Abstract
Background
Regaining walking ability is a key target in geriatric rehabilitation. This study evaluated the prevalence of walking ability at (pre‐)admission and related clinical characteristics in a cohort of geriatric rehabilitation inpatients; in inpatients without walking ability, feasibility and effectiveness of progressive resistance exercise training (PRT) were assessed.
Methods
Inpatients within RESORT, an observational, longitudinal cohort of geriatric rehabilitation inpatients, were stratified in those with and without ability to walk independently (defined by Functional Ambulation Classification (FAC) score ≤ 2) at admission; further subdivision was performed by pre‐admission walking ability. Clinical characteristics at admission, length of stay, and changes in physical and functional performance throughout admission were compared depending on (pre‐)admission walking ability. Feasibility (relative number of PRT sessions given and dropout rate) and effectiveness [change in Short Physical Performance Battery, FAC, independence in (instrumental) activities of daily living (ADL/IADL)] of PRT (n = 11) in a subset of inpatients without ability to walk independently at admission (able to walk pre‐admission) were investigated compared with usual care (n = 11) (LIFT‐UP study).
Results
Out of 710 inpatients (median age 83.5 years; 58.0% female), 52.2% were not able to walk independently at admission, and 7.6% were not able to walk pre‐admission. Inpatients who were not able to walk independently at admission, had a longer length of stay, higher prevalence of cognitive impairment and frailty and malnutrition risk scores, and a lower improvement in independence in (I)ADL compared with inpatients who were able to walk at both admission and pre‐admission. In LIFT‐UP, the relative median number of PRT sessions given compared with the protocol (twice per weekday) was 11 out of 44. There were no dropouts. PRT improved FAC (P = 0.028) and ADL (P = 0.034) compared with usual care.
Conclusions
High prevalence of inpatients who are not able to walk independently and its negative impact on independence in (I)ADL during geriatric rehabilitation highlights the importance of tailored interventions such as PRT, which resulted in improvement in FAC and ADL.
Keywords: Aged, Dependent ambulation, Physical functional performance, Rehabilitation, Resistance training, Walking
Introduction
Walking ability is likely to decline during acute hospitalization of older patients with up to 65% of inpatients losing their ability to walk independently, 1 defined as a Functional Ambulation Classification (FAC) score ≤ 2 points. 2 Besides the reason for admission, bed rest and lack of mobilization during hospitalization contribute to the loss of walking ability. 1 Inability to walk is associated with poor outcome in older adults, such as activities of daily living (ADL) impairment, institutionalization, and death. 3 Geriatric rehabilitation consists of multidisciplinary interventions, including physical and occupational therapy, to promote independent functioning and muscle strength recovery in older adults after hospitalization. 4 These interventions, although designed according to the patient's needs, may need further tailoring accounting for walking ability. This includes considering the underlying reason for walking inability such as a lack of strength, endurance, cognition, pain, or a combination of factors.
Up to 50% of geriatric rehabilitation patients are not able to walk independently at admission. 5 , 6 But whether walking ability prior to and at admission to geriatric rehabilitation is related to clinical characteristics of inpatients and whether it influences physical and functional performance recovery have not been studied. These insights are important to guide the need for tailored interventions in patients who are unable to walk. Moreover, evidence lacks on the type of interventions needed to regain walking ability, in addition to standard rehabilitation. Several interventions aimed at improving walking ability in community dwelling older adults have been studied, including a combination of resistance, endurance, and balance training, 7 as well as visual‐related training. 8 However, feasibility and efficacy of these interventions to improve mobility and independence in ADL in geriatric rehabilitation inpatients without walking ability have not been investigated. Progressive resistance exercise training (PRT) may be beneficial as it has proven to be effective to improve muscle strength and power, balance, gait speed, and independence in ADL in (acutely hospitalized) older adults. 9 , 10 , 11
This study aims to (1) identify the prevalence of independent walking ability at admission to geriatric rehabilitation and pre‐admission in a large cohort; (2) compare admission characteristics, length of stay, and changes in physical and functional performance throughout the admission to geriatric rehabilitation stratified by walking ability at admission and pre‐admission; (3) investigate the feasibility (relative number of PRT sessions given, dropout rate, and related adverse events) and effectiveness of PRT on changes in physical and functional performance from baseline to discharge compared with usual care in a subgroup of inpatients without walking ability at admission.
Methods
Study design and population
REStOring health of acutely unwell adulTs (RESORT) is an observational, longitudinal inception cohort of geriatric rehabilitation inpatients admitted to the Royal Melbourne Hospital (Melbourne, Victoria, Australia). Inpatients were assessed using Comprehensive Geriatric Assessment (CGA) performed by physicians, nurses, physiotherapists, occupational therapists, and dietitians within 48 h of admission and 48 h before discharge. 12 Exclusion criteria were (1) inpatient's inability to provide informed consent and no nominated proxy to consent and (2) palliative admission at admission. The study was approved by the Melbourne Health Human Research Ethics Committee (HREC/17/MH/103) and was conducted in accordance with the Declaration of Helsinki. 13 Of the 2692 inpatients admitted from 16 October 2017 and discharged by 18 March 2020, 446 were excluded, and 356 refused to consent; a total of 1890 inpatients were included in the RESORT cohort. The FAC was implemented in wave 3, and therefore, for the present analysis, 710 inpatients were included. LIFT‐UP is a feasibility intervention sub study within the RESORT cohort from 15 May 2019 to 18 March 2020, assessing the feasibility and effectiveness of PRT in inpatients without walking ability. LIFT‐UP was conducted on four wards; the PRT programme was enrolled in two of the four wards; the other two wards served as usual care group. Of the 360 admitted inpatients, 164 were able to walk, 98 were excluded for other reasons including inability to follow instructions, end‐stage disease, and severe hemiparesis, and 41 refused to consent to RESORT (Figure 1). Of the 28 inpatients, 12 inpatients were in the PRT group and 16 in the usual care group based on the ward allocation.
Figure 1.

Flowchart of RESORT patients included in LIFT‐UP. FAC, Functional Ambulation Classification; PRT, progressive resistance exercise training.
Inpatient characteristics
Age, sex, primary reason for acute admission, and length of stay in geriatric rehabilitation were collected from medical records. Disease burden was assessed by physicians with the 56‐point Cumulative Illness Rating Scale (CIRS) 14 and 37‐point Charlson co‐morbidity index (CCI). 15 Cognitive impairment was assessed by physicians and defined by a dementia diagnosis or mild cognitive impairment/minor neurocognitive disorder reported in medical records, CCI or CIRS or by a cognitive score below cuff‐off values for one of the following tests: standardized Mini‐Mental State Examination (sMMSE) < 24 points, 16 Rowland Universal Dementia Assessment Scale (RUDAS) < 23 points, 17 or Montreal Cognitive Assessment (MoCA) < 26 points. 18 Frailty status was assessed by physicians using the Clinical Frailty Scale (CFS) on a scale from 1 (very fit) to 9 (terminally ill). 19 Anthropometric measurements were performed by nurses. Standing height was measured barefoot; if the inpatient was unable to stand, knee height was measured with a sliding calliper, and height was estimated using the Chumlea equation. 20 Body weight was measured using a calibrated weighing scale, weighing chair, or hoist without shoes or heavy clothes. Body mass index (BMI) was calculated by dividing weight by height squared (kg/m2). Risk of malnutrition was assessed by nurses using the Malnutrition Screening Tool (MST) on a scale from 0 to 5. 21 Muscle strength and physical performance were assessed by physiotherapists. Handgrip strength (HGS) was assessed with a handheld dynamometer (JAMAR, Sammons Preston, Inc. Boling‐Brook, IL, USA). Inpatients were asked to squeeze with maximum effort three times on both hands, alternating between right and left, in a seated position with the elbow bent at a 90° angle, adjacent to the body and unsupported. 22 The maximum score in kilograms was used. The Short Physical Performance Battery (SPPB) includes the standing balance test, the timed chair stand test and the timed 4‐m walk test; score ranges from 0 to 12 points. 23 Functional performance was assessed by occupational therapists using the Katz index for ADL (0–6 points) 24 and the Lawton and Brody scale for instrumental ADL (IADL) (0–8 points). 25
Walking ability
Walking ability pre‐admission to hospital was assessed as part of a patient survey with the following question: ‘In the month before you were admitted to hospital, were you able to walk (with or without a walking aid)?’. Independent ambulation, here defined as walking ability, at admission to geriatric rehabilitation was assessed by physiotherapists and defined as a FAC score ≤ 2. 2
LIFT‐UP
Inclusion criteria were a FAC score ≤ 2 points and being able to follow basic instructions in English or gestures. Exclusion criteria encompassed not being to walk pre‐admission to hospital, weight‐bearing restrictions, inability to follow instructions, end‐stage disease, severe hemiparesis, and spinal lesions. There was no blinding due to the nature of the intervention. Usual care consisted of a maximum of daily physiotherapy sessions, depending on the needs of the inpatient, including functional (walking, transfer from bed/chair, and stair climbing), balance, endurance, and resistance training (variety of upper‐ and lower‐body exercises). The treatment goals were determined together with the patient. There were four types of physiotherapy sessions: case management (multidisciplinary meeting to discuss progress, individual not present), assessment (evaluation), intervention (session alone with physiotherapist), and exercise class (group session). Details on the frequency, duration, and type of physiotherapy received during admission in the RESORT cohort are described elsewhere. 26 In the PRT group, bi‐daily resistance training sessions were provided in addition to usual care. The PRT programme was a one‐on‐one supervised programme implemented by the treating physiotherapist during the total length of stay in geriatric rehabilitation, designed using the 16‐item checklist Consensus on Exercise Reporting Template (CERT). 27 The programme consisted of seven exercises (chair raise, hip thrust, glute abduction, lateral pulldown, chest fly, shoulder abduction, and prone hold) performed every weekday split into two 15‐min sessions (morning‐afternoon); one for the lower and one for the upper body. The progression model was designed to be performed on the bed and eventually progressed to complete stance. The progression model incorporated the Oxford Scale, starting with performing the movement assisted and with gravity eliminated with the goal to progress the movement against gravity with resistance. The intensity was assessed by the treating physiotherapist using the rate of perceived exertion (RPE), a scale of 1 (very easy) to 10 (maximal), 28 with a desired intensity for each exercise of eight. If eight repetitions of the exercise could be performed at less than RPE eight, the movement was either progressed to the next variation, or resistance was added using elastic bands by Thera‐Band (Hygenic Corp., Akron, USA) with three levels: yellow (low resistance), red (moderate resistance), and green (high resistance).
Feasibility of the PRT programme was assessed as the (1) relative number of PRT sessions given, defined as number of PRT sessions given relative to the total number of sessions planned per protocol (twice per weekday – two assessment sessions); (2) number of dropouts (patients who decided to prematurely withdraw from LIFT‐UP), excluding inpatients who were transferred back to acute hospitalization (considered as discontinuation); and (3) percentage of inpatients with adverse events related to PRET. The outcomes of LIFT‐UP were the changes in SPPB, FAC, ADL, and IADL scores from baseline (inclusion LIFT‐UP) at discharge.
Statistical analysis
Median and interquartile ranges (IQR) were reported for non‐normally distributed continuous variables and frequencies (n) with percentages (%) for categorical variables. Changes in SPPB, FAC, ADL, and IADL during geriatric rehabilitation were calculated by subtracting the admission from the discharge score. Differences in characteristics dependent on walking ability were assessed with Kruskal–Wallis tests for continuous/ordinal variables and chi‐square tests for categorical variables. Bonferroni corrections were applied for pairwise comparisons resulting in P values < 0.008 to be considered statistically significant (six comparisons). For LIFT‐UP, inpatients who discontinued the study (transfer to acute hospitalization) were excluded from the analyses. Differences in baseline characteristics between the PRT and the usual care group were assessed with Mann–Whitney U tests for continuous/ordinal variables and chi‐square tests for categorical variables. Within‐group differences in SPPB, FAC, ADL, and IADL scores between baseline and discharge were assessed with Wilcoxon‐signed rank tests and between‐group differences with Mann–Whitney U tests. P‐values of <0.05 were considered statistically significant. All statistical analyses were performed using the Statistical Package for the Social Sciences (IBM SPSS Advanced Statistics 27.0).
Results
Median length of stay (n = 710) was 20 days [IQR: 13–32], and median SPPB, ADL, and IADL scores at admission were 1 [IQR: 0–4], 2 [IQR: 1–2], and 1 [IQR: 0–2], respectively (Table 1). In the LIFT‐UP study (n = 28), one inpatient discontinued (transfer to acute hospitalization) in the PRT group and five in the usual care group. The PRT group (n = 11) had a lower BMI, and a lower prevalence of cognitive impairment compared with the usual care group (n = 11) (Table 1).
Table 1.
Characteristics at admission to geriatric rehabilitation of inpatients included in RESORT (n = 710) and LIFT‐UP (n = 22)
| RESORT (n = 710) | LIFT‐UP (n = 22) | ||||||
|---|---|---|---|---|---|---|---|
| PRT group (n = 11) | Usual care group (n = 11) | ||||||
| Characteristics | n | Total | n | Total | n | Total | P a |
| Age (years) | 710 | 83.5 [77.8–89.4] | 11 | 85.9 [79.5–92.2] | 11 | 79.5 [77.4–86.2] | 0.193 |
| Female, n (%) | 710 | 412 (58.0) | 11 | 9 (81.8) | 11 | 6 (54.5) | 0.170 |
| Primary reason for acute admission, n (%) | 710 | 11 | 11 | 0.363 | |||
| Musculoskeletal | 338 (47.6) | 7 (63.6) | 5 (45.5) | ||||
| Neurological | 100 (14.1) | 1 (9.1) | 2 (18.2) | ||||
| Respiratory | 53 (7.5) | 1 (9.1) | 0 (0.0) | ||||
| Cardiac | 50 (7.0) | 0 (0.0) | 1 (9.1) | ||||
| Other | 169 (23.8) | 2 (18.2) | 2 (18.2) | ||||
| Length of stay c (days) | 710 | 20 [13–32] | 11 | 22 [17–32] | 11 | 38 [17–49] | 0.151 |
| CIRS score [0–56] (points) | 710 | 12 [8–16] | 11 | 15 [10–19] | 11 | 15 [11–17] | 1.000 |
| CCI score [0–37] (points) | 710 | 2 [1–3] | 11 | 2 [2–4] | 11 | 3 [1–4] | 0.847 |
| Cognitive impairment, n (%) | 710 | 466 (65.6) | 11 | 5 (45.5) | 11 | 11 (100) | 0.011 |
| Clinical Frailty Scale [1–9] (points) | 652 | 6 [5–7] | 10 | 6 [6–7] | 11 | 6 [6–7] | 0.426 |
| Body mass index (kg/m2) | 694 | 25.7 [22.2–29.8] | 11 | 23.4 [20.4–27.2] | 11 | 30.6 [27.0–35.0] | 0.013 |
| Malnutrition Screening Tool [0–5] (points) | 695 | 1 [0–2] | 11 | 2 [1–4] | 11 | 0 [0–2] | 0.056 |
| Handgrip strength (kg) | 547 | 16.0 [11.5–22.0] | 8 | 15.5 [8.0–19.5] | 10 | 16.0 [11.0–19.0] | 0.829 |
| Female | 291 | 13.0 [9.0–17.6] | 7 | 14.0 [8.0–18.0] | 5 | 12.0 [8.5–17.5] | b |
| Male | 241 | 21.7 [16.0–26.0] | 1 | 28.0 [28.0–28.0] | 5 | 16.0 [14.5–24.0] | b |
| Physical performance | |||||||
| SPPB score [0–12] (points) | 655 | 1 [0–4] | 9 | 0 [0–0] | 11 | 0 [0–1] | 0.882 |
| FAC score [0–5] (points) | 710 | 2 [0–3] | 11 | 1 [0–2] | 11 | 1 [0–2] | 0.847 |
| Functional performance | |||||||
| Katz‐ADL score [0–6] (points) | 709 | 2 [1–2] | 11 | 1 [1–1] | 11 | 1 [1–2] | 0.562 |
| Lawton‐IADL score [0–12] (points) | 709 | 1 [0–2] | 11 | 1 [0–2] | 11 | 1 [0–1] | 0.300 |
All results are presented as median [IQR] unless otherwise indicated. Between‐group difference significant at the 5% level, indicated in bold.
(I)ADL, (instrumental) activities of daily living; CCI, Charlson co‐morbidity index; CIRS, Cumulative Illness Rating Scale; FAC, Functional Ambulation Classification; IQR, interquartile range; PRT, progressive resistance exercise training; SPPB, Short Physical Performance Battery.
The difference between the PRT group and control group of the LIFT‐UP study.
No P‐value presented because of the small sample size.
In geriatric rehabilitation.
Walking ability
Overall, 7.6% (n = 54) of inpatients were not able to walk independently pre‐admission, and 52.2% (n = 371) were not able to walk independently at admission. When combining pre‐admission and admission, 45.4% (n = 322) of inpatients were able to walk at both pre‐admission and admission (able/able), 2.4% (n = 17) were not able to walk independently pre‐admission but were able at admission (not able/able), 47.0% (n = 334) were able to walk independently pre‐admission but not at admission (able/not able), and 5.2% (n = 37) were not able to walk independently at both pre‐admission and admission (not able/not able) (Figure 2).
Figure 2.
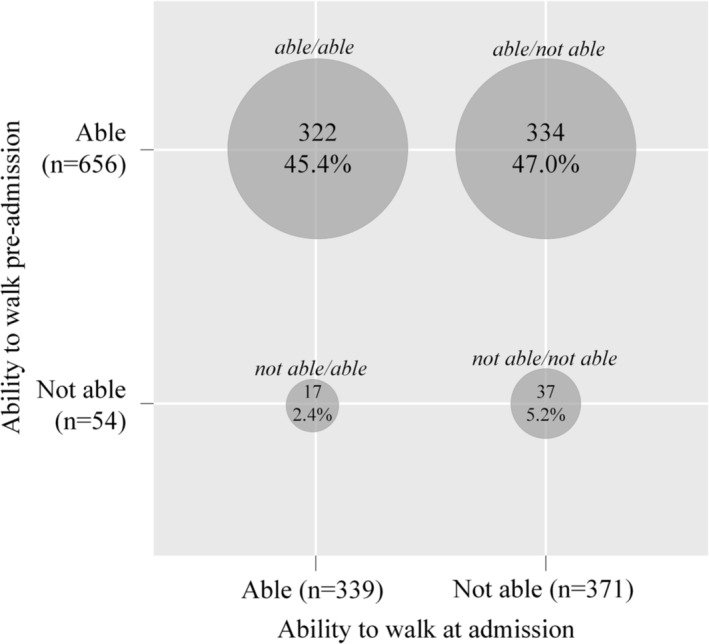
Prevalence of walking ability pre‐admission (before acute hospital admission) and at admission to geriatric rehabilitation (n = 710).
Clinical characteristics, length of stay, and changes in physical and functional performance stratified by walking ability
Inpatients who were not able to walk independently at admission (able/not able – not able/not able groups) had a longer length of stay, worse cognition, higher frailty and malnutrition risk, lower independence in IADL at admission, and a lower improvement in independence in IADL during admission compared with inpatients who were able to walk at admission and pre‐admission (able/able group) (Table 2). Inpatients who were not able to walk independently at admission but able to walk pre‐admission (able/not able group) had a higher improvement in mobility (FAC) during admission compared with inpatients who were able to walk at both admission and pre‐admission (able/able group). Inpatients who were not able to walk independently at both admission and pre‐admission (not able/not able group) had a lower improvement in independence in ADL during admission compared with inpatients who were able to walk at both admission and pre‐admission (able/able group). There were no differences in age, sex, reason for admission, morbidity, BMI, handgrip strength, and change in physical performance between groups.
Table 2.
Clinical characteristics at admission to geriatric rehabilitation and change in physical and functional performance during admission in the RESORT cohort, stratified by walking ability (n = 710)
| Walking ability | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Able/able a (n = 322) | Not able/able a (n = 17) | Able/not able a (n = 334) | Not able/not able a (n = 37) | ||||||
| Characteristics | n | Total | n | Total | n | Total | n | Total | P b |
| Age (years) | 322 | 83.1 [77.4–88.9] | 17 | 80.2 [76.9–84.9] | 334 | 84.1 [78.8–90.0] | 37 | 85.3 [79.2–88.6] | 0.076 |
| Female, n (%) | 322 | 178 (55.3) | 17 | 15 (88.2) | 334 | 197 (59.0) | 37 | 22 (59.5) | 0.057 |
| Primary reason for acute admission, n (%) | 322 | 17 | 334 | 37 | 0.083 | ||||
| Musculoskeletal | 145 (45.0) | 9 (52.9) | 169 (50.6) | 15 (40.5) | |||||
| Neurological | 34 (10.6) | 2 (11.8) | 54 (16.2) | 10 (27.0) | |||||
| Respiratory | 30 (9.3) | 0 (0.0) | 20 (6.0) | 3 (8.1) | |||||
| Cardiac | 33 (10.2) | 1 (5.9) | 15 (4.5) | 1 (2.7) | |||||
| Other | 80 (24.8) | 5 (29.4) | 76 (22.8) | 8 (21.6) | |||||
| Length of stay c (days) | 322 | 15 [10–23] d , e | 17 | 19 [10–24] | 334 | 24 [17–39] | 37 | 26 [14–41] | <0.001 |
| CIRS [0–56] (points) | 322 | 11 [7–15] | 17 | 14 [8–16] | 334 | 12 [8–16] | 37 | 11 [8–14] | 0.117 |
| CCI [0–37] (points) | 322 | 2 [1–3] | 17 | 2 [1–4] | 334 | 2 [1–4] | 37 | 2 [1–4] | 0.112 |
| Cognitive impairment, n (%) | 322 | 196 (60.9) d , e | 17 | 9 (52.9) | 334 | 233 (69.8) | 28 | 21 (75.7) | 0.035 |
| Clinical Frailty Scale [1–9] (points) | 289 | 6 [4–6] d , e | 15 | 5 [4–6] | 314 | 7 [6–7] | 34 | 6 [5–7] | <0.001 |
| Body mass index (kg/m2) | 317 | 26.3 [22.7–30.1] | 17 | 27.2 [23.0–31.3] | 324 | 25.3 [21.7–29.0] | 36 | 25.1 [22.9–31.9] | 0.106 |
| Malnutrition Screening Tool [0–5] (points) | 317 | 0 [0–2] d , e | 17 | 1 [0–2] | 325 | 1 [0–2] | 36 | 2 [0–2] | <0.001 |
| Handgrip strength (kg) | 269 | 18.0 [12.0–22.5] | 15 | 12.0 [8.0–18.0] | 241 | 15.0 [10.0–20.5] | 22 | 14.0 [6.0–22.0] | 0.008 |
| Physical performance | |||||||||
| SPPB score at admission [0–12] (points) | 291 | 4 [2–6] d , e | 16 | 2 [1–4] d , e | 313 | 0 [0–0] | 35 | 0 [0–0] | <0.001 |
| Change from admission to discharge | 251 | 1 [0–3] | 12 | 1 [0–2] | 250 | 1 [0–3] | 31 | 0 [0–3] | 0.345 |
| FAC score [0–5] (points) | |||||||||
| Change from admission to discharge | 302 | 1 [0–1] d | 14 | 1 [0–1] | 298 | 2 [0–3] | 33 | 1 [0–3] | <0.001 |
| Functional performance | |||||||||
| Katz‐ADL score [0–6] (points) | 322 | 2 [1–4] d , e | 17 | 2 [2–3] d , e | 333 | 1 [0–2] | 37 | 1 [0–2] | <0.001 |
| Change from admission to discharge | 314 | 2 [1–3] e | 16 | 2 [1–3] | 305 | 1 [0–3] | 36 | 0 [0–2] | 0.005 |
| Lawton‐IADL score [0–12] (points) | 322 | 1 [1–2] d , e | 17 | 1 [0–3] | 333 | 1 [0–2] | 37 | 0 [0–1] | <0.001 |
| Change from admission to discharge | 315 | 2 [0–4] d , e | 16 | 2 [0–3] | 305 | 1 [0–2] | 36 | 0 [0–2] | <0.001 |
All results are presented as median [IQR] unless otherwise indicated. Between‐group difference significant at the 0.8% level, indicated in bold.
Able or not able before the backlash refers to walking ability pre‐acute admission and after the backlash to walking ability at admission.
Statistical difference between the four groups; pairwise comparisons adjusted with Bonferroni correction (P < 0.008).
In geriatric rehabilitation.
Significant difference from the ‘able/not able’ group.
Significant difference from the ‘not able/not able’ group.
Feasibility and effectiveness of progressive resistance exercise training in the LIFT‐UP study
The duration of the intervention varied between 8 and 48 days (until discharge) and relative median number of PRT sessions given compared with protocol was 11 out of 44 (Table 3). There were no dropouts in both the PRT and usual care group. In the PRT group (n = 11), SPPB, FAC, ADL, and IADL scores significantly improved between baseline and discharge (Table 4). In the usual care group (n = 11), SPPB and FAC significantly improved between baseline and discharge, and ADL and IADL scores did not improve. PRT improved change in FAC (P = 0.028) and ADL (P = 0.034) between baseline and discharge compared with usual care, but not SPPB (P = 0.837) and IADL (P = 0.243).
Table 3.
Relative number of progressive resistance exercise training sessions given compared with protocol (two sessions per day) for the LIFT‐UP study (n = 11)
| Patient a | Study duration (days) | Weekend Days | Number of PRT sessions given | Relative number of sessions | ||
|---|---|---|---|---|---|---|
| Morning | Afternoon | Total | Given/maximum per protocol b | |||
| 1 | 13 | 2 | 8 | 3 | 11 | 11/20 |
| 2 | 8 | 2 | 3 | 2 | 5 | 5/10 |
| 3 | 16 | 4 | 4 | 6 | 10 | 10/22 |
| 4 | 32 | 10 | 13 | 6 | 19 | 19/42 |
| 5 | 48 | 14 | 19 | 4 | 23 | 23/66 |
| 6 | 31 | 8 | 6 | 5 | 11 | 11/44 (median) |
| 7 | 9 | 2 | 3 | 0 | 3 | 3/12 |
| 8 | 14 | 4 | 3 | 0 | 3 | 3/18 |
| 9 | 27 | 8 | 5 | 0 | 5 | 5/36 |
| 10 | 18 | 4 | 1 | 0 | 1 | 1/26 |
| 11 | 24 | 6 | 1 | 0 | 1 | 1/34 |
PRT, progressive resistance exercise training.
Ordered in descending order of relative number of PRT sessions given.
Maximum number of sessions per protocol = [(study duration (days) − number of weekend days)*2] − 2.
Table 4.
Effect of progressive resistance exercise training on physical and functional performance change during admission compared with usual care in geriatric rehabilitation inpatients for the LIFT‐UP study (n = 22)
| Outcome | PRT group (n = 11) | Usual care group (n = 11) | Difference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Discharge | Change a | P b | Baseline | Discharge | Change a | P b | P c | |
| Physical performance | |||||||||
| SPBB score [0–12] (points) | 0 [0–1] | 3 [1–6] | 3 [0–5] | 0.041 | 0 [0–1] | 3 [0–6] | 3 [0–6] | 0.027 | 0.837 |
| FAC score [0–5] (points) | 1 [0–2] | 4 [3–4] | 2 [2–4] | 0.003 | 1 [0–2] | 2 [0–4] | 0 [0–2] | 0.042 | 0.028 |
| Functional performance | |||||||||
| Katz‐ADL score [0–6] (points) | 1 [1–1] | 5 [1–5] | 3 [1–4] | 0.007 | 1 [1–2] | 1 [1–4] | 0 [0–2] | 0.168 | 0.034 |
| Lawton‐IADL score [0–12] (points) | 1 [0–2] | 2 [1–5] | 1 [1–4] | 0.011 | 1 [0–1] | 1 [0–4] | 0 [0–3] | 0.078 | 0.243 |
All results are presented as median [IQR]. Significant at the 5% level, indicated in bold.
(I)ADL, (instrumental) activities of daily living; FAC, Functional Ambulation Classification; IQR, interquartile range; PRT, progressive resistance exercise training; SPPB, Short Physical Performance Battery.
Change in score from baseline to discharge.
Within‐group difference between baseline and discharge scores.
Between‐group (PRT vs. usual care) difference in change in score from baseline to discharge.
Discussion
In this cohort of geriatric rehabilitation inpatients, half were not able to walk independently at admission. (Pre‐)admission walking ability had a significant impact on clinical characteristics at admission and improvements observed during rehabilitation. Notably, inpatients who were not able to walk independently at admission had a longer length of stay, worse cognition, higher frailty and malnutrition risk, and a lower improvement in independence in (I)ADL compared with inpatients who were able to walk at (pre‐)admission. Progressive resistance training improved changes in FAC and independence in ADL but not in SPPB and independence in IADL between baseline and discharge when compared with usual care in inpatients without walking ability.
Previous studies in older adults at discharge from acute hospitalization showed lower prevalence of inability to walk (16.8%) 29 and similar prevalence (45.0%) 30 compared with this cohort. This may be due to difference in assessment as the former study used subjective patient reports, which may overestimate walking ability. 31 Moreover, lower walking ability can be expected in geriatric rehabilitation compared with acute hospitalization as patients who are able to walk are more likely to be discharged home directly after acute admission. Previous studies in geriatric rehabilitation also showed around 50% of patients were not able to walk at admission. 5 , 6 The decline in walking ability between pre‐admission and admission is in line previous studies 1 although inability to walk pre‐admission is commonly solely described by the use of a walking aid. 32 Future research should investigate whether there are specific risk factors of prolonged inability to walk.
Similarly to our findings, low physical function (Barthel Index/Katz‐ADL/gait speed/balance) at admission 33 and reduced pre‐fracture mobility (use of a walking aid) 34 were associated with a longer length of stay in hospitalized older adults. Moreover, poor mobility and pre‐admission loss of independence in ADL have shown to be predictors of functional decline during acute hospitalization in older adults. 35 , 36 This highlights the importance of tailored interventions in patients without walking ability to improve function recovery during rehabilitation, as this is crucial to facilitate discharge home. The higher improvement in mobility (FAC) in inpatients who are not able to walk is likely to be caused by a ceiling effect as inpatients who can already walk at admission will have less room for improvement. Specifically, physical therapy interventions in geriatric rehabilitation are designed to enable patients to achieve a satisfactory level of independent functioning following discharge; walking outside and climbing stairs may not be trained if not deemed necessary. In line with our findings, hip fracture patients with worse pre‐fracture nutritional status assessed by the Mini Nutritional Assessment were less likely to regain their pre‐fracture mobility. 37 Improving nutritional interventions prior and during rehabilitation may thus play a key role to promote walking ability. Similarly to our results, in hospitalized older adults, cognitive impairment had a negative impact on mobility recovery (Tinetti Performance‐Oriented Mobility Assessment) 38 and lead to an increased risk of functional decline. 39 Cognitive impairment therefore also needs to be considered when tailoring rehabilitation interventions to recover walking ability. Previous studies did not take pre‐admission walking ability into account. The present results show most differences in clinical characteristics occur based on admission walking ability. However, the small group size of inpatients who were not able to walk pre‐admission may have influenced the results.
Interestingly, while inability to walk independently at admission impeded improvement in ADL, PRT in patients who were unable to walk at admission showed to significantly improve change in ADL and FAC during geriatric rehabilitation admission compared with usual care. Although further research is needed with a larger sample size, this shows that the addition of PRT to the rehabilitation plan of patients who are unable to walk may improve their recovery. Systematic reviews investigating the effect of PRT in older adults after a hip fracture surgery, 7 community‐dwelling older adults, 8 and nursing homes residents 11 have also shown significant improvements in muscle strength, functional performance, and physical function. PRT twice daily was not feasible in most inpatients. Lack of patients' knowledge on the importance of PRT for muscle strength, 40 other interventions and medical appointments, and visitors may have limited feasibility. Understanding the reasons why PRT sessions were not given (patient and not patient related) is needed to assess feasibility further.
Strengths and limitations
To the best of our knowledge, this is one of the first studies assessing (pre‐) admission walking ability and thereto related clinical characteristics in a large cohort of geriatric rehabilitation inpatients. Furthermore, all inpatients were assessed using a CGA, which promotes validity and standardization of measurements in older patients. A limitation of this study is the self‐reported assessment of walking ability pre‐admission to hospital, which may have overestimated walking ability. 31 Furthermore, the LIFT‐UP study consisted of a small sample size due to the nature of an exploratory study and BMI and cognitive impairment prevalence were significantly higher in the PRT group compared with the usual care group, warranting caution with results interpretation. Finally, this was a single‐site study, which could limit generalisability to other geriatric rehabilitation settings and countries. Further research is therefore needed to inform clinical practice.
Conclusions
The prevalence of inability to walk independently at admission was high and associated with a longer length of stay, worse cognition, and nutritional status at admission, as well as worse functional performance improvement during admission compared with inpatients who were able to walk, which emphasizes the importance of interventions tailored for walking ability. Progressive resistance exercise training improved FAC and independence in ADL but not SPPB and IADL independence in the feasibility intervention study. Further research with a larger sample size is needed.
Funding
This work was supported by an unrestricted grant of the University of Melbourne received by Prof. Andrea B. Maier and the Medical Research Future Fund (MRFF) provided by the Melbourne Academic Centre for Health (MACH). This work is also part of a collaboration project co‐funded by the PPP Allowance made available by Health~Holland (grant number TKI‐LSHM19069‐H049), Top Sector Life Sciences & Health, to stimulate public‐private partnerships, and Top Sector Agri & Food (grant number LWV19287). The collaboration project also includes an in‐cash and in‐kind contribution from Danone Nutricia Research.
Conflict of interest
Andrea B. Maier reports grants from Danone Nutricia Research outside the conduct of the study. The other authors declare that they have no conflicts of interest.
Acknowledgements
The authors thank the multidisciplinary team of the Royal Melbourne Hospital involved in the RESORT cohort for their clinical work and data collection, especially Anthony Kamleh and the physiotherapy team at Royal Park Campus for their work on LIFT‐UP. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.
Verstraeten LMG, Reijnierse EM, Spoelstra T, Meskers CGM, Maier AB. The impact of mobility limitations on geriatric rehabilitation outcomes: Positive effects of resistance exercise training (RESORT). Journal of Cachexia, Sarcopenia and Muscle 2024; 10.1002/jcsm.13557.
Esmee M. Reijnierse and Thom Spoelstra contributed equally to this manuscript.
References
- 1. Gazineo D, Godino L, Decaro R, Calogero P, Pinto D, Chiari P, et al. Assisted walking program on walking ability in in‐hospital geriatric patients: a randomized trial. J Am Geriatr Soc 2021;69:637–643. [DOI] [PubMed] [Google Scholar]
- 2. Viosca E, Martínez JL, Almagro PL, Gracia A, González C. Proposal and validation of a new functional ambulation classification scale for clinical use. Arch Phys Med Rehabil 2005;86:1234–1238. [DOI] [PubMed] [Google Scholar]
- 3. Brown CJ, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc 2004;52:1263–1270. [DOI] [PubMed] [Google Scholar]
- 4. Smit EB, Bouwstra H, Hertogh CM, Wattel EM, van der Wouden JC. Goal‐setting in geriatric rehabilitation: a systematic review and meta‐analysis. Clin Rehabil 2019;33:395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chu CL, Lee TH, Chen YP, Ro LS, Hsu JL, Chu YC, et al. Recovery of walking ability in stroke patients through postacute care rehabilitation. Biom J 2023;46:100550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Groenendijk I, Kramer CS, den Boeft LM, Hobbelen HSM, van der Putten GJ, de Groot LCPGM. Hip fracture patients in geriatric rehabilitation show poor nutritional status, dietary intake and muscle health. Nutrients 2020;12:2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cadore EL, Rodríguez‐Mañas L, Sinclair A, Izquierdo M. Effects of different exercise interventions on risk of falls, gait ability, and balance in physically frail older adults: a systematic review. Rejuvenation Res 2013;16:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mak TCT, Wong TWL, Ng SSM. Visual‐related training to improve balance and walking ability in older adults: a systematic review. Exp Gerontol 2021;156:111612. [DOI] [PubMed] [Google Scholar]
- 9. Lee SY, Yoon BH, Beom J, Ha YC, Lim JY. Effect of lower‐limb progressive resistance exercise after hip fracture surgery: a systematic review and meta‐analysis of randomized controlled studies. J Am Med Dir Assoc 2017;18:1096.e19–1096.e26. [DOI] [PubMed] [Google Scholar]
- 10. Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev 2009; 2009;CD002759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Valenzuela T. Efficacy of progressive resistance training interventions in older adults in nursing homes: a systematic review. J Am Med Dir Assoc 2012;13:418–428. [DOI] [PubMed] [Google Scholar]
- 12. Ellis G, Gardner M, Tsiachristas A, Langhorne P, Burke O, Harwood RH, et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev 2017;9:CD006211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Medical Association . Declaration of Helsinki. Ethical principles for medical research involving human subject. In 64th WMA General Assembly in Fortaleza, Vol. 310. Brazil: World Medical Association; 2013. p 2191–2194. [DOI] [PubMed] [Google Scholar]
- 14. Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. J Psychiatr Res 1992;41:237–248. [DOI] [PubMed] [Google Scholar]
- 15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 16. Folstein MF, Folstein SE, McHugh PR. ‘Mini‐mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 17. Storey JE, Rowland JTJ, Conforti DA, Dickson HG. The Rowland Universal Dementia Assessment Scale (RUDAS): a multicultural cognitive assessment scale. Int Psychogeriatr 2004;16:13–31. [DOI] [PubMed] [Google Scholar]
- 18. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 19. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CAMJ 2005;173:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chumlea WC, Roche AF, Steinbaugh ML. Estimating stature from knee height for persons 60 to 90 years of age. J Am Geriatr Soc 1985;33:116–120. [DOI] [PubMed] [Google Scholar]
- 21. Ferguson M, Capra S, Bauer J, Banks M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition 1999;15:458–464. [DOI] [PubMed] [Google Scholar]
- 22. Reijnierse EM, de Jong N, Trappenburg MC, Blauw GJ, Butler‐Browne G, Gapeyeva H, et al. Assessment of maximal handgrip strength: how many attempts are needed? J Cachexia Sarcopenia Muscle 2017;8:466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self‐reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 24. Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist 1970;10:20–30. [DOI] [PubMed] [Google Scholar]
- 25. Lawton MP, Brody EM. Assessment of older people: self‐maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–186. [PubMed] [Google Scholar]
- 26. Verstraeten LMG, Sacchi F, van Wijngaarden JP, Meskers CGM, Maier AB. Sarcopenia, malnutrition and cognition affect physiotherapy frequency during geriatric rehabilitation: RESORT cohort. Ann Phys Rehabil Med 2023;66:101735. [DOI] [PubMed] [Google Scholar]
- 27. Slade SC, Dionne CE, Underwood M, Buchbinder R. Consensus on Exercise Reporting Template (CERT): explanation and elaboration statement. Br J Sports Med 2016;50:1428–1437. [DOI] [PubMed] [Google Scholar]
- 28. Foster C, Florhaug JA, Franklin J, Gottschall L, Hrovatin LA, Parker S, et al. A new approach to monitoring exercise training. J Strength Cond Res 2001;15:109–115. [PubMed] [Google Scholar]
- 29. Mahoney JE, Sager MA, Jalaluddin M. New walking dependence associated with hospitalization for acute medical illness: incidence and significance. J Gerontol A Biol Sci Med Sci 1998;53:M307–M312. [DOI] [PubMed] [Google Scholar]
- 30. Sager MA, Franke T, Inouye SK, Landefeld CS, Morgan TM, Rudberg MA, et al. Functional outcomes of acute medical illness and hospitalization in older persons. Arch Intern Med 1996;156:645–652. [PubMed] [Google Scholar]
- 31. Sager MA, Dunham NC, Schwantes A, Mecum L, Halverson K, Harlowe D. Measurement of activities of daily living in hospitalized elderly: a comparison of self‐report and performance‐based methods. J Am Geriatr Soc 1992;40:457–462. [DOI] [PubMed] [Google Scholar]
- 32. Loyd C, Markland AD, Zhang Y, Fowler M, Harper S, Wright NC, et al. Prevalence of hospital‐associated disability in older adults: a meta‐analysis. J Am Med Dir Assoc 2020;21:455–461.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Campbell SE, Seymour DG, Primrose WR. A systematic literature review of factors affecting outcome in older medical patients admitted to hospital. Age Ageing 2004;33:110–115. [DOI] [PubMed] [Google Scholar]
- 34. Richards T, Glendenning A, Benson D, Alexander S, Thati S. The independent patient factors that affect length of stay following hip fractures. Ann R Coll Surg Engl 2018;100:556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Geyskens L, Jeuris A, Deschodt M, van Grootven B, Gielen E, Flamaing J. Patient‐related risk factors for in‐hospital functional decline in older adults: a systematic review and meta‐analysis. Age Ageing 2022;51:afac007. [DOI] [PubMed] [Google Scholar]
- 36. Hoogerduijn JG, Schuurmans MJ, Duijnstee MS, De Rooij SE, Grypdonck MF. A systematic review of predictors and screening instruments to identify older hospitalized patients at risk for functional decline. J Clin Nurs 2007;16:46–57. [DOI] [PubMed] [Google Scholar]
- 37. Goisser S, Schrader E, Singler K, Bertsch T, Gefeller O, Biber R, et al. Malnutrition according to mini nutritional assessment is associated with severe functional impairment in geriatric patients before and up to 6 months after hip fracture. J Am Med Dir Assoc 2015;16:661–667. [DOI] [PubMed] [Google Scholar]
- 38. Ariza‐Vega P, Lozano‐Lozano M, Olmedo‐Requena R, Martín‐Martín L, Jiménez‐Moleón JJ. Influence of cognitive impairment on mobility recovery of patients with hip fracture. Am J Phys Med Rehabil 2017;96:109–115. [DOI] [PubMed] [Google Scholar]
- 39. Hartley P, Gibbins N, Saunders A, Alexander K, Conroy E, Dixon R, et al. The association between cognitive impairment and functional outcome in hospitalised older patients: a systematic review and meta‐analysis. Age Ageing 2017;46:559–567. [DOI] [PubMed] [Google Scholar]
- 40. Manini TM, Druger M, Ploutz‐Snyder L. Misconceptions about strength exercise among older adults. J Aging Phys Act 2005;13:422–433. [DOI] [PubMed] [Google Scholar]


