Abstract
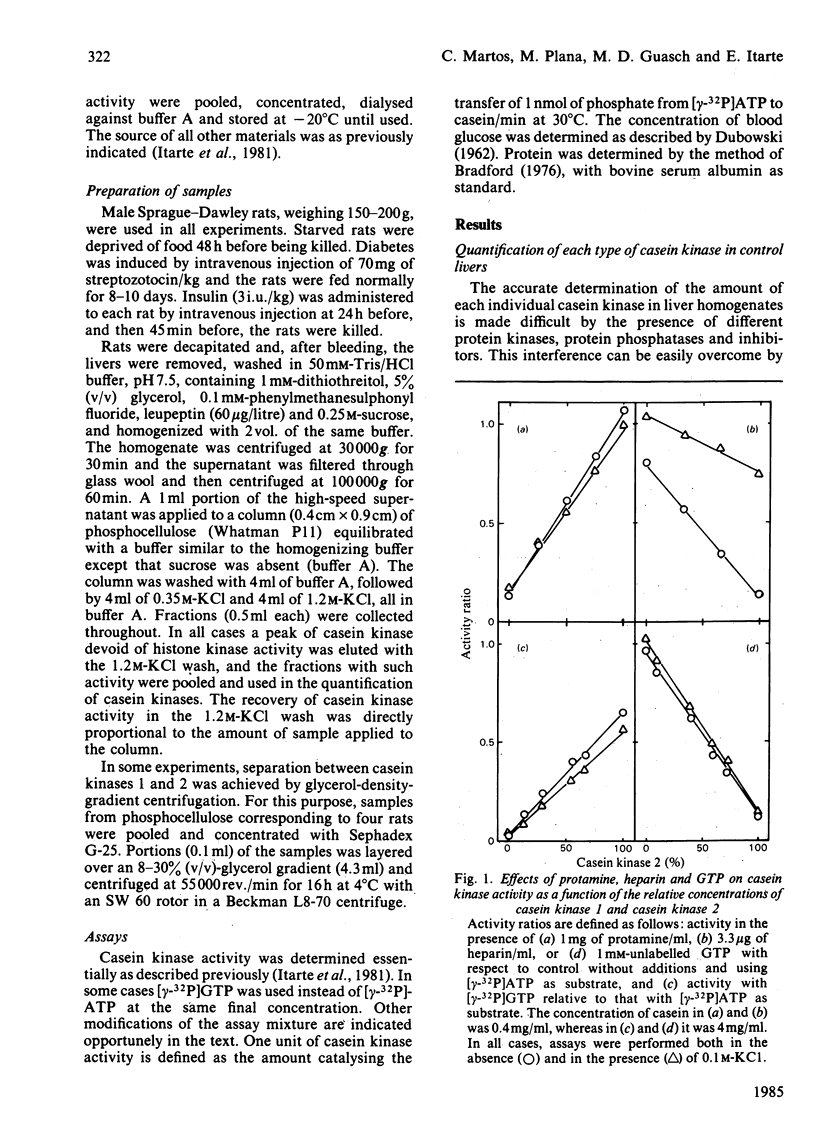
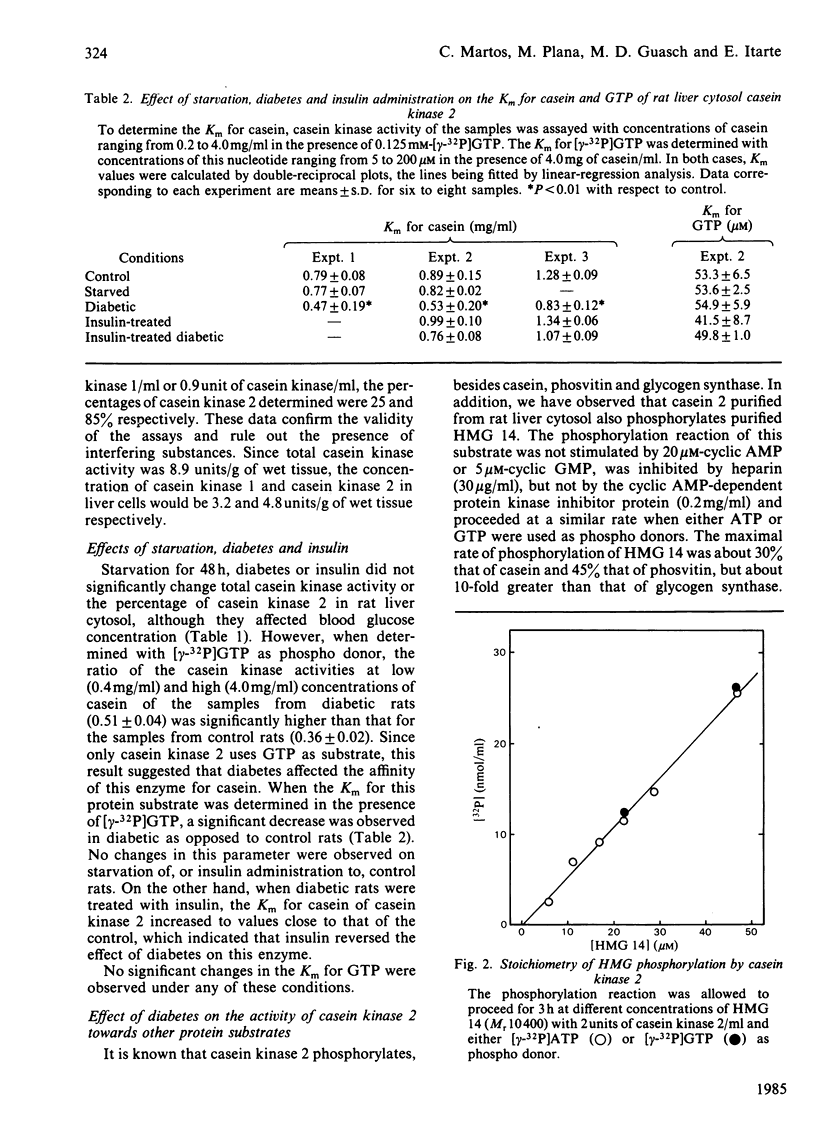
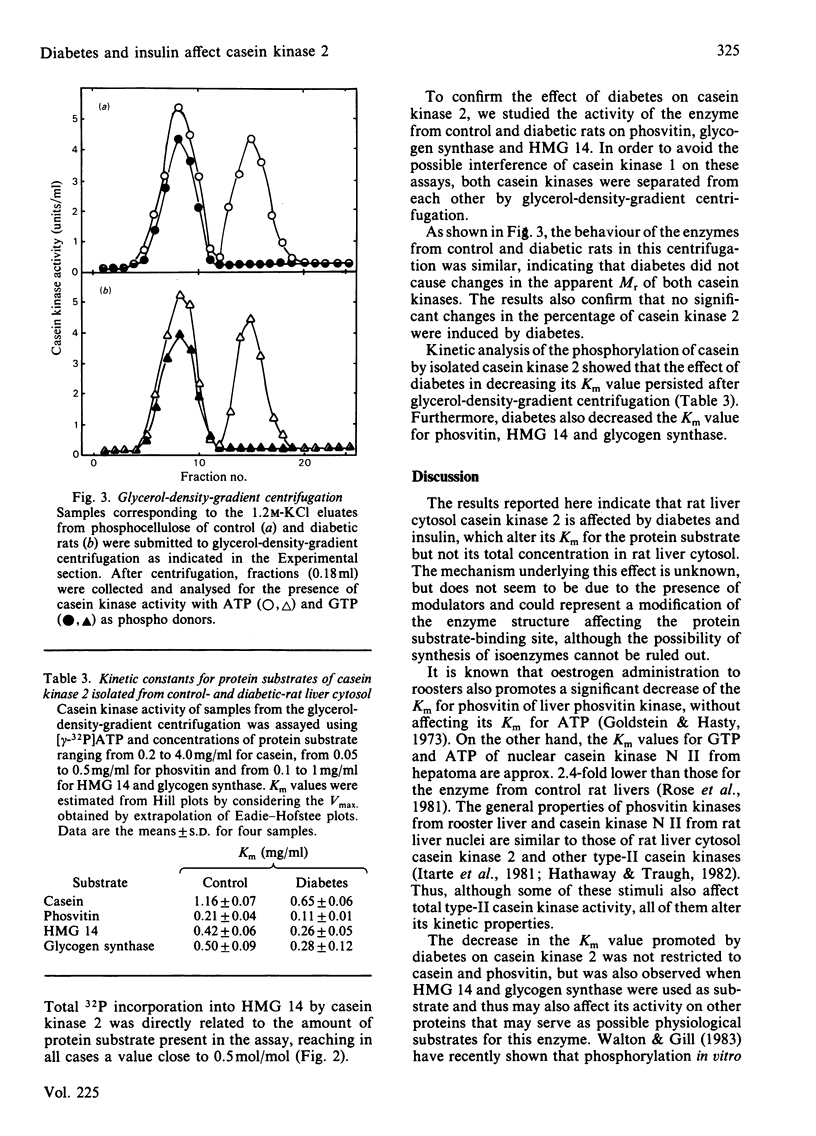
Starvation, diabetes and insulin did not alter the concentration of casein kinases in rat liver cytosol. However, the Km for casein of casein kinase 2 from diabetic rats was about 2-fold lower than that from control animals. Administration of insulin to control rats did not alter this parameter, but increased the Km for casein of casein kinase 2 in diabetic rats. Starvation did not affect the kinetic constants of casein kinases. The effect of diabetes on casein kinase 2 persisted after partial purification of the enzyme by glycerol-density-gradient centrifugation and affected also its activity on other protein substrates such as phosvitin, high-mobility-group protein 14 and glycogen synthase. The results indicate that rat liver cytosol casein kinase 2 is under physiological control.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad Z., DePaoli-Roach A. A., Roach P. J. Purification and characterization of a rabbit liver calmodulin-dependent protein kinase able to phosphorylate glycogen synthase. J Biol Chem. 1982 Jul 25;257(14):8348–8355. [PubMed] [Google Scholar]
- Bertomeu J. F., Guasch M. D., Plana M., Itarte E. Rat liver cytosol contains an inhibitor of the casein kinases 1 and 2 from the same source. FEBS Lett. 1981 Feb 23;124(2):261–264. doi: 10.1016/0014-5793(81)80151-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bustin M., Neihart N. K. Antibodies against chromosomal HMG proteins stain the cytoplasm of mammalian cells. Cell. 1979 Jan;16(1):181–189. doi: 10.1016/0092-8674(79)90199-5. [DOI] [PubMed] [Google Scholar]
- Carmichael D. F., Geahlen R. L., Allen S. M., Krebs E. G. Type II regulatory subunit of cAMP-dependent protein kinase. Phosphorylation by casein kinase II at a site that is also phosphorylated in vivo. J Biol Chem. 1982 Sep 10;257(17):10440–10445. [PubMed] [Google Scholar]
- Cohen P., Yellowlees D., Aitken A., Donella-Deana A., Hemmings B. A., Parker P. J. Separation and characterisation of glycogen synthase kinase 3, glycogen synthase kinase 4 and glycogen synthase kinase 5 from rabbit skeletal muscle. Eur J Biochem. 1982 May;124(1):21–35. doi: 10.1111/j.1432-1033.1982.tb05902.x. [DOI] [PubMed] [Google Scholar]
- DUBOWSKI K. M. An o-toluidine method for body-fluid glucose determination. Clin Chem. 1962 May-Jun;8:215–235. [PubMed] [Google Scholar]
- DePaoli-Roach A. A., Ahmad Z., Camici M., Lawrence J. C., Jr, Roach P. J. Multiple phosphorylation of rabbit skeletal muscle glycogen synthase. Evidence for interactions among phosphorylation sites and the resolution of electrophoretically distinct forms of the subunit. J Biol Chem. 1983 Sep 10;258(17):10702–10709. [PubMed] [Google Scholar]
- DePaoli-Roach A. A., Ahmad Z., Roach P. J. Characterization of a rabbit skeletal muscle protein kinase (PC0.7) able to phosphorylate glycogen synthase and phosvitin. J Biol Chem. 1981 Sep 10;256(17):8955–8962. [PubMed] [Google Scholar]
- DePaoli-Roach A. A., Roach P. J. Heparin inhibition and polyamine stimulation of a glycogen synthase kinase (PC0.7) from rabbit skeletal muscle. Arch Biochem Biophys. 1982 Aug;217(1):305–311. doi: 10.1016/0003-9861(82)90506-9. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Hasty M. A. Phosvitin kinase from the liver of the rooster. Purification and partial characterization. J Biol Chem. 1973 Sep 25;248(18):6300–6307. [PubMed] [Google Scholar]
- Goodwin G. H., Rabbani A., Nicolas P. H., Johns E. W. The isolation of the high mobility group non-histone chromosomal protein HMG 14. FEBS Lett. 1977 Aug 15;80(2):413–416. doi: 10.1016/0014-5793(77)80488-2. [DOI] [PubMed] [Google Scholar]
- Hathaway G. M., Traugh J. A. Casein kinases--multipotential protein kinases. Curr Top Cell Regul. 1982;21:101–127. [PubMed] [Google Scholar]
- Hemmings B. A., Aitken A., Cohen P., Rymond M., Hofmann F. Phosphorylation of the type-II regulatory subunit of cyclic-AMP-dependent protein kinase by glycogen synthase kinase 3 and glycogen synthase kinase 5. Eur J Biochem. 1982 Oct;127(3):473–481. doi: 10.1111/j.1432-1033.1982.tb06896.x. [DOI] [PubMed] [Google Scholar]
- Huang K. P., Itarte E., Singh T. J., Akatsuka A. Phosphorylation of glycogen synthase by cyclic AMP-independent casein kinase-2 from rabbit skeletal muscle. J Biol Chem. 1982 Mar 25;257(6):3236–3242. [PubMed] [Google Scholar]
- Itarte E., Mor M. A., Salavert A., Pena J. M., Bertomeu J. F., Guinovart J. J. Purification and characterization of two cyclic AMP-independent casein/glycogen synthase kinases from rat liver cytosol. Biochim Biophys Acta. 1981 Apr 14;658(2):334–347. doi: 10.1016/0005-2744(81)90304-1. [DOI] [PubMed] [Google Scholar]
- Itarte E., Robinson J. C., Huang K. P. Total conversion of glycogen synthase from the I- to the D-form by a cyclic AMP-independent protein kinase from rabbit skeletal muscle. J Biol Chem. 1977 Feb 25;252(4):1231–1234. [PubMed] [Google Scholar]
- Keller R. K., Chandra T., Schrader W. T., O'Malley B. W. Protein kinases of the chick oviduct: a study of the cytoplasmic and nuclear enzymes. Biochemistry. 1976 May 4;15(9):1958–1967. doi: 10.1021/bi00654a025. [DOI] [PubMed] [Google Scholar]
- Miyazaki K., Miyamoto E., Maeyama M., Uchida M. Specific regulation by steroid hormones of protein kinases in the endometrium. 1. Alteration by estrogen and progesterone in levels of protein kinases in rabbit endometrium. Eur J Biochem. 1980 Mar;104(2):535–542. doi: 10.1111/j.1432-1033.1980.tb04456.x. [DOI] [PubMed] [Google Scholar]
- Pena J. M., Itarte E., Domingo A., Cussó R. Cyclic adenosine 3':5'-monophosphate-dependent and -independent protein kinases in human leukemic cells. Cancer Res. 1983 Mar;43(3):1172–1175. [PubMed] [Google Scholar]
- Picton C., Woodgett J., Hemmings B., Cohen P. Multisite phosphorylation of glycogen synthase from rabbit skeletal muscle. Phosphorylation of site 5 by glycogen synthase kinase-5 (casein kinase-II) is a prerequisite for phosphorylation of sites 3 by glycogen synthase kinase-3. FEBS Lett. 1982 Dec 13;150(1):191–196. doi: 10.1016/0014-5793(82)81332-x. [DOI] [PubMed] [Google Scholar]
- Plana M., Guasch M. D., Itarte E. Modulators of rat liver cytosol casein kinases 1 and 2. Biochem Biophys Res Commun. 1982 Dec 31;109(4):1284–1290. doi: 10.1016/0006-291x(82)91916-7. [DOI] [PubMed] [Google Scholar]
- Rose K. M., Bell L. E., Siefken D. A., Jacob S. T. A heparin-sensitive nuclear protein kinase. Purification, properties, and increased activity in rat hepatoma relative to liver. J Biol Chem. 1981 Jul 25;256(14):7468–7477. [PubMed] [Google Scholar]
- Seyedin S. M., Kistler W. S. Levels of chromosomal protein high mobility group 2 parallel the proliferative activity of testis, skeletal muscle, and other organs. J Biol Chem. 1979 Nov 25;254(22):11264–11271. [PubMed] [Google Scholar]
- Singh T. J., Akatsuka A., Huang K. P., Sharma R. K., Tam S. W., Wang J. H. A multifunctional cyclic nucleotide- and Ca2+-independent protein kinase from rabbit skeletal muscle. Biochem Biophys Res Commun. 1982 Jul 30;107(2):676–683. doi: 10.1016/0006-291x(82)91544-3. [DOI] [PubMed] [Google Scholar]
- Walton G. M., Gill G. N. Identity of the in vivo phosphorylation site in high mobility group 14 protein in HeLa cells with the site phosphorylated by casein kinase II in vitro. J Biol Chem. 1983 Apr 10;258(7):4440–4446. [PubMed] [Google Scholar]
- Woodgett J. R., Tonks N. K., Cohen P. Identification of a calmodulin-dependent glycogen synthase kinase in rabbit skeletal muscle, distinct from phosphorylase kinase. FEBS Lett. 1982 Nov 1;148(1):5–11. doi: 10.1016/0014-5793(82)81231-3. [DOI] [PubMed] [Google Scholar]


