 2006;14(12):1307–1314. doi: 10.1007/s00167-006-0124-8. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg A., Mitchell K., Soans J., Kim L., Zaidi R. The use of mesenchymal stem cells for cartilage repair and regeneration: a systematic review. J Orthop Surg Res. 2017;12(1):39. doi: 10.1186/s13018-017-0534-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim S.H., Ha C.-W., Park Y.-B., Nam E., Lee J.-E., Lee H.-J. Intra-articular injection of mesenchymal stem cells for clinical outcomes and cartilage repair in osteoarthritis of the knee: a meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg. 2019;139(7):971–980. doi: 10.1007/s00402-019-03140-8. [DOI] [PubMed] [Google Scholar]
- 36.Freitag J., Ford J., Bates D., Boyd R., Hahne A., Wang Y., et al. Adipose derived mesenchymal stem cell therapy in the treatment of isolated knee chondral lesions: design of a randomised controlled pilot study comparing arthroscopic microfracture versus arthroscopic microfracture combined with postoperative mesenchymal stem cell injections. BMJ Open. 2015;5(12) doi: 10.1136/bmjopen-2015-009332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diekman B.O., Rowland C.R., Lennon D.P., Caplan A.I., Guilak F. Chondrogenesis of adult stem cells from adipose tissue and bone marrow: induction by growth factors and cartilage-derived matrix. Tissue Eng. 2010;16(2):523–533. doi: 10.1089/ten.TEA.2009.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray I.R., Péault B. Q&A: mesenchymal stem cells - where do they come from and is it important? BMC Biol. 2015;13:99. doi: 10.1186/s12915-015-0212-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen W.C., Park T.S., Murray I.R., Zimmerlin L., Lazzari L., Huard J., et al. Cellular kinetics of perivascular MSC precursors. Stem Cell Int. 2013;2013 doi: 10.1155/2013/983059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kouroupis D., Sanjurjo-Rodriguez C., Jones E., Correa D. Mesenchymal stem cell functionalization for enhanced therapeutic applications. Tissue Eng Part B. 2019;25(1):55–77. doi: 10.1089/ten.TEB.2018.0118. [DOI] [PubMed] [Google Scholar]
- 41.Filardo G., Boffa A., Andriolo L., Poggi A., Di Martino A. In: Orthobiologics: injectable therapies for the musculoskeletal system. Filardo G., Mandelbaum B.R., Muschler G.F., Rodeo S.A., Nakamura N., editors. Springer International Publishing; 2022. Cartilage lesions and osteoarthritis of the knee: biologics; pp. 315–327. [DOI] [Google Scholar]
- 42.Riddle D.L., Jiranek W.A., Hayes C.W. Use of a validated algorithm to judge the appropriateness of total knee arthroplasty in the United States: a multicenter longitudinal cohort study. Arthritis Rheumatol. 2014;66(8):2134–2143. doi: 10.1002/art.38685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuah D., Sivell S., Longworth T., James K., Guermazi A., Cicuttini F., et al. Safety, tolerability and efficacy of intra-articular Progenza in knee osteoarthritis: a randomized double-blind placebo-controlled single ascending dose study. J Transl Med. 2018;16(1) doi: 10.1186/s12967-018-1420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamo-Espinosa J.M., Blanco J.F., Sánchez M., Moreno V., Granero-Moltó F., Sánchez-Guijo F., et al. Phase II multicenter randomized controlled clinical trial on the efficacy of intra-articular injection of autologous bone marrow mesenchymal stem cells with platelet rich plasma for the treatment of knee osteoarthritis. J Transl Med. 2020;18(1):356. doi: 10.1186/s12967-020-02530-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ankrum J.A., Ong J.F., Karp J.M. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boehme K.A., Schleicher S.B., Traub F., Rolauffs B. Chondrosarcoma: a rare misfortune in aging human cartilage? the role of stem and progenitor cells in proliferation, malignant degeneration and therapeutic resistance. Int J Mol Sci. 2018;19(1) doi: 10.3390/ijms19010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chahla J., Piuzzi N.S., Mitchell J.J., Dean C.S., Pascual-Garrido C., LaPrade R.F., et al. Intra-articular cellular therapy for osteoarthritis and focal cartilage defects of the knee: a systematic review of the literature and study quality analysis. J Bone Joint Surg Am. 2016;98(18):1511–1521. doi: 10.2106/JBJS.15.01495. [DOI] [PubMed] [Google Scholar]
- 48.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., et al. Cochrane Handbook for systematic reviews of interventions version 6.3. Cochrane, 2022; 2022. Cochrane Handbook for systematic reviews of interventions version 6.3.www.training.cochrane.org/handbookhttp://www.training.cochrane.org/handbook February 2022. [Google Scholar]
- 49.Page M., McKenzie J., Bossuyt P., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Altman R., Brandt K., Hochberg M., Moskowitz R., Bellamy N., Bloch D.A., et al. Design and conduct of clinical trials in patients with osteoarthritis: recommendations from a task force of the Osteoarthritis Research Society. Results from a workshop. Osteoarthritis Cartilage. 1996;4(4):217–243. doi: 10.1016/s1063-4584(05)80101-3. [DOI] [PubMed] [Google Scholar]
- 51.FDA (U.S Food and Drug Administration). What is a serious adverse event? FDA 2016. U.S Food and Drug Administration. What is a serious adverse event? 2016. 2016. https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event
- 52.Jüni P., Reichenbach S., Dieppe P. Osteoarthritis: rational approach to treating the individual. Best Pract Res Clin Rheumatol. 2006;20(4):721–740. doi: 10.1016/j.berh.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 53.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1) doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.The Cochrane Collaboration . The Cochrane Collaboration; 2020. Review manager.http://revman.cochrane.org [Google Scholar]
- 55.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 56.Guyatt G.H., Oxman A.D., Vist G.E., Kunz R., Falck-Ytter Y., Alonso-Coello P., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347. [AD] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schünemann H., Brożek J., Guyatt G., Oxman A. GRADE Handbook for grading quality of evidence and strength of recommendations. 2013. http://guidelinedevelopment.org/handbook
- 58.GRADEpro Guideline Development Tool . McMaster University and Evidence Prime; 2022. GRADEpro GDT. [Google Scholar]
- 59.Akgun I., Unlu M.C., Erdal O.A., Ogut T., Erturk M., Ovali E., et al. Matrix-induced autologous mesenchymal stem cell implantation versus matrix-induced autologous chondrocyte implantation in the treatment of chondral defects of the knee: a 2-year randomized study. Arch Orthop Trauma Surg. 2015;135(2):251–263. doi: 10.1007/s00402-014-2136-z. [DOI] [PubMed] [Google Scholar]
- 60.Bastos R., Mathias M., Andrade R., Amaral R.J.F.C., Schott V., Balduino A., et al. Intra-articular injection of culture-expanded mesenchymal stem cells with or without addition of platelet-rich plasma is effective in decreasing pain and symptoms in knee osteoarthritis: a controlled, double-blind clinical trial. Knee Surg Sports Traumatol Arthrosc. 2020;28(6):1989–1999. doi: 10.1007/s00167-019-05732-8. [DOI] [PubMed] [Google Scholar]
- 61.Chen C.F., Hu C.C., Wu C.T., Wu H.H., Chang C.S., Hung Y.P., et al. Treatment of knee osteoarthritis with intra-articular injection of allogeneic adipose-derived stem cells (ADSCs) ELIXCYTE®: a phase I/II, randomized, active-control, single-blind, multiple-center clinical trial. Stem Cell Res Ther. 2021;12(1):562. doi: 10.1186/s13287-021-02631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Emadedin M., Labibzadeh N., Liastani M.G., Karimi A., Jaroughi N., Bolurieh T., et al. Intra-articular implantation of autologous bone marrow-derived mesenchymal stromal cells to treat knee osteoarthritis: a randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy. 2018;20(10):1238–1246. doi: 10.1016/j.jcyt.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 63.Fiolin J., Dilogo I.H., Lubis A.M.T., Pawitan J.A., Liem I.K., Pandelaki J., et al. Functional and radiological comparison of umbilical cord mesenchymal stem cells, somatotropin, and hyaluronic acid injection for cartilage repair in early osteoarthritis of the knee: a randomized controlled trial. Orthop J Sports Med. 2020;8(5_suppl5) doi: 10.1177/2325967120S00045. [DOI] [Google Scholar]
- 64.Freitag J., Bates D., Wickham J., Shah K., Huguenin L., Tenen A., et al. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regen Med. 2019;14(3):213–230. doi: 10.2217/rme-2018-0161. [DOI] [PubMed] [Google Scholar]
- 65.Gupta P.K., Chullikana A., Rengasamy M., Shetty N., Pandey V., Agarwal V., et al. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res Ther. 2016;18(1) doi: 10.1186/s13075-016-1195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gupta P.K., Maheshwari S., Cherian J., Goni V., Sharma A., Tripathy S.K., et al. Mesenchymal stem/stromal cells: allogeneic, off the shelf, pooled, Bm - Mscs (Stempeucel®) – a potential break through therapy for grade ii and Iii osteoarthritis knee. Cytotherapy. 2022;24(5):S32–S33. doi: 10.1016/S1465-3249(22)00136-0. [DOI] [Google Scholar]
- 67.Hashimoto Y., Nishida Y., Takahashi S., Nakamura H., Mera H., Kashiwa K., et al. Transplantation of autologous bone marrow-derived mesenchymal stem cells under arthroscopic surgery with microfracture versus microfracture alone for articular cartilage lesions in the knee: a multicenter prospective randomized control clinical trial. Regenerative Therapy. 2019;11:106–113. doi: 10.1016/j.reth.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ho K.K.-W., Lee W.Y.-W., Griffith J.F., Ong M.T.-Y., Li G. Randomized control trial of mesenchymal stem cells versus hyaluronic acid in patients with knee osteoarthritis - a Hong Kong pilot study. J Orthop Translat. 2022;37:69–77. doi: 10.1016/j.jot.2022.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shadmanfar S., Labibzadeh N., Emadedin M., Jaroughi N., Azimian V., Mardpour S., et al. Intra-articular knee implantation of autologous bone marrow-derived mesenchymal stromal cells in rheumatoid arthritis patients with knee involvement: results of a randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy. 2018;20(4):499–506. doi: 10.1016/j.jcyt.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 70.Lamo-Espinosa J.M., Mora G., Blanco J.F., Granero-Moltó F., Nuñez-Córdoba J.M., Sánchez-Echenique C., et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: multicenter randomized controlled clinical trial (phase I/II) J Transl Med. 2016;14(1) doi: 10.1186/s12967-016-0998-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee W.-S., Kim H.J., Kim K.-I., Kim G.B., Jin W. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Transl Med. 2019;8(6):504–511. doi: 10.1002/sctm.18-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu L., Dai C., Du H., Li S., Ye P., Zhang L., et al. Intra-articular injections of allogeneic human adipose-derived mesenchymal progenitor cells in patients with symptomatic bilateral knee osteoarthritis: a Phase I pilot study. Regen Med. 2020;15(5):1625–1636. doi: 10.2217/rme-2019-0106. [DOI] [PubMed] [Google Scholar]
- 73.Matas J., Orrego M., Amenabar D., Infante C., Tapia-Limonchi R., Cadiz M.I., et al. Umbilical cord-derived mesenchymal stromal cells (MSCs) for knee osteoarthritis: repeated MSC dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized phase I/II trial. Stem Cells Transl Med. 2019;8(3):215–224. doi: 10.1002/sctm.18-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sadat-Ali M., AlOmran A.S., AlMousa S.A., AlSayed H.N., AlTabash K.W., Azam M.Q., et al. Autologous bone marrow-derived chondrocytes for patients with knee osteoarthritis: a randomized controlled trial. Adv Orthop. 2021;2021 doi: 10.1155/2021/2146722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khalifeh Soltani S., Forogh B., Ahmadbeigi N., Hadizadeh Kharazi H., Fallahzadeh K., Kashani L., et al. Safety and efficacy of allogenic placental mesenchymal stem cells for treating knee osteoarthritis: a pilot study. Cytotherapy. 2019;21(1):54–63. doi: 10.1016/j.jcyt.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 76.Vega A., Martín-Ferrero M.A., Del Canto F., Alberca M., García V., Munar A., et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99(8):1681–1690. doi: 10.1097/TP.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 77.Wakitani S., Imoto K., Yamamoto T., Saito M., Murata N., Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10(3):199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 78.Wang Y., Jin W., Liu H., Cui Y., Mao Q., Fei Z., et al. [curative effect of human umbilical cord mesenchymal stem cells by intra-articular injection for degenerative knee osteoarthritis] Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2016;30(12):1472–1477. doi: 10.7507/1002-1892.20160305. [DOI] [PubMed] [Google Scholar]
- 79.Wong K.L., Lee K.B.L., Tai B.C., Law P., Lee E.H., Hui J.H.P. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years' follow-up. Arthroscopy. 2013;29(12):2020–2028. doi: 10.1016/j.arthro.2013.09.074. [DOI] [PubMed] [Google Scholar]
- 80.Zhao X., Ruan J., Tang H., Li J., Shi Y., Li M., et al. Multi-compositional MRI evaluation of repair cartilage in knee osteoarthritis with treatment of allogeneic human adipose-derived mesenchymal progenitor cells. Stem Cell Res Ther. 2019;10(1) doi: 10.1186/s13287-019-1406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pham T., van der Heijde D., Altman R.D., Anderson J.J., Bellamy N., Hochberg M., et al. OMERACT-OARSI initiative: osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis Cartilage. 2004;12(5):389–399. doi: 10.1016/j.joca.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 82.Hawker G.A., Mian S., Kendzerska T., French M. Measures of adult pain: visual analog scale for pain (VAS pain), numeric rating scale for pain (NRS pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short form-36 bodily pain scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP) Arthritis Care Res. 2011;63(Suppl 11):S240–S252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 83.Clement N.D., Bardgett M., Weir D., Holland J., Gerrand C., Deehan D.J. What is the minimum clinically important difference for the WOMAC index after TKA? Clin Orthop Relat Res. 2018;476(10):2005–2014. doi: 10.1097/CORR.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Y., Han Z.-B., Song Y.-P., Han Z.C. Safety of mesenchymal stem cells for clinical application. Stem Cell Int. 2012;2012 doi: 10.1155/2012/652034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu C., Zhou X., Zorzela L.M., Ju K., Furuya-Kanamori L., Lin L., et al. Utilization of the evidence from studies with no events in meta-analyses of adverse events: an empirical investigation. BMC Med. 2021;19(1) doi: 10.1186/s12916-021-02008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zorzela L., Golder S., Liu Y., Pilkington K., Hartling L., Joffe A., et al. Quality of reporting in systematic reviews of adverse events: systematic review. BMJ. 2014;348 doi: 10.1136/bmj.f7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yubo M., Yanyan L., Li L., Tao S., Bo L., Lin C. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: a meta-analysis. PLoS One. 2017;12(4) doi: 10.1371/journal.pone.0175449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shoukrie S.I., Venugopal S., Dhanoa R.K., Selvaraj R., Selvamani T.Y., Zahra A., et al. Safety and efficacy of injecting mesenchymal stem cells into a human knee joint to treat osteoarthritis: a systematic review. Cureus. 2022;14(5) doi: 10.7759/cureus.24823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jevotovsky D.S., Alfonso A.R., Einhorn T.A., Chiu E.S. Osteoarthritis and stem cell therapy in humans: a systematic review. Osteoarthritis Cartilage. 2018;26(6):711–729. doi: 10.1016/j.joca.2018.02.906. [DOI] [PubMed] [Google Scholar]
- 90.Song Y., Zhang J., Xu H., Lin Z., Chang H., Liu W., et al. Mesenchymal stem cells in knee osteoarthritis treatment: a systematic review and meta-analysis. J Orthop Translat. 2020;24:121–130. doi: 10.1016/j.jot.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wiggers T.G., Winters M., Van den Boom N.A., Haisma H.J., Moen M.H. Autologous stem cell therapy in knee osteoarthritis: a systematic review of randomised controlled trials. Br J Sports Med. 2021;55(20):1161–1169. doi: 10.1136/bjsports-2020-103671. [DOI] [PubMed] [Google Scholar]
- 92.Pas H.I., Winters M., Haisma H.J., Koenis M.J., Tol J.L., Moen M.H. Stem cell injections in knee osteoarthritis: a systematic review of the literature. Br J Sports Med. 2017;51(15):1125–1133. doi: 10.1136/bjsports-2016-096793. [DOI] [PubMed] [Google Scholar]
- 93.Álvarez Hernández P., de la Mata Llord J. Expanded Mesenchymal Stromal Cells in knee osteoarthritis: a systematic literature review. Reumatol Clínica. 2022;18(1):49–55. doi: 10.1016/j.reumae.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 94.Kyriakidis T., Pitsilos C., Iosifidou M., Tzaveas A., Gigis I., Ditsios K., et al. Stem cells for the treatment of early to moderate osteoarthritis of the knee: a systematic review. J EXP ORTOP. 2023;10(1):102. doi: 10.1186/s40634-023-00665-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.