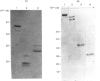
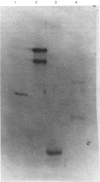
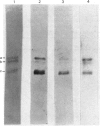
Abstract
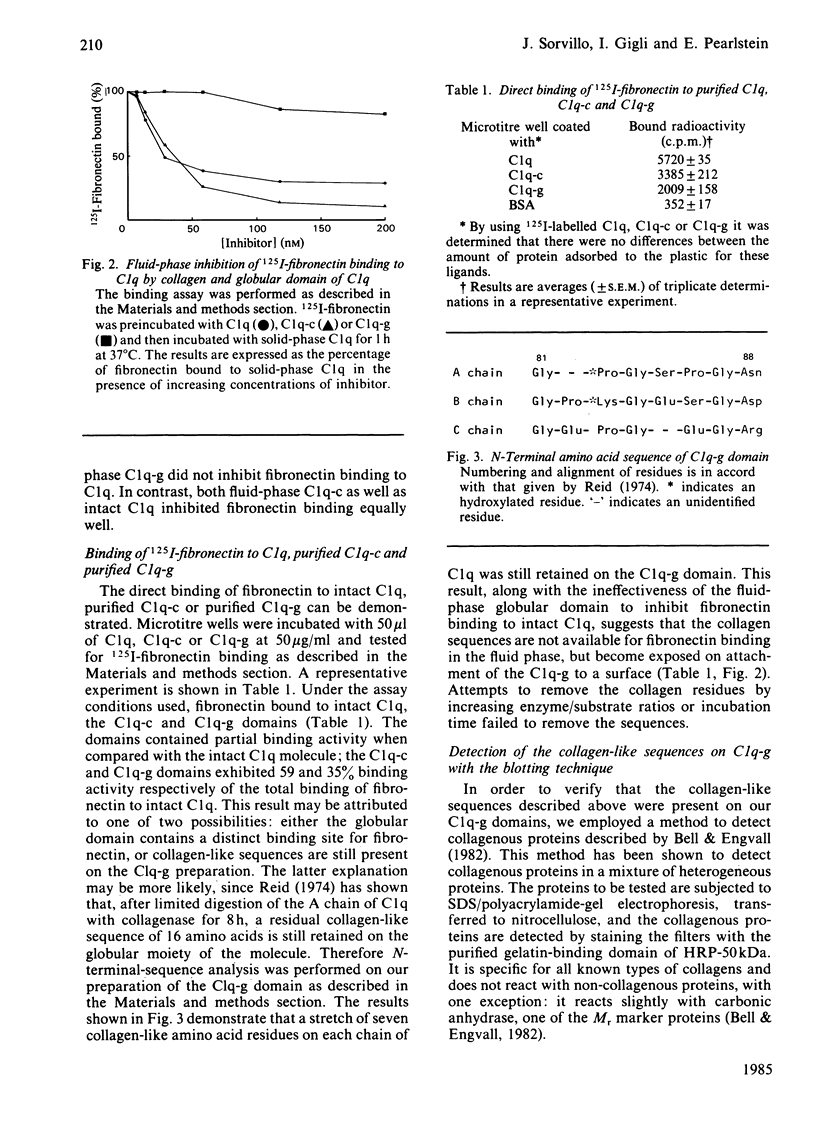
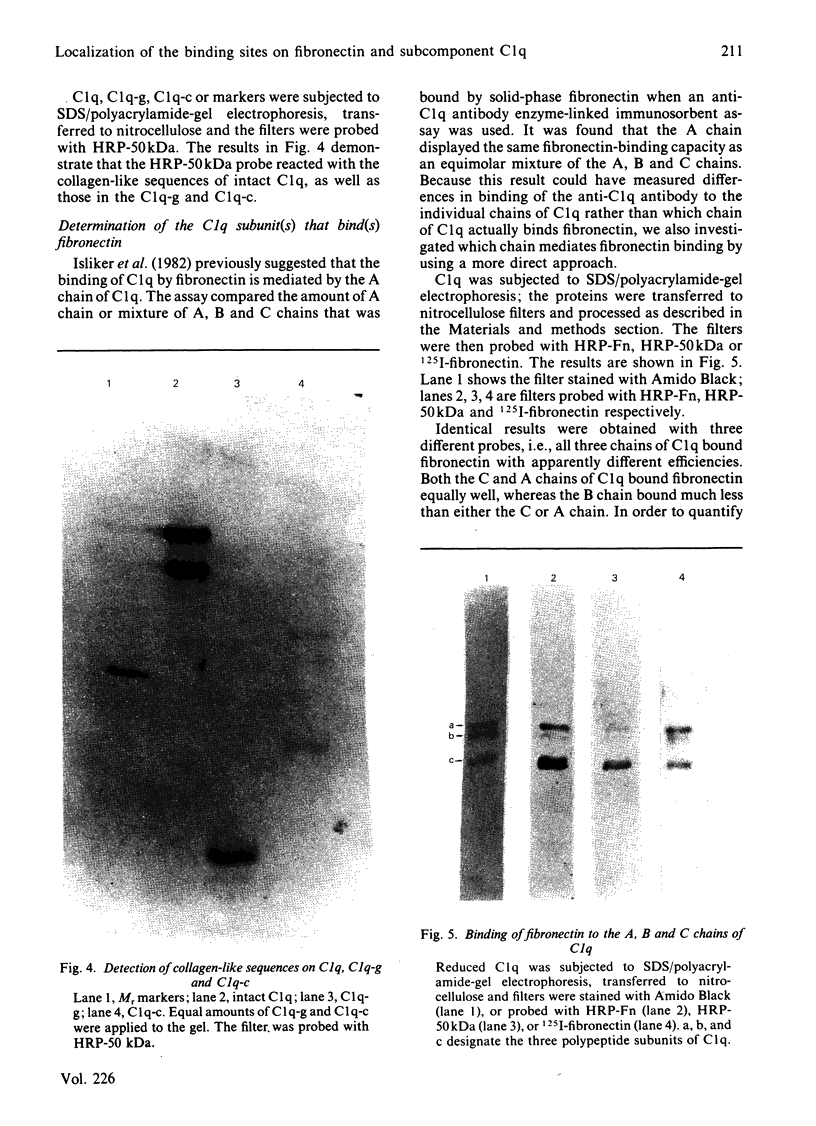
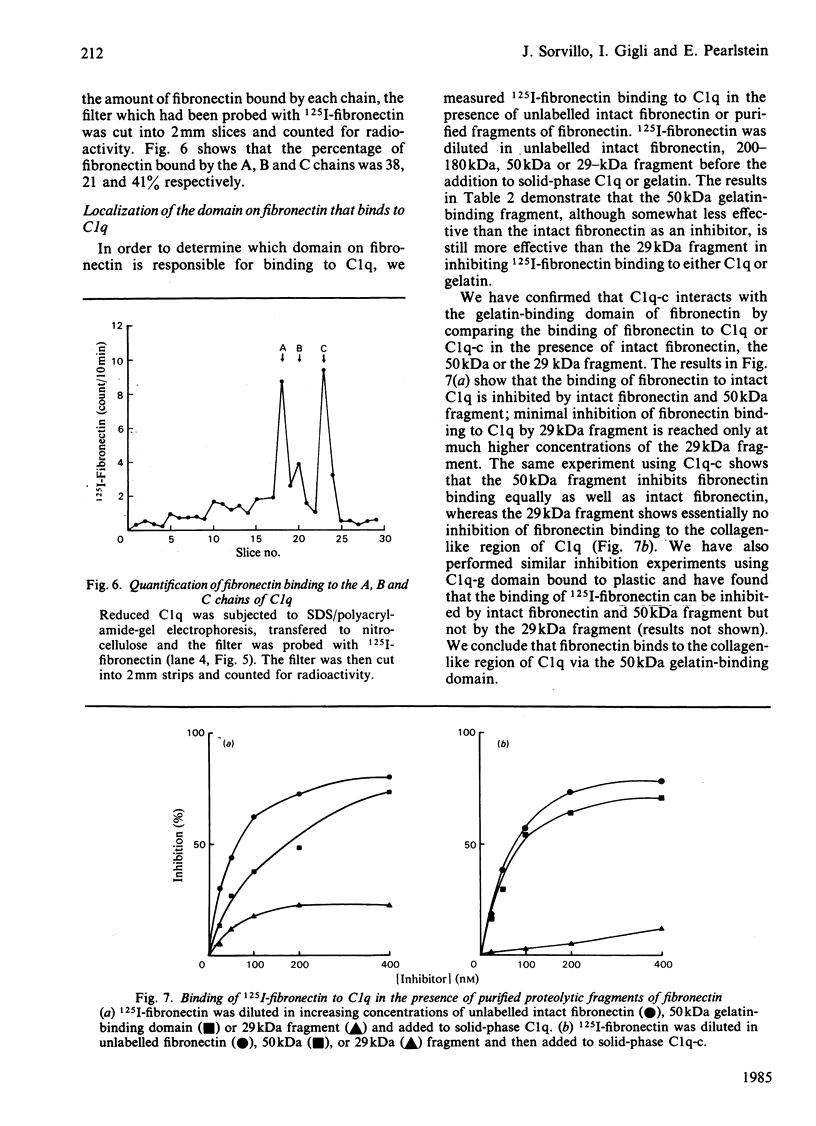
The interaction of purified human plasma fibronectin with the C1q subcomponent of complement was investigated by using a solid-phase radiobinding assay. 125I-fibronectin binding to native C1q, purified collagen domain (C1q-c) or globular domain (C1q-g) was compared. When the purified domains were insolubilized by binding to plastic, the C1q-c exhibited 59% of the binding demonstrated with intact C1q, whereas the C1q-g exhibited 35% of the binding. N-Terminal sequencing of the globular domain showed that a sequence of seven collagen-like amino acids was retained on each chain of the C1q-g fragment. 125I-fibronectin binding to C1q could be inhibited equally well by fluid-phase C1q and C1q-c, but not by fluid-phase C1q-g, implying that the collagen-like region retained on the C1q-g is masked in the fluid phase. In addition, studies were performed to determine which subunit(s) of C1q bind(s) fibronectin. The percentages of fibronectin bound by the A, B, and C chain of C1q were found to be 38, 21 and 41% respectively. Inhibition studies with purified 200-180 kDa, 50 kDa or 29 kDa fragments of fibronectin show that the binding site on fibronectin for C1q is the 50 kDa gelatin-binding domain.
Full text
PDF

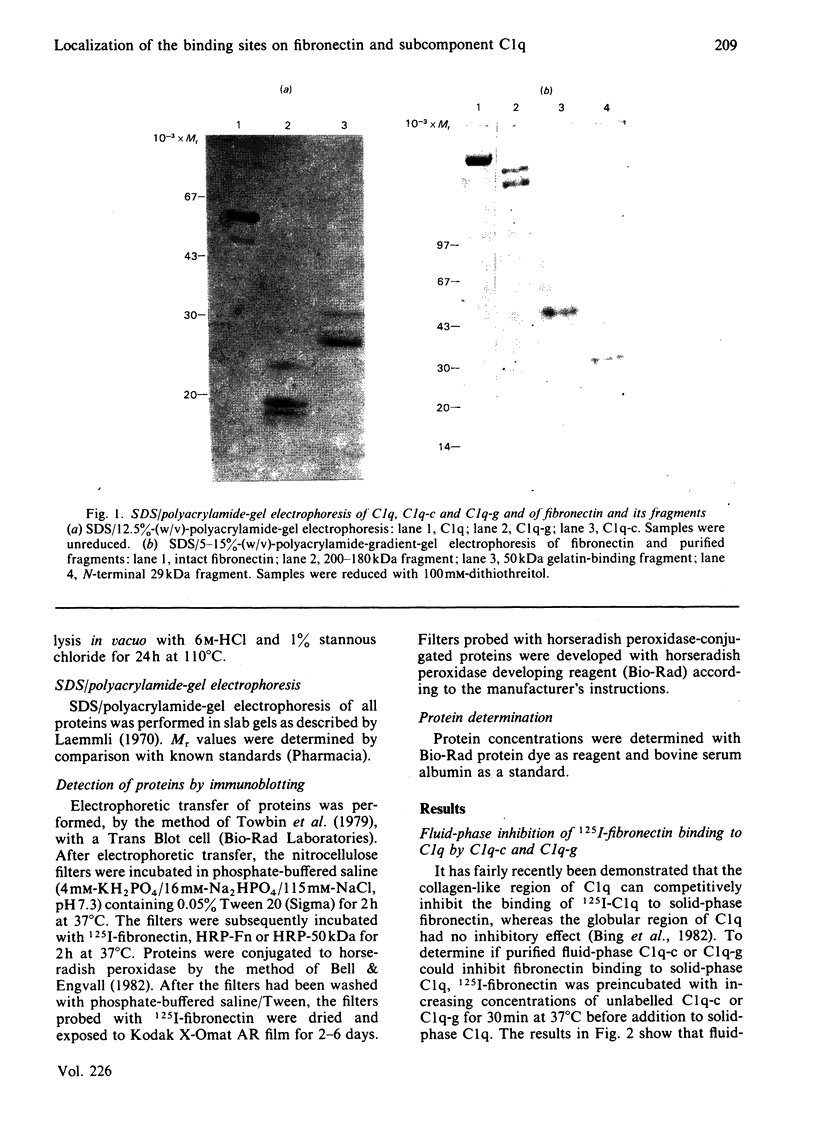



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz S. J., Isliker H. Antibody-independent interactions between Escherichia coli J5 and human complement components. J Immunol. 1981 Nov;127(5):1748–1754. [PubMed] [Google Scholar]
- Bing D. H., Almeda S., Isliker H., Lahav J., Hynes R. O. Fibronectin binds to the C1q component of complement. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4198–4201. doi: 10.1073/pnas.79.13.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerling M. R., Johnson C. D., Mosher D. F., Lipton B. H., Lilien J. E. Cross-linking and binding of fibronectin with asymmetric acetylcholinesterase. Biochemistry. 1981 May 26;20(11):3242–3247. doi: 10.1021/bi00514a040. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Garcia-Pardo A., Pearlstein E., Frangione B. Primary structure of human plasma fibronectin. The 29,000-dalton NH2-terminal domain. J Biol Chem. 1983 Oct 25;258(20):12670–12674. [PubMed] [Google Scholar]
- Giclas P. C., Pinckard R. N., Olson M. S. In vitro activation of complement by isolated human heart subcellular membranes. J Immunol. 1979 Jan;122(1):146–151. [PubMed] [Google Scholar]
- Golan M. D., Burger R., Loos M. Conformational changes in C1q after binding to immune complexes: detection of neoantigens with monoclonal antibodies. J Immunol. 1982 Aug;129(2):445–447. [PubMed] [Google Scholar]
- Gold L. I., Garcia-Pardo A., Frangione B., Franklin E. C., Pearlstein E. Subtilisin and cyanogen bromide cleavage products of fibronectin that retain gelatin-binding activity. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4803–4807. doi: 10.1073/pnas.76.10.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C., Roth S., Schmid F. R., Anderson B. Binding of fibronectin to Clq; inhibition of binding by aggregated IgG. Immunol Commun. 1981;10(7):601–609. doi: 10.3109/08820138109050713. [DOI] [PubMed] [Google Scholar]
- Hughes-Jones N. C., Gardner B. Reaction between the isolated globular sub-units of the complement component C1q and IgG-complexes. Mol Immunol. 1979 Sep;16(9):697–701. doi: 10.1016/0161-5890(79)90010-5. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Yamada K. M. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982 Nov;95(2 Pt 1):369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham K. C., Brew S. A., Miekka S. I. Interaction of plasma fibronectin with gelatin and complement C1q. Mol Immunol. 1983 Mar;20(3):287–295. doi: 10.1016/0161-5890(83)90068-8. [DOI] [PubMed] [Google Scholar]
- Isliker H., Bing D. H., Lahan J., Hynes R. O. Fibronectin interacts with Clq, a subcomponent of the first component of complement. Immunol Lett. 1982 Jan;4(1):39–43. doi: 10.1016/0165-2478(82)90075-x. [DOI] [PubMed] [Google Scholar]
- Kono I., Sakurai T., Kabashima T., Yamane K., Kashiwagi H. Fibronectin binds to C1q: possible mechanisms for their co-precipitation in cryoglobulins from patients with systemic lupus erythematosus. Clin Exp Immunol. 1983 May;52(2):305–310. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loos M., Wellek B., Thesen R., Opferkuch W. Antibody-independent interaction of the first component of complement with Gram-negative bacteria. Infect Immun. 1978 Oct;22(1):5–9. doi: 10.1128/iai.22.1.5-9.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel E. J., Smolen J. S., Liotta L., Reid K. B. Interaction of fibronectin with C1q and its collagen-like fragment (CLF). FEBS Lett. 1981 Jun 29;129(1):188–192. doi: 10.1016/0014-5793(81)80787-9. [DOI] [PubMed] [Google Scholar]
- Mosher D. F. Fibronectin. Prog Hemost Thromb. 1980;5:111–151. [PubMed] [Google Scholar]
- Nielsen H., Blom J., Larsen S. O. Human blood monocyte function in relation to age. Acta Pathol Microbiol Immunol Scand C. 1984 Feb;92(1):5–10. doi: 10.1111/j.1699-0463.1984.tb00045.x. [DOI] [PubMed] [Google Scholar]
- Pearlstein E., Baez L. A solid-phase radioimmunoassay for the determination of fibronectin levels in plasma. Anal Biochem. 1981 Sep 15;116(2):292–297. doi: 10.1016/0003-2697(81)90359-6. [DOI] [PubMed] [Google Scholar]
- Pearlstein E., Gold L. I., Garcia-Pardo A. Fibronectin: a review of its structure and biological activity. Mol Cell Biochem. 1980 Feb 8;29(2):103–128. doi: 10.1007/BF00220304. [DOI] [PubMed] [Google Scholar]
- Pearlstein E., Sorvillo J., Gigli I. The interaction of human plasma fibronectin with a subunit of the first component of complement, C1q. J Immunol. 1982 May;128(5):2036–2039. [PubMed] [Google Scholar]
- Porter R. R., Reid K. B. Activation of the complement system by antibody-antigen complexes: the classical pathway. Adv Protein Chem. 1979;33:1–71. doi: 10.1016/s0065-3233(08)60458-1. [DOI] [PubMed] [Google Scholar]
- Porter R. R., Reid K. B. The biochemistry of complement. Nature. 1978 Oct 26;275(5682):699–704. doi: 10.1038/275699a0. [DOI] [PubMed] [Google Scholar]
- Reid K. B. A collagen-like amino acid sequence in a polypeptide chain of human C1q (a subcomponent of the first component of complement). Biochem J. 1974 Jul;141(1):189–203. doi: 10.1042/bj1410189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B. Isolation, by partial pepsin digestion, of the three collagen-like regions present in subcomponent Clq of the first component of human complement. Biochem J. 1976 Apr 1;155(1):5–17. doi: 10.1042/bj1550005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba T. M., Jaffe E. Plasma fibronectin (opsonic glycoprotein): its synthesis by vascular endothelial cells and role in cardiopulmonary integrity after trauma as related to reticuloendothelial function. Am J Med. 1980 Apr;68(4):577–594. doi: 10.1016/0002-9343(80)90310-1. [DOI] [PubMed] [Google Scholar]
- Sorvillo J., Gigli I., Pearlstein E. Requirements for the binding of human plasma fibronectin to the C1q subunit of the first component of complement. J Immunol. 1983 Sep;131(3):1400–1404. [PubMed] [Google Scholar]
- Tenner A. J., Lesavre P. H., Cooper N. R. Purification and radiolabeling of human C1q. J Immunol. 1981 Aug;127(2):648–653. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. The influence of intracellular levels of cyclic nucleotides on cell proliferation and the induction of antibody synthesis. J Exp Med. 1975 Jan 1;141(1):97–111. doi: 10.1084/jem.141.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]