Abstract
Introduction
Clinical trials often enroll nonrepresentative participant samples, limiting generalizability of trial findings. The current analysis explores the influences of remote recruitment and screening protocols on participation in a digital health intervention (DHI) to promote the evidence-based Dietary Approaches to Stop Hypertension (DASH) eating pattern.
Methods
Nourish was a 12-month randomized controlled trial comparing the effectiveness of a DHI to an attention control arm among US adults with hypertension. Participants were recruited using digital approaches; eligible individuals completed several screening steps. We examined associations between sociodemographics and mobile technology use and completion of each screening step and compared those characteristics between randomized and nonrandomized participants (those consented but were screened out before randomization).
Results
A total of 678 adults consented to participate in Nourish; 44% of those consented were randomized (n = 301). Those randomized possessed a higher education level (p < 0.0001); were more likely to use health-related apps (p < 0.0001) and wearables (p < 0.0001); and were older (p = 0.01) than nonrandomized individuals. Randomized adults were more likely to use a desktop/laptop/tablet for Internet access (vs mobile phones) (p = 0.01). No significant association was observed existed between sex, race, ethnicity, income, or geographic density of residence and subsequent randomization.
Conclusions
Participants with lower education levels or limited experience in using mobile technologies may require additional support to participate in DHIs. Future research is needed to evaluate remote clinical trial procedures and impacts on generalizability to achieve equitable clinical trial participation.
Keywords: Hypertension, remote clinical trials, cardiovascular, lifestyle, recruitment, diversity
Introduction
Nearly half of USA adults have hypertension (HTN), 1 a risk factor for heart failure, stroke, and kidney disease. 2 A first-line treatment approach for adults with HTN is the Dietary Approaches to Stop Hypertension (DASH) diet, an evidence-based dietary pattern shown to lower blood pressure. 3 For many adults, following DASH requires significant behavioral change and resources, such as access to transportation and nutritious food; consequently, DASH adherence remains sub-optimal and varies across population groups. 4 For example, DASH adherence is often less common among those with lower health literacy and educational achievement. 5 Accessible dissemination strategies for diverse communities are needed to increase DASH adherence.
Clinical trials are needed to identify effective dissemination strategies for health interventions like DASH. Yet clinical trial participant populations often do not reflect the individuals experiencing chronic disease burden. This limits the generalizability of trial results to diverse populations, and ultimately, the uptake of evidence-based interventions. For instance, an analysis of clinical trials supporting FDA approval of treatment for cardiovascular conditions showed that participation among Black adults was only 2.9%. 6 The authors found that the participation-to-prevalence ratio, calculated by dividing the percent of participants in a trial population by the percent of participants among a disease population, was highest among HTN trials, yet Black adults were still underrepresented. 6 Other demographic factors and participant characteristics have also been identified as predictors of clinical trial enrollment; for example, individuals with lower education levels and those living in rural areas are less likely to participate.7,8
Clinical trial procedures, such as recruitment, screening, and data collection, can be inaccessible or burdensome to complete for research participants; this in turn may influence study enrollment and representation. Remote procedures and digital health strategies offer an opportunity to improve accessibility of and inclusive enrollment practices in clinical trials. 9 For example, these strategies can alleviate participant barriers related to distance to research sites and medical centers, access to transportation, and schedule flexibility. Leveraging health technology to disseminate health interventions may also improve equitable access to evidence-based treatments, such as DASH. On the other hand, the use of digital health strategies simultaneously has the opportunity to introduce conflicting concerns, such as the need for digital access and literacy. 10 As digital health strategies for clinical trial delivery continue to grow, it is important to understand the impacts on diversity in clinical trial enrollment and participation.
Within this landscape, we launched Nourish, a randomized controlled trial to test the effectiveness of a tailored digital health intervention to increase the dissemination of and adherence to DASH among US adults with HTN. 11 Here, we describe analyses we conducted to elucidate the influence of our remote recruitment and screening protocols on our randomized participant sample. The objectives of these specific analyses were to:
Describe recruitment yields and sociodemographic characteristics of enrollees in the Nourish trial.
Evaluate the association between sociodemographics, mobile technology use characteristics, and the completion of screening steps in the Nourish trial.
Assess the differences in sociodemographics and mobile technology use between individuals who were randomized and those not randomized in the Nourish trial.
Methods
Study design, population, and intervention
The Nourish trial has been previously described and is registered on Clinicaltrials.gov, NCT03875768.11,12 In brief, Nourish was a 12-month, two-arm randomized controlled trial that compared the efficacy of a digital health HTN intervention to an attention control arm. The primary outcome was 6-month change in DASH adherence, and the secondary outcome was 6-month change in blood pressure. The intervention was delivered via a smartphone application (app) customized for the Nourish trial.
Nourish randomized 301 adults from across the USA with self-reported HTN, defined as a systolic blood pressure of 120–159 mmHg; a diastolic blood pressure of 80–99 mmHg 13 ; and/or those on blood pressure-lowering medication for the treatment of HTN. Other major eligibility criteria included having a smartphone with a data plan; having an active email account; being willing to receive daily text messages; and being able to write and read in English. Participants received skills training on the DASH dietary pattern and were asked to track their daily food and beverage intake within the Nourish app for 12 months. Those randomized to the intervention arm also received daily nutrient goals; personalized text message feedback; and responsive health coaching by a registered dietitian. The personalized feedback and responsive health coaching were informed by their daily dietary tracking and goal progress. All Nourish procedures were approved by the Duke Health Institutional Review Board. Recruitment for the trial began in September 2020.
Recruitment, screening, and enrollment procedures
Recruitment
Using an adaptive approach, we deployed several recruitment methods to meet our recruitment goal of enrolling 40% of participants from a racial or ethnic group traditionally underrepresented in research (racial/ethnic minorities) and 30% of participants who identified as male. Recruitment methods included messaging patients in Duke's electronic health record (EHR) patient portal, ResearchMatch, online listings (e.g. Craigslist), and social media (e.g. Facebook, Twitter, and Reddit). We also leveraged connections with local networks of health agencies and other organizations that serve patients with HTN to share trial information. Lastly, we posted information about the Nourish trial on ClinicalTrials.gov and the Duke University clinical trials website.
Throughout active recruitment, we monitored recruitment yields on a weekly basis. This included breakdown by key demographic criteria to ensure we were meeting our goals for diversity in sex, race, and ethnicity. Following, we would adjust our outreach approach. For example, we would refine our queries in the EHR or ResearchMatch to prioritize individuals that were not adequately represented (e.g. males or adults who identify as Black or African American).
Screening and enrollment
Individuals interested in participating in Nourish were asked to complete several steps before enrollment and randomization. All steps could be completed from a smartphone device. This included a survey to determine initial eligibility status; informed consent; a baseline sociodemographic survey; two Automated Self-Administered 24-h Dietary Assessment Tool (ASA24®) dietary recalls14,15; an introductory study visit via Zoom; a dietary tracking run-in period; and a final eligibility visit. All study visits, including those during the screening processes, were conducted using Zoom video conferencing. Each screening step is described in further detail below.
Eligibility survey
Anyone interested in participating in the Nourish trial was directed to a brief, online prescreening eligibility survey via REDCap (Research Electronic Data Capture).16,17 Survey information determined initial eligibility status, including owning a smartphone with email and a data plan and being able to participate in Zoom videoconferencing. 18 If eligible, participants watched a short video explaining the study and were directed to the online informed consent process. Written electronic informed consent was completed by all eligible participants at this screening stage using REDCap.
Baseline survey
Following informed consent, participants completed a baseline survey assessing sociodemographic characteristics such as age, gender, race, ethnicity, education level, socioeconomic status, and address. Addresses were used to determine their geography (rural versus urban), described below. Lastly, participants were asked about their Internet use and previous use of health and fitness-related apps and digital devices. This included the following questions, “How do you access the Internet most frequently?”; “Do you use apps?”; “On a scale of 0–10, with 0 being ‘not at all comfortable’ and 10 being ‘very comfortable’, how comfortable are you using applications (‘apps’) on your smartphone?”; “Have you ever used an electronic or wearable device to track: blood pressure, physical activity, diet, etc.?” This survey took approximately 20–30 min to complete.
24-h dietary recalls
After completing the baseline survey, participants were emailed a request to complete two 24-h dietary recalls. These were administered using the ASA24 recall tool developed by the National Cancer Institute.14,15 The first ASA24 request was sent to participants the day following baseline survey completion. Participants were asked to complete an ASA24 dietary recall on one weekday and one weekend. Thus, depending on the day of the week the first ASA24 recall was completed, the second ASA24 recall request was sent for the next available weekend or weekday. They were then emailed automated reminders to complete these dietary recalls every 3 days during a 14-day period. If both ASA24s were completed, potential participants were emailed to schedule an introductory visit (Visit 1) with a research team member. If both ASA24s were not completed within 14 days, they were emailed and thanked for their time and told they were ineligible to move forward with trial participation.
Visit 1: introduction to Nourish
Visit 1 provided an overview of the Nourish trial, including study timeline and intervention activities and introduced potential participants to the Nourish study app. During this visit, a research team member assisted the potential participant with downloading the Nourish app and provided instructions on app navigation, including logging all food and drinks. They would also schedule the final eligibility visit (Visit 2). Participant mailing addresses were verified to ship an at-home blood pressure monitor and other study materials. No eligibility criteria were evaluated at this screening step; the primary purpose was to provide technical support and guidance on app navigation and food tracking. Visit 1 lasted approximately 30 min.
Tracking run-in
The tracking run-in period started the day after Visit 1 was completed. During this period, participants were asked to log all their food and drinks within the Nourish app for the next week to continue to Visit 2. If they did not successfully log food and drinks during that first week, they were given an opportunity to track for the following 7 days. If they did not track their food for a week during the run-in period, they were thanked for their time and informed they were ineligible to move forward with participation.
Visit 2: final eligibility and randomization
Visit 2 was conducted to determine final eligibility, which was established by measuring blood pressure. Blood pressure assessment occurred via Zoom video conferencing, using the at-home blood pressure monitor that was mailed after Visit 1. Full procedures for remote blood pressure assessment were previously described. 12 If the individual remained eligible following blood pressure assessment, Visit 2 concluded with randomization and onboarding procedures. Visit 2 took approximately 45 min.
Key metrics and statistical analysis
Recruitment yields
We calculated the proportion of individuals initially screened and consented by recruitment method type. We also examined sociodemographic characteristics by recruitment method type using percentages and means (standard deviations), depending on variable type. Lastly, we examined the proportion of individuals yielded from each recruitment type by sociodemographic variable, and the distribution of recruitment methods within sociodemographic subgroups. We repeated these analyses for individuals who were randomized. The following categories were used for these comparisons: (a) ResearchMatch; (b) Duke EHR patient portal messaging; (c) online community forums, such as Reddit, Craigslist, and Nextdoor; (d) social media, such as Facebook and Twitter; (e) other clinical trial platforms or websites, such as ClinicalTrials.gov and the Duke University clinical trials website; and (f) word of mouth. Any recruitment methods that did not fit the aforementioned categories were considered “other” (e.g. a google search).
Completion of screening steps
We examined the proportion of individuals that were ineligible following the eligibility survey and the reasons for ineligibility, specifically related to mobile technology use. Following, we examined associations between several sociodemographic and mobile technology use characteristics and completion of each screening step. Sociodemographic and mobile technology characteristics were obtained from the baseline survey. The number of individuals compared is based on who answered that respective question at baseline survey; therefore, the total number assessed for each variable may vary (i.e., variables at the start of the survey had higher completion rates than variables toward the end). For each subsequent screening step, we explored these associations within the subsample of adults who completed the previous screening step, beginning with those who had initially consented to participate in the study and reported data for the characteristic of interest. Categorical variables were compared using χ2 tests, and continuous variables were explored using t-tests to compare the means of adults who completed each step with those who did not.
Differences in randomization
We summarized baseline sociodemographic and mobile technology use characteristics from the baseline survey for all participants who consented and were randomized using proportions and means for categorical and continuous variables, respectively. Geographic density was derived by matching participant addresses to their US Census tract and using the USDA Food Access Research Atlas categorization of census tracts by rural or urban. 19
Following, we compared sociodemographic and mobile technology use characteristics of participants who completed all screening steps and were randomized, to the sample of those who initially consented to participate in the study but were screened out at any point prior to randomization. We refer to these two groups of individuals as randomized and nonrandomized, respectively. As in the screening completion analysis, we were limited to those participants who reported data for the characteristic of interest prior to exiting the study. We used χ2 tests and t-tests to compare categorical and continuous variables, respectively.
Results
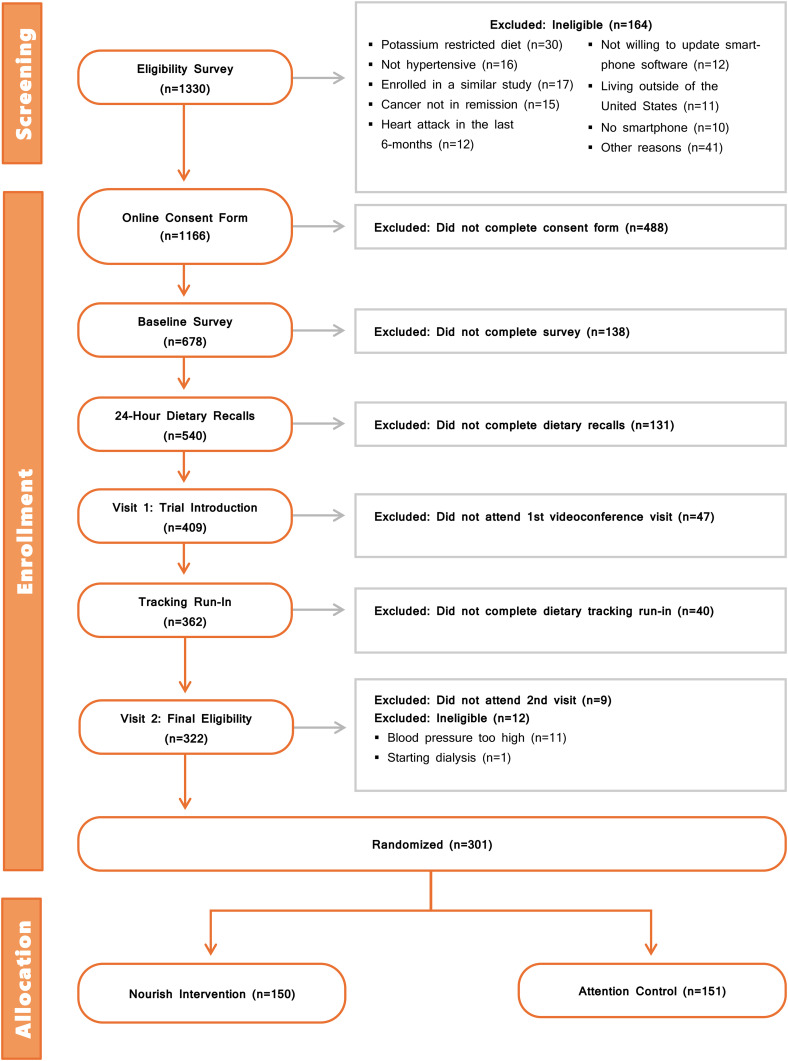
A total of 1638 individuals started the initial eligibility survey for Nourish, of whom 71% (n = 1166) completed the eligibility survey and were eligible to continue to informed consent procedures. Primary reasons for ineligibility related to mobile technology use included not owning a smartphone (n = 10), not being willing to update software (n = 12), and not being willing to video conference (n = 2). Fifty-eight percent (n = 678) of these eligible individuals provided their informed consent. Ultimately, 44% of those consented (n = 301) were randomized to Nourish. A full breakdown of attrition and eligibility by screening step is detailed in Figure 1.
Figure 1.
CONSORT diagram for the Nourish trial.
Recruitment yields
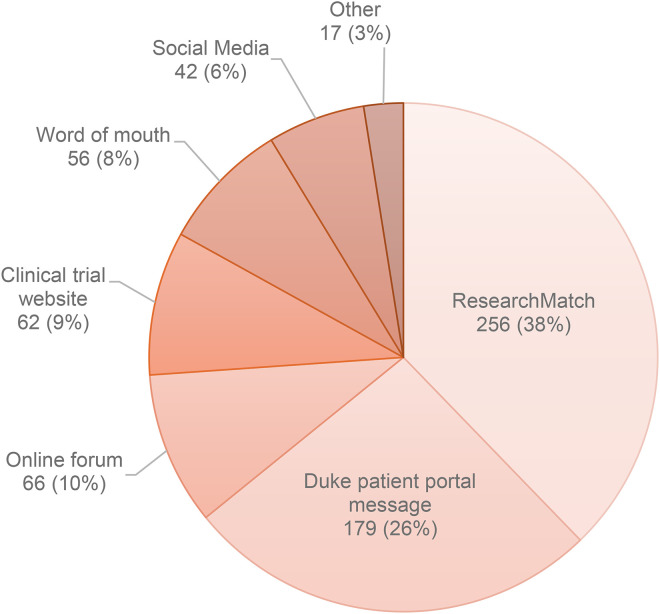
The highest proportion of individuals initially screened and consented (38%) were recruited via ResearchMatch, followed by EHR patient portal messaging (26%), and online forums (10%). A small number of participants heard about the Nourish study through a clinical trial website (9%), word of mouth (8%), or social media (6%). These trends were similar at enrollment and randomization (see Figure 2).
Figure 2.
Recruitment source for consented participants in Nourish (n = 678).
Table 1 describes the sociodemographics of individuals who consented and completed the baseline survey, by recruitment method. There was variation in mean age by recruitment method. For instance, the mean (SD) age of individuals recruited via online forums was 43.5 (10.3), while the mean (SD) age of individuals recruited via the EHR patient portal was 56.5 (12.5). All modalities reached a majority women, however, ResearchMatch and online forums were the most successful at enrolling men (>40%). In fact, 43% of the consented males resulted from ResearchMatch outreach. Among all outreach methods, EHR patient portal outreach observed the highest proportion of African American or Black individuals screened (44%) and was the most successful outreach method among African American and Black adults consented. ResearchMatch resulted in the largest number of Hispanic adults consented in total (n = 15), accounting for 35% of individuals who consented and identified as Hispanic in our sample. A full summary of sociodemographic characteristics at consent by recruitment method is in Table 1. Several of these trends were maintained at randomization (Supplemental Table 1).
Table 1.
Sociodemographic characteristics for consented participants in Nourish by recruitment method (n = 678).
| – N (column %, row %) or Mean ± SD, N; (n = 678 a ) | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | ResearchMatch (n = 256) | Duke patient portal message (n = 179) | Online forum (n = 66) | Social media (n = 42) | Clinical trial website (n = 62) | Word of mouth (n = 56) | Other (n = 17) |
| Age at consent, years | 54.1 ± 13.2, 229 | 56.5 ± 12.5, 158 | 43.5 ± 10.3, 62 | 48.9 ± 10.8, 39 | 53.4 ± 14.5, 57 | 50.9 ± 13.7, 50 | 54.3 ± 11.1, 15 |
| Sex at birth | |||||||
| Male | 104 (41%, 43%) | 67 (37%, 28%) | 28 (42%, 12%) | 6 (14%, 2%) | 18 (29%, 7%) | 15 (27%, 6%) | 3 (18%, 1%) |
| Female | 152 (59%, 35%) | 112 (63%, 26%) | 38 (58%, 9%) | 36 (86%, 8%) | 44 (71%, 10%) | 41 (73%, 9%) | 14 (82%, 3%) |
| Race/ethnicity | |||||||
| White | 127 (50%, 41%) | 44 (25%, 14%) | 44 (67%, 14%) | 27 (64%, 9%) | 33 (53%, 11%) | 26 (46%, 8%) | 10 (59%, 3%) |
| Black or African American | 73 (29%, 36%) | 78 (44%, 39%) | 9 (14%, 4%) | 6 (14%, 3%) | 16 (26%, 8%) | 16 (29%, 8%) | 4 (24%, 2%) |
| Hispanic (includes all races) | 15 (6%, 35%) | 12 (7%, 28%) | 5 (8%, 12%) | 1 (2%, 2%) | 4 (6%, 9%) | 5 (9%, 12%) | 1 (6%, 2%) |
| Another race | 41 (16%, 34%) | 45 (25%, 37%) | 8 (12%, 7%) | 8 (19%, 7%) | 9 (15%, 7%) | 9 (16%, 7%) | 2 (12%, 2%) |
| Income | |||||||
| Less than $50,000 | 78 (35%, 43%) | 43 (29%, 24%) | 11 (19%, 6%) | 12 (32%, 7%) | 23 (42%, 13%) | 12 (26%, 7%) | 3 (23%, 2%) |
| $50,000 through $99,999 | 79 (36%, 39%) | 47 (32%, 23%) | 23 (39%, 11%) | 11 (29%, 5%) | 20 (36%, 10%) | 21 (45%, 10%) | 3 (23%, 1%) |
| $100,000 or greater | 64 (29%, 33%) | 59 (40%, 30%) | 25 (42%, 13%) | 15 (39%, 8%) | 12 (22%, 6%) | 14 (30%, 7%) | 7 (54%, 4%) |
| Missing | 35 | 30 | 7 | 4 | 7 | 9 | 4 |
| Education | |||||||
| Less than four years of college | 71 (31%, 37%) | 50 (32%, 26%) | 19 (31%, 10%) | 9 (23%, 5%) | 25 (45%, 13%) | 12 (24%, 6%) | 4 (27%, 2%) |
| College, four years or more (College graduate) | 157 (69%, 38%) | 107 (68%, 26%) | 43 (69%, 10%) | 30 (77%, 7%) | 31 (55%, 7%) | 38 (76%, 9%) | 11 (73%, 3%) |
| Missing | 28 | 22 | 4 | 3 | 6 | 6 | 2 |
| Geographic density of census tract | |||||||
| Rural | 28 (11%, 24%) | 33 (20%, 28%) | 16 (25%, 13%) | 10 (25%, 8%) | 17 (28%, 14%) | 11 (22%, 9%) | 4 (25%, 3%) |
| Urban | 217 (89%, 41%) | 134 (80%, 26%) | 48 (75%, 9%) | 30 (75%, 6%) | 43 (72%, 8%) | 40 (78%, 8%) | 12 (75%, 2%) |
| Missing | 11 | 12 | 2 | 2 | 2 | 5 | 1 |
| Most frequent method of internet access | |||||||
| Cell phone | 120 (52%, 34%) | 81 (51%, 23%) | 42 (68%, 12%) | 32 (82%, 9%) | 34 (60%, 10%) | 31 (62%, 9%) | 8 (53%, 2%) |
| Desktop/laptop | 90 (39%, 41%) | 64 (40%, 29%) | 18 (29%, 8%) | 3 (8%, 1%) | 21 (37%, 10%) | 16 (32%, 7%) | 5 (33%, 2%) |
| Tablet | 21 (9%, 43%) | 15 (9%, 31%) | 2 (3%, 4%) | 4 (10%, 8%) | 2 (4%, 4%) | 3 (6%, 6%) | 2 (13%, 4%) |
| Missing | 25 | 19 | 4 | 3 | 5 | 6 | 2 |
| Uses any health apps | |||||||
| None reported | 91 (36%, 45%) | 58 (32%, 29%) | 11 (17%, 5%) | 8 (19%, 4%) | 16 (26%, 8%) | 14 (25%, 7%) | 4 (24%, 2%) |
| Any reported | 165 (64%, 35%) | 121 (68%, 25%) | 55 (83%, 12%) | 34 (81%, 7%) | 46 (74%, 10%) | 42 (75%, 9%) | 13 (76%, 3%) |
| Uses any health wearables | |||||||
| None reported | 100 (39%, 42%) | 63 (35%, 27%) | 19 (29%, 8%) | 14 (33%, 6%) | 20 (32%, 8%) | 14 (25%, 6%) | 6 (35%, 3%) |
| Any reported | 156 (61%, 35%) | 116 (65%, 26%) | 47 (71%, 11%) | 28 (67%, 6%) | 42 (68%, 10%) | 42 (75%, 10%) | 11 (65%, 2%) |
| Comfort using apps (rating 1 = not at all comfortable to 10 = very comfortable) | 9.4 ± 1.2, 230 | 9.3 ± 1.3, 154 | 9.7 ± 0.8, 62 | 9.3 ± 1.5, 39 | 9.5 ± 1.2, 57 | 9.2 ± 1.2, 50 | 9.1 ± 1.6, 15 |
Counts may not sum to 678, due to missing data.
Completion of screening steps
Completion of screening steps differed by several baseline sociodemographic characteristics and mobile technology use characteristics. Differences in these characteristics at each step are described below and in Table 2.
Table 2.
Comparisons of the completion rates of each subsequent protocol step within sociodemographic groupings of consenting adults—n (row %) or mean ± SD, n, (N = 678 a ).
| Characteristic | n a | Protocol step completed | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Survey | Dietary recalls | Visit 1 | Tracking run-in | Visit 2 | ||||||||||||
| Yes | No | p | Yes | No | p | Yes | No | p | Yes | No | p | Yes | No | p | ||
| Sex at birth | ||||||||||||||||
| Male | 241 | 196 (81%) | 45 (19%) | 0.42 | 146 (74%) | 50 (26%) | 0.61 | 130 (89%) | 16 (11%) | 0.80 | 114 (88%) | 16 (12%) | 0.57 | 109 (96%) | 5 (4%) | 0.29 |
| Female | 437 | 344 (78%) | 93 (22%) | 263 (76%) | 81 (24%) | 232 (88%) | 31 (12%) | 208 (90%) | 24 (10%) | 204 (98%) | 4 (2%) | |||||
| Race/ethnicity | ||||||||||||||||
| White | 311 | 275 (88%) | 36 (12%) | 0.97 | 216 (79%) | 59 (21%) | 0.37 | 188 (87%) | 28 (13%) | 0.89 | 173 (92%) | 15 (8%) | 0.10 | 168 (97%) | 5 (3%) | 0.02 |
| Black or African American | 202 | 179 (89%) | 23 (11%) | 130 (73%) | 49 (27%) | 117 (90%) | 13 (10%) | 97 (83%) | 20 (17%) | 97 (100%) | 0 | |||||
| Hispanic (includes all races) | 43 | 39 (91%) | 4 (9%) | 30 (77%) | 9 (23%) | 27 (90%) | 3 (10%) | 25 (93%) | 2 (7%) | 22 (88%) | 3 (12%) | |||||
| Another race | 48 | 42 (88%) | 6 (12%) | 29 (69%) | 13 (31%) | 26 (90%) | 3 (10%) | 23 (88%) | 3 (12%) | 22 (96%) | 1 (4%) | |||||
| Income | ||||||||||||||||
| Less than $50,000 | 182 | 161 (88%) | 21 (12%) | 0.51 | 125 (78%) | 36 (22%) | 0.75 | 102 (82%) | 23 (18%) | 0.01 | 88 (86%) | 14 (14%) | 0.24 | 86 (98%) | 2 (2%) | 0.90 |
| $50,000–$99,999 | 204 | 178 (87%) | 26 (13%) | 132 (74%) | 46 (26%) | 118 (89%) | 14 (11%) | 104 (88%) | 14 (12%) | 102 (98%) | 2 (2%) | |||||
| $100,000 or greater | 196 | 178 (91%) | 18 (9%) | 136 (76%) | 42 (24%) | 127 (93%) | 9 (7%) | 118 (93%) | 9 (7%) | 114 (97%) | 4 (3%) | |||||
| Education | ||||||||||||||||
| Less than 4 years of college | 190 | 160 (84%) | 30 (16%) | 0.03 | 114 (71%) | 46 (29%) | 0.13 | 91 (80%) | 23 (20%) | 0.0004 | 80 (88%) | 11 (12%) | 0.74 | 74 (93%) | 6 (8%) | 0.009 |
| College, 4 + years (College graduate) | 417 | 377 (90%) | 40 (10%) | 292 (77%) | 85 (23%) | 269 (92%) | 23 (8%) | 240 (89%) | 29 (11%) | 237 (99%) | 3 (1%) | |||||
| Geographic density of census tract | ||||||||||||||||
| Rural | 119 | 96 (81%) | 23 (19%) | 0.41 | 67 (70%) | 29 (30%) | 0.11 | 58 (87%) | 9 (13%) | 0.55 | 52 (90%) | 6 (10%) | 0.85 | 48 (92%) | 4 (8%) | 0.03 |
| Urban | 524 | 439 (84%) | 85 (16%) | 340 (77%) | 99 (23%) | 303 (89%) | 37 (11%) | 269 (90%) | 34 (11%) | 265 (99%) | 4 (1%) | |||||
| Most frequent method of internet access | ||||||||||||||||
| Cell phone | 348 | 301 (86%) | 47 (14%) | 0.39 | 222 (74%) | 79 (26%) | 0.20 | 194 (87%) | 28 (13%) | 0.73 | 170 (88%) | 24 (12%) | 0.54 | 163 (96%) | 7 (4%) | 0.45 |
| Desktop/laptop | 217 | 196 (90%) | 21 (10%) | 150 (77%) | 46 (23%) | 135 (90%) | 15 (10%) | 121 (90%) | 14 (10%) | 119 (98%) | 2 (2%) | |||||
| Tablet | 49 | 43 (88%) | 6 (12%) | 37 (86%) | 6 (14%) | 33 (89%) | 4 (11%) | 31 (94%) | 2 (6%) | 31 (100%) | 0 | |||||
| Use any health apps | ||||||||||||||||
| No | 202 | 121 (60%) | 81 (40%) | <0.0001 | 87 (72%) | 34 (28%) | 0.26 | 73 (84%) | 14 (16%) | 0.13 | 66 (90%) | 7 (10%) | 0.66 | 66 (100%) | 0 | 0.12 |
| Yes | 476 | 419 (88%) | 57 (12%) | 322 (77%) | 97 (23%) | 289 (90%) | 33 (10%) | 256 (89%) | 33 (11%) | 247 (96%) | 9 (4%) | |||||
| Use any wearables | ||||||||||||||||
| No | 236 | 151 (64%) | 85 (36%) | <0.0001 | 110 (73%) | 41 (27%) | 0.33 | 97 (88%) | 13 (12%) | 0.90 | 87 (90%) | 10 (10%) | 0.79 | 85 (98%) | 2 (2%) | 1.00 |
| Yes | 442 | 389 (88%) | 53 (12%) | 299 (77%) | 90 (23%) | 265 (89%) | 34 (11%) | 235 (89%) | 30 (11%) | 228 (97%) | 7 (3%) | |||||
| Recruitment method | ||||||||||||||||
| ResearchMatch | 297 | 207 (81%) | 90 (19%) | 0.41 | 157 (76%) | 50 (24%) | 0.14 | 136 (87%) | 21 (13%) | nc b | 121 (89%) | 15 (11%) | nc b | 118 (98%) | 3 (2%) | nc b |
| Duke patient portal message | 179 | 135 (75%) | 44 (25%) | 92 (68%) | 43 (32%) | 87 (95%) | 5 (5%) | 77 (89%) | 10 (11%) | 75 (97%) | 2 (3%) | |||||
| Online forum | 66 | 58 (88%) | 8 (12%) | 48 (83%) | 10 (17%) | 42 (88%) | 6 (13%) | 39 (93%) | 3 (7%) | 37 (95%) | 2 (5%) | |||||
| Social Media | 42 | 34 (81%) | 8 (19%) | 24 (71%) | 10 (29%) | 22 (92%) | 2 (8%) | 19 (86%) | 3 (14%) | 19 (100%) | 0 | |||||
| Clinical trial website | 62 | 51 (82%) | 11 (18%) | 43 (84%) | 8 (16%) | 35 (81%) | 8 (19%) | 29 (83%) | 6 (17%) | 28 (97%) | 1 (3%) | |||||
| Word of mouth | 56 | 42 (75%) | 14 (25%) | 35 (83%) | 7 (17%) | 30 (86%) | 5 (14%) | 27 (90%) | 3 (10%) | 26 (96%) | 1 (4%) | |||||
| Other | 17 | 13 (76%) | 4 (24%) | 10 (77%) | 3 (23%) | 10 (100%) | 0 | 10 (100%) | 0 | 10 (100%) | 0 | |||||
| Age at enrollment, years | ||||||||||||||||
| Mean ± SD, n | 601 | 53.4 ± 13.3, 539 | 50.1 ± 12.6, 71 | 0.049 | 53.4 ± 13.7, 409 | 53.3 ± 11.8, 130 | 0.95 | 53.8 ± 13.6, 362 | 50.3 ± 14.2, 47 | 0.10 | 54.1 ± 13.5, 322 | 51.6 ± 14.5, 40 | 0.28 | 54.4 ± 13.4, 313 | 49.4 ± 16.7, 9 | 0.30 |
| Comfort using apps, rating 1 = not at all comfortable to 10 = very comfortable | ||||||||||||||||
| Mean ± SD, n | 607 | 9.4 ± 1.2, 536 | 9.3 ± 1.2, 71 | 0.73 | 9.4 ± 1.2, 406 | 9.4 ± 1.2, 130 | 0.87 | 9.4 ± 1.2, 359 | 9.4 ± 1.2, 47 | 0.93 | 9.4 ± 1.2, 319 | 9.7 ± 0.8, 40 | 0.09 | 9.4 ± 1.2, 310 | 9.6 ± 1.0, 9 | 0.45 |
NB: Counts may not sum to 678, due to missing data.
Number of consented adults with data. The N for the baseline survey step varies due to the consented adults stopping at variable points in the data collection.
The final three steps of recruitment method were not able to be statistically compared due to small cell sizes in the non-completion groups.
Baseline survey
Baseline survey completion differed by age and education, such that older adults were more likely to complete the baseline survey (mean (SD): 53.4 (13.3) years vs 50.1 (12.6) years, p = 0.049) and individuals with four years or more of college education had a higher rate of survey completion (90%) as compared to individuals with less education (84%), p = 0.03. Baseline survey completion also significantly differed by mobile technology use. Individuals who used health apps were more likely to complete the baseline survey than those who did not (88% vs 60%, p < 0.0001). Similarly, individuals who used wearables for health tracking were significantly more likely to complete the baseline survey than those who did not (88% vs 64%, p < 0.0001).
24-h dietary recall
Completion of the 24-h dietary recalls did not differ by sociodemographics (i.e., race, age, etc.) or mobile technology use characteristics.
Visit 1: introduction to Nourish
Completion of Visit 1 differed by income and education status. Specifically, individuals with incomes of $100,000 or more were more likely than individuals with incomes of $50,000–$99,999 or less than $50,000 to complete Visit 1 (93% vs 89% vs 82%, p = 0.01). Similar to the differences observed at the baseline survey, individuals with four years or more of college education had a higher rate of Visit 1 completion (92%) as compared to individuals with less education (80% completion, p = 0.0004). This trend was also true for education at Visit 2.
Tracking run-in
There were no significant differences in completion of the tracking run-in screening step by baseline characteristics.
Visit 2: final eligibility and randomization
Individuals of any race who identified as having Hispanic ethnicity were less likely to complete Visit 2 (88%) than non-Hispanic White individuals (97%), non-Hispanic Black individuals (100%), and non-Hispanic individuals of any other race (96%, p = 0.02). As mentioned above, individuals with four years or more of college education had a higher rate of Visit 2 completion (99%), as compared to individuals with less education (93% completion, p = 0.009). Additionally, individuals living in urban US Census tracts were more likely to complete Visit 2 than those living in rural tracts (99% vs 92%, p = 0.03).
There were no significant trends in screening step completion by sex assigned at birth. There were also no differences in the completion of screening steps by participant comfort in using apps at baseline.
Differences in randomization
The differences observed in screening step completion affected those who were eligible for and subsequently randomized within Nourish (Table 3). Among all consented individuals, randomized adults (compared to nonrandomized adults) possessed a higher education level (p < 0.0001); were more likely to use health-related apps (p< 0.0001) and wearables (p < 0.0001); and were older (p = 0.01).
Table 3.
Comparisons of consented adults who were never randomized and those who were randomized by categorical sociodemographic characteristics—N (%) or mean ± SD, N; (N = 678 a ).
| Characteristic | Not randomized | Randomized | p-value |
|---|---|---|---|
| Sex assigned female at birth | 241 (64%) | 196 (65%) | 0.75 |
| Race/ethnicity | 0.52 | ||
| White | 150 (49%) | 161 (54%) | |
| Black or African American | 109 (36%) | 93 (31%) | |
| Hispanic (includes all races) | 21 (7%) | 22 (7%) | |
| Another race | 27 (9%) | 21 (7%) | |
| Income | 0.17 | ||
| Less than $50,000 | 99 (34%) | 83 (29%) | |
| $50,000–$99,999 | 104 (36%) | 100 (34%) | |
| $100,000 or greater | 88 (30%) | 108 (37%) | |
| Less than four years of college education | 119 (39%) | 71 (24%) | <0.0001 |
| Rural census tract | 72 (21%) | 47 (16%) | 0.07 |
| Most frequent method of Internet access | 0.01 | ||
| Cell phone | 194 (62%) | 154 (51%) | |
| Desktop/laptop | 101 (32%) | 116 (39%) | |
| Tablet | 18 (6%) | 31 (10%) | |
| Previous use of any health apps | 239 (63%) | 237 (79%) | <0.0001 |
| Previous use of any health wearables | 221 (59%) | 221 (73%) | <0.0001 |
| Recruitment method | 0.57 | ||
| ResearchMatch | 144 (38%) | 112 (37%) | |
| Duke patient portal message | 107 (28%) | 72 (24%) | |
| Online forum | 31 (8%) | 35 (12%) | |
| Social Media | 23 (6%) | 19 (6%) | |
| Clinical trial website | 34 (9%) | 8 (9%) | |
| Word of mouth | 31 (8%) | 25 (8%) | |
| Other | 7 (2%) | 10 (3%) | |
| Age at enrollment, years | 51.6 ± 12.9, 309 | 54.4 ± 13.4, 301 | 0.01 |
| Comfort using apps (rating 1 = not at all comfortable to 10 = very comfortable) | 9.4 ± 1.2, 309 | 9.3 ± 1.3, 298 | 0.32 |
NB: Counts may not sum to 678, due to missing data.
Out of participants reporting perceived discrimination in getting medical care (n = 61).
Although significant differences were observed at a specific screening step for some variables, these were insufficient to result in a significant difference in the randomized group of adults as compared to all nonrandomized adults. Specifically, no significant results were observed between the nonrandomized and randomized groups for sex, race, ethnicity, income, or geographic density of the home Census tract.
We observed one significant association not observed during the screening step analysis. Within the sample of consented adults, randomized individuals were more likely to use a desktop, laptop, or tablet for Internet access than nonrandomized individuals, who were more likely to use a cell phone to access the Internet (p = 0.01). The full details of baseline sociodemographic characteristics for those who consented and were randomized can be found in Table 3.
Discussion
In a fully remote dietary intervention for US adults with HTN, we found that older adults, adults with higher education levels, and adults with previous use of health-related apps and wearables were more likely to complete screening steps and be randomized into the clinical trial. We also found that individuals who used a desktop, laptop, or tablet for Internet access were more likely to be randomized than those who used a smartphone. These findings demonstrate that evaluating clinical trial procedures that precede enrollment may help investigators identify opportunities to enhance diversity in participant enrollment. Our results also highlight areas where additional support can be provided or strategies can be tailored to improve the reach and accessibility of fully remote and digital clinical trials, such as optimizing intervention components to be delivered on mobile devices.
Major funding agencies have made several efforts to increase diversity in clinical trials.20,21 However, progress has been slow in achieving adequate representation, particularly as it pertains to race and ethnicity.22–25 One major contributor may be the lack of adequate planning for the recruitment and engagement of underrepresented communities. For instance, in a systematic review of NIH-funded cardiovascular trials, only 21% stated a goal for recruitment of underrepresented populations in their protocol, resulting in most trials not identifying a recruitment plan for engaging racial and ethnic minority populations. 24 Our recruitment goals for traditionally underrepresented groups in research encouraged our team to evaluate enrollment and modify our recruitment approaches to attain goals throughout the recruitment period. This adaptive approach was supported by adding recruitment methods, such as ResearchMatch and patient portal messaging, that allow researchers to prioritize specific populations. These methods resulted in the highest proportion of Black and Hispanic adults recruited in our study, respectively, and thus aligned with priorities from the NIH and our research team to increase our demographic diversity. Other online approaches used, such as social media, were less successful for our team, but have shown promise in previous studies.26–28 A major difference is that we did not pay for advertisements on social media, such as Facebook, which is likely to influence the present findings. Given recruitment methods’ variable success rates across studies, we recommend research teams include monitoring and evaluation procedures as part of their recruitment plans and as one of their strategies to promote diverse enrollment.
Similar to recruitment methods, there are steps prior to enrollment, such as run-in periods, that can pose a threat to external validity if it leads to one or more groups of individuals discontinuing the screening processes due to personal choice or eligibility. 29 For example, an extensive run-in process may screen out many eligible individuals and only leave those with the most motivation or fewest barriers to participation. In Nourish, there were no significant differences in completion of the tracking during run-in by baseline characteristics, including by sex, race, or ethnicity. However, our comprehensive findings show differential attrition by sociodemographic characteristics and mobile technology use at various steps of screening. These findings provide an additional example of how clinical trial processes that precede enrollment that may affect participation. Study teams should carefully consider screening procedures and the unintentional bias that they may introduce and how they may exacerbate or address disparities in enrollment.
In our sample, individuals with a higher education level (college graduate or higher) were more likely to be randomized, as compared to individuals with lower levels of education. This finding aligns with previous literature.30,31 Our further analyses showed that this difference resulted from individuals with higher education being more likely to complete the baseline survey, Visit 1, and Visit 2, with the largest difference observed in Visit 1 completion. This may be due to the burden of completing the ASA24 prior to Visit 1. For some participants, the ASA24 may have been lengthy and potentially challenging to complete. Regardless, these differences represent important findings, as they emphasize the value of expanding data collection and evaluation early in clinical trial procedures, a practice we recommend to study teams with complex screening procedures and workflows. Conducting such evaluation provides insight on opportunities to modify protocols or increase support for groups where higher attrition rates are being observed, such as among individuals with lower education levels in the current study. They may also provide insight into the trial's limitations for dissemination, should it be effective.
Another difference in screening step completion we observed was that participants who previously used health apps or wearable, such as Fitbits, were more likely to express interest in the study. This finding may point to the importance of initial recruitment messaging: How can we encourage and support individuals with little-to-no experience using health-related technology to participate in digital health interventions? Our recruitment materials emphasized the trial's digital approach and thus requirement to have a smartphone, which may have resulted in individuals with minimal experience using health apps being hesitant to pursue enrollment. However, once the baseline survey was completed, there was no longer a difference in the completion of screening steps between individuals who previously used these technologies. It is possible that interaction with a study team member sooner in the screening procedures could have mitigated some of this earlier attrition and may have supported continued interest and participation among individuals with lower levels of mobile technology experience.
Despite showing greater upfront interest, individuals with previous health app use were less likely to complete the tracking run-in period and Visit 2 compared to non-users. Although this difference was not significant, it highlights an interesting pattern. One reason for this might be that participants who are frequent health app users may have higher expectations regarding what a research study app can do to support and track behaviors. Smartphone users spend about 4 h per day on the Internet, and most of that time is spent using apps; 32 there are hundreds of commercial health apps, and they are constantly improving on the user experience. 33 Unfortunately, many research studies do not have the capability to create such apps; they are limited by time and resources, while the digital health industry is able to create and refine apps quickly and efficiently. This is a complex problem faced by many study teams exploring digital health solutions, as the decision to use an existing commercial app has limitations, such as more extensive privacy and security requirements for handling participant data. 34 Further, there is no guarantee that the app will remain in existence throughout the duration of the study, possibly impeding data collection and trial fidelity. However, leveraging commercial apps may improve the likelihood of high user engagement, 35 a key determinant of behavior change and improved health outcomes. 36 As the use of digital strategies for healthcare delivery continues to grow, researchers and practitioners must carefully weigh the strengths and limitations of partnering with commercial companies versus establishing new infrastructure to support care delivery.
One of our goals in using a smartphone app for intervention delivery was to improve the dissemination potential of the intervention. Smartphone ownership rates are high across diverse groups, including populations underrepresented in research, such as Black and Latino adults and adults who have lower education levels. These groups are also more likely to be smartphone dependent for Internet access. 37 Our recruitment materials reflected that this study could be completed via smartphone, and at the baseline survey, 59% reported using their smartphone as their primary source of Internet access. However, randomized individuals were more likely to use a tablet, desktop, or laptop device as their primary source. This finding may be due to the large proportion of randomized individuals with high education and income levels, both of which are associated with higher rates of home broadband. 37 It is also possible that the trial procedures, though accessible, were difficult to complete solely on smartphone devices, which discouraged individuals who are smartphone dependent from participating. This reiterates the importance of designing user-friendly intervention components for mobile devices to improve equitable access. In addition, taken together with our finding on previous health technology use, this finding highlights the need for future work to disentangle the predicators of engagement in digital health interventions to refine interventions for populations with variable device access and digital health literacy levels and technology access.
An advantage of remote clinical trial procedures is that they may improve accessibility for individuals that reside a significant distance from the research site and/or do not have access to transportation. Our participant sample comprised 16% of individuals who resided in rural areas, defined by the USDA Food Access Research Atlas categorization of rural and urban census tracts. 19 This is less than the proportion of adults in the USA who reside in rural areas (20%); 38 however, it shows promise for digital health interventions to reach rural populations. In our trial, the largest proportion of rural residents came from individuals contacted through EHR patient portal messaging. This may reflect the high proportion of rural residents living in North Carolina.38,39 Nonetheless, future research is needed to understand how remote clinical trial procedures may impact participation among individuals residing in rural areas, in addition to how clinical trial information can be effectively disseminated to reach this underrepresented population in research.
Strengths and limitations
There are limitations of this study that should be considered. Due to our approach of making multiple comparisons in our analyses, there is a higher likelihood of type 1 error. In addition, the results presented who completed as part of an ad hoc analyses, and therefore, no power analysis was performed for the present study. Yet, we still believe the differences observed in screening step completion offer important considerations for researchers conducting clinical trials with several processes prior to enrollment. Second, not every participant who expressed interest ultimately completed the baseline survey. Therefore, we do not have full data for each characteristic and there could be a possibility that we underestimated the differences in screening completion. Third, there were instances where potential participants requested support completing a component of the screening steps and the team provided assistance. However, we did not systematically document this process, and it is possible this support may have influenced participation. Fourth, EHR patient portal messaging was a primary source of recruitment, resulting in us reaching a population that is already connected to care and using technology for health purposes, which may have influenced their interest in the study and ability to complete screening steps. Fifth, the questions used to assess mobile health technology use do not originate from a validated scale and do not represent a validated measure of digital literacy. Lastly, the current exploratory analysis was limited to bivariate associations and therefore, cannot tease apart independent factors predicting exclusion or withdrawal prior to randomization. Future research is needed to expand on this area of inquiry and inform future refinement of clinical trial procedures for diverse populations.
Despite the aforementioned limitations, this study has many strengths. We collected sociodemographic data early in the screening process, which enabled us to conduct an evaluation of the representation before and after each screening step, a process not reported by many studies with run-in periods or other similar screening methods. 29 Similarly, our data collection and reporting included a comprehensive set of sociodemographic variables, including income and education, which are extensively underreported in clinical trials. 40 Third, our recruitment and screening processes were supported by digital technologies and able to be conducted fully remote, offering insight on the potential utility of these applications in increasing the accessibility of trials, while also highlighting groups that may need additional support to engage. Finally, our approach to data collection and evaluation offers insights for investigators when developing documents required by sponsor agencies, such as the NIH Recruitment and Retention Plan and Plan for Enhancing Diverse Perspectives.
Conclusion
The use of digital methods for clinical trial recruitment, intervention delivery, and data collection is on the rise. These remote and digital procedures could improve clinical trial accessibility and diversity. However, thoughtful data collection and subsequent evaluation and reporting are needed to understand their impact on representation fully. In our analysis of a digital HTN intervention, we found that individuals with lower levels of education and with limited experience in using digital health technology may require additional support to participate. These findings underscore the critical need for routinely evaluating clinical trial procedures, regardless of delivery method, to understand their potential impact on generalizability and to work toward more inclusive, equitable and widely applicable clinical trial design and findings.
Supplemental Material
Supplemental material, sj-docx-1-dhj-10.1177_20552076241281216 for Sociodemographic predictors of successful screening and subsequent randomization in a digital health hypertension intervention by Hailey N Miller, Sandy Askew, Miriam B Berger, Elizabeth Trefney, Loneke T Blackman Carr, Melissa C Kay, Cherie Barnes, Qing Yang, Crystal C Tyson, Laura Svetkey, Ryan J Shaw, Dori M Steinberg and Gary G Bennett in DIGITAL HEALTH
Supplemental material, sj-docx-2-dhj-10.1177_20552076241281216 for Sociodemographic predictors of successful screening and subsequent randomization in a digital health hypertension intervention by Hailey N Miller, Sandy Askew, Miriam B Berger, Elizabeth Trefney, Loneke T Blackman Carr, Melissa C Kay, Cherie Barnes, Qing Yang, Crystal C Tyson, Laura Svetkey, Ryan J Shaw, Dori M Steinberg and Gary G Bennett in DIGITAL HEALTH
Acknowledgements
We would like to thank our funder, all research team members, and collaborators, including Alexandra Bennion, Jamiyla Bolton, Jasmine Burroughs, Miriam Chisholm, Taylor Crimmins, Mia de Leon, Megan Freed, Gabrielle Fogg, Norma Garcia Ortiz, Aaliyah Goodman, Nour Hammad, Christina Hopkins, Hannah Kelly, Monika Kraus, Heather Parnell, Kristen Rigsby, Meghana Sai Iragavarapu, Samantha Sette, Cayla Treadway, and Tia Willis. Most importantly, we would like to thank the research participants who joined Nourish.
Footnotes
Contributorship: HNM conceived of the recruitment analyses and drafted the manuscript. SA and QY led the analyses and provided statistical input. ET, MCK, CCT, LS, LBC, CB, and RS provided study input and edited the manuscript. CCT and LS consulted on data safety and execution of the study. MBB was involved in protocol development, gaining ethical approval and editing the manuscript. DS conceived the study, acquired study funding, and led initial study design. GGB provided subsequent oversight, study design, data collection, and evaluation input. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DMS has equity in Equip Health. GGB is on the scientific advisory board for WeightWatchers. RJS is a consultant for Cerner Enviza. All other authors have no conflicts of interests to declare.
Ethical approval: The Duke Health Institutional Review Board approved this study (protocol number: Pro00101689).
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This trial is funded by the National Heart, Lung, and Blood Institute at the National Institutes of Health (R01HL146768). The funder had no role in study design, data collection, data analysis, and interpretation of data, in the writing of the report, and in the decision to submit this article for publication. HNM was supported by an American Heart Association Strategically Focused Research Network (953550). MK was supported by the National Institutes of Health, National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number 1KL2TR002554. CCT was supported K01HL143116 by the NHLBI.
Guarantor: HNM.
ORCID iDs: Miriam B Berger https://orcid.org/0000-0001-7236-8734
Ryan J Shaw https://orcid.org/0000-0001-6800-6503
Trial number: NCT03875768.
Supplemental material: Supplemental material for this article is available online.
References
- 1.Centers for Disease Control and Prevention. Hypertension cascade: hypertension prevalence, treatment and control estimates among US adults aged 18 years and older applying the criteria from the American College of Cardiology and American Heart Association’s 2017 hypertension guideline—NHANES 2017–2020. https://millionhearts.hhs.gov/data-reports/hypertension-prevalence.html (2023, accessed August 7 2023).
- 2.Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension 2020; 75: 285–292. 20191223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conlin PR. The dietary approaches to stop hypertension (DASH) clinical trial: implications for lifestyle modifications in the treatment of hypertensive patients. Cardiol Rev 1999; 7: 284–288. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg D, Bennett GG, Svetkey L. The DASH diet, 20 years later. Jama 2017; 317: 1529–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lou SP, Han D, Kuczmarski MF, et al. Health literacy, numeracy, and dietary approaches to stop hypertension accordance among hypertensive adults. Health Educ Behav 2023; 50: 49–57. 20220311. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Li J. Participation of black US residents in clinical trials of 24 cardiovascular drugs granted FDA approval, 2006–2020. JAMA Network Open 2021; 4: e212640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang S, Hong YA. Clinical trial participation in America: the roles of eHealth engagement and patient–provider communication. Digital Health 2021; 7: 20552076211067658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S-H, Tanner A, Friedman DB, et al. Barriers to clinical trial participation: a comparison of rural and urban communities in South Carolina. J Community Health 2014; 39: 562–571. [DOI] [PubMed] [Google Scholar]
- 9.Stewart J, Krows ML, Schaafsma TT, et al. Comparison of racial, ethnic, and geographic location diversity of participants enrolled in clinic-based vs 2 remote COVID-19 clinical trials. JAMA Network Open 2022; 5: e2148325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw RJ. Access to technology and digital literacy as determinants of health and health care. Creat Nurs 2023; 29: 258–263. [DOI] [PubMed] [Google Scholar]
- 11.Miller HN, Berger MB, Askew S, et al. The nourish protocol: a digital health randomized controlled trial to promote the DASH eating pattern among adults with hypertension. Contemp Clin Trials 2021; 109: 106539. 20210813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller HN, Berger MB, Askew S, et al. Implementation of an at-home blood pressure measurement protocol in a hypertension management clinical trial during the COVID-19 pandemic. J Cardiovasc Nurs 2022; 37: 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension 2017; 2018: 1269–1324. 20171113. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute. Automated self-administered 24-hour (ASA24®) dietary assessment tool, https://epi.grants.cancer.gov/asa24/ (2020, accessed September 1 2020).
- 15.Subar AF, Kirkpatrick SI, Mittl B, et al. The automated self-administered 24-h dietary recall (ASA24): a resource for researchers, clinicians, and educators from the National Cancer Institute. J Acad Nutr Diet 2012; 112: 1134–1137. 20120615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019; 95: 103208. 20190509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. 20080930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zoom Video Communications Inc. https://zoom.us/ (2020, accessed February 14 2024).
- 19.Economic Research Service (ERS) USDoAU. Food access research atlas, https://www.ers.usda.gov/data-products/food-access-research-atlas/ (2021, accessed October 1, 2023).
- 20.National Institutes of Health. NIH’s oversight processes to ensure diversity among human subjects enrolled in clinical trials, https://www.nhlbi.nih.gov/sites/default/files/2017-11/NHLBI-Strategic-Vision-2016_FF.pdf (2017, accessed January 13 2024).
- 21.Food and Drug Administration. Draft guidance. Diversity plans to improve enrollment of participants from underrepresented racial and ethnic populations in clinical trials guidance for industry. Bethesda, Maryland, USA: Food and Drug Administration, 2022. https://www.fda.gov/media/157635/download . [Google Scholar]
- 22.Turner BE, Steinberg JR, Weeks BT, et al. Race/ethnicity reporting and representation in US clinical trials: a cohort study. Lancet Reg Health Am 2022; 11: 20220410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mak WWS, Law RW, Alvidrez J, et al. Gender and ethnic diversity in NIMH-funded clinical trials: review of a decade of published research. Adm Policy Ment Health – Ment Health Serv Res 2007; 34: 497–503. [DOI] [PubMed] [Google Scholar]
- 24.Prasanna A, Miller HN, Wu Y, et al. Recruitment of black adults into cardiovascular disease trials. J Am Heart Assoc 2021; 10: e021108. 20210825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Y, Prasanna A, Miller HN, et al. Female recruitment into cardiovascular disease trials. Am J Cardiol 2023; 198: 88–91. 20230519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darmawan I, Bakker C, Brockman TA, et al. The role of social media in enhancing clinical trial recruitment: scoping review. J Med Internet Res 2020; 22: e22810. 20201026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darko EM, Kleib M, Olson J. Social Media use for research participant recruitment: integrative literature review. J Med Internet Res 2022; 24: e38015. 20220804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feyz L, Wang Y, Pathak A, et al. Using social media to recruit study participants for a randomized trial for hypertension. Eur Heart J Digit Health 2020; 1: 71–74. 20201130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laursen DRT, Paludan-Müller AS, Hróbjartsson A. Randomized clinical trials with run-in periods: frequency, characteristics and reporting. Clin Epidemiol 2019; 11: 169–184. 20190211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams CP, Senft Everson N, Shelburne N, et al. Demographic and health behavior factors associated with clinical trial invitation and participation in the United States. JAMA Network Open 2021; 4: e2127792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stopponi MA, Alexander GL, McClure JB, et al. Recruitment to a randomized web-based nutritional intervention trial: characteristics of participants compared to non-participants. J Med Internet Res 2009; 11: e38. 20090826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Insider Intelligence. The majority of Americans’ mobile time spent takes place in apps, https://www.insiderintelligence.com/content/the-majority-of-americans-mobile-time-spent-takes-place-in-apps (2020, accessed August 28 2023).
- 33.Dehling T, Gao F, Schneider S, et al. Exploring the far side of mobile health: information security and privacy of mobile health apps on iOS and android. JMIR Mhealth Uhealth 2015; 3: e8. 20150119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sezgin E. Can we use commercial mobile apps instead of research mobile apps in healthcare research? Front Public Health 2021; 9: 685439. 20210723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel ML, Brooks TL, Bennett GG. Consistent self-monitoring in a commercial app-based intervention for weight loss: results from a randomized trial. J Behav Med 2020; 43: 391–401. 20190808. [DOI] [PubMed] [Google Scholar]
- 36.Spaulding EM, Marvel FA, Piasecki RJ, et al. User engagement with smartphone apps and cardiovascular disease risk factor outcomes: systematic review. JMIR Cardio 2021; 5: e18834. 20210203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pew Research Center. Mobile fact sheet, https://www.pewresearch.org/internet/fact-sheet/mobile/ (2024, accessed February 19 2024).
- 38.United States Census Bureau. Nation’s urban and rural populations shift following 2020 census, https://www.census.gov/newsroom/press-releases/2022/urban-rural-populations.html (2022, 2024).
- 39.North Carolina Office of State Budget and Management. Making sense of the new “Urban Area” definitions. The US census bureau alters what it considers urban areas, https://www.osbm.nc.gov/blog/2023/01/09/making-sense-new-urban-area-definitions (accessed February 22 2024).
- 40.Orkin AM, Nicoll G, Persaud N, et al. Reporting of sociodemographic variables in randomized clinical trials, 2014–2020. JAMA Netw Open 2021; 4: e2110700. 20210601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-dhj-10.1177_20552076241281216 for Sociodemographic predictors of successful screening and subsequent randomization in a digital health hypertension intervention by Hailey N Miller, Sandy Askew, Miriam B Berger, Elizabeth Trefney, Loneke T Blackman Carr, Melissa C Kay, Cherie Barnes, Qing Yang, Crystal C Tyson, Laura Svetkey, Ryan J Shaw, Dori M Steinberg and Gary G Bennett in DIGITAL HEALTH
Supplemental material, sj-docx-2-dhj-10.1177_20552076241281216 for Sociodemographic predictors of successful screening and subsequent randomization in a digital health hypertension intervention by Hailey N Miller, Sandy Askew, Miriam B Berger, Elizabeth Trefney, Loneke T Blackman Carr, Melissa C Kay, Cherie Barnes, Qing Yang, Crystal C Tyson, Laura Svetkey, Ryan J Shaw, Dori M Steinberg and Gary G Bennett in DIGITAL HEALTH