Abstract
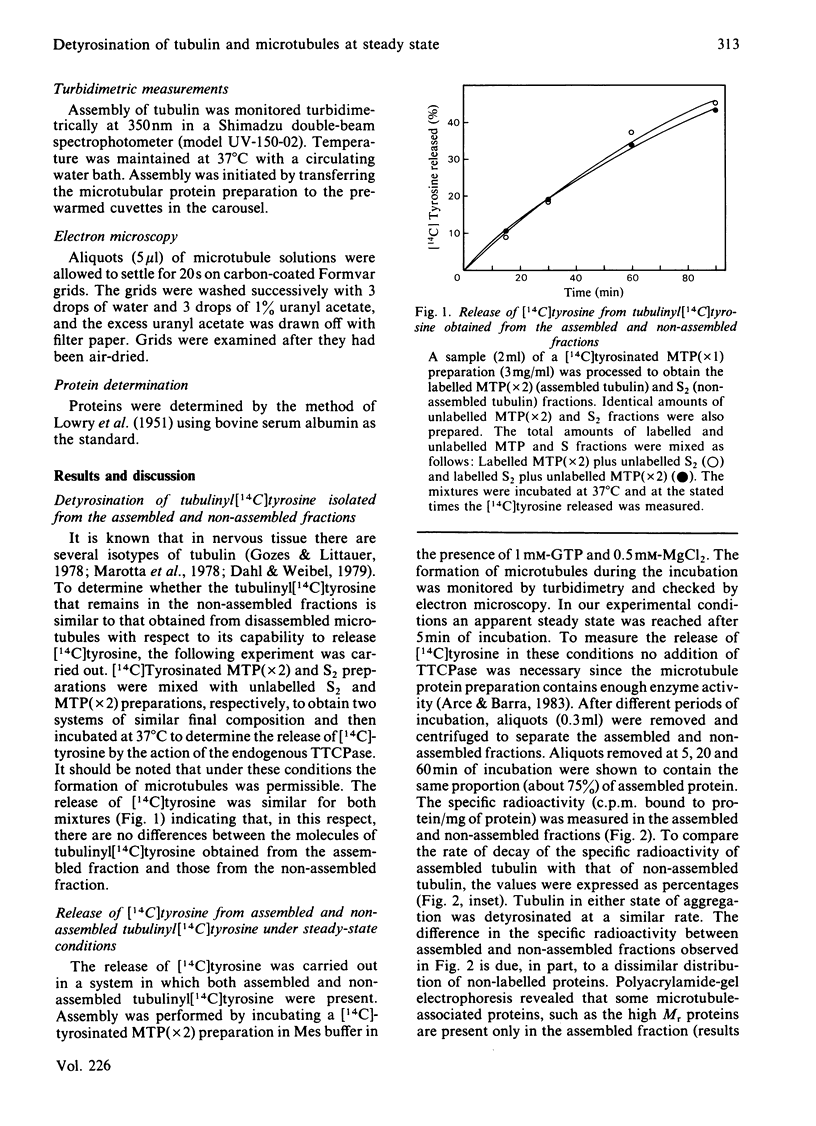
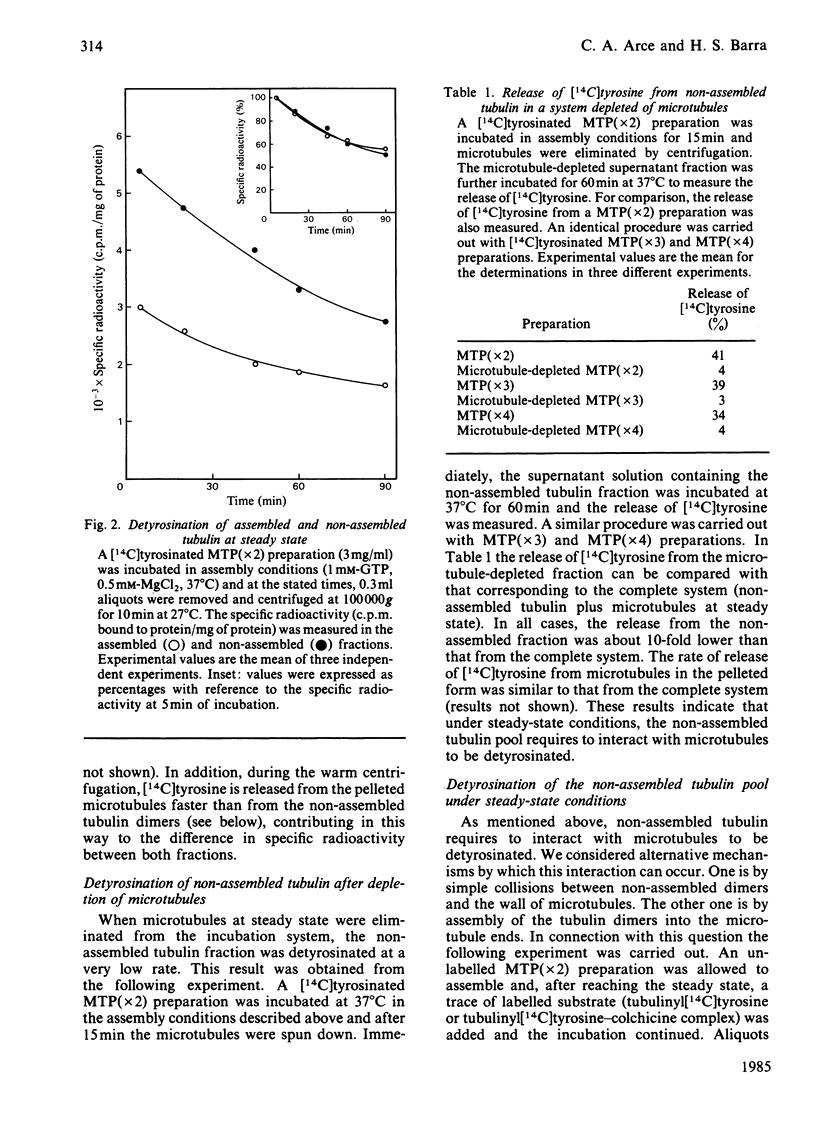
Microtubule protein preparations purified by cycles of assembly-disassembly contain the enzyme tubulinyltyrosine carboxypeptidase (TTCPase). Using these preparations, containing tubulinyl[14C]tyrosine, we studied the release of [14C]tyrosine from assembled and non-assembled tubulin under steady-state conditions. It was found that both states of aggregation were detyrosinated at similar rates by the action of the endogenous TTCPase. However, practically no release of [14C]tyrosine from the non-assembled tubulin pool was found when microtubules were previously eliminated from the incubation mixture. These results indicated that non-assembled tubulin requires to interact with microtubules to be detyrosinated. This interaction seems to occur through the incorporation of dimers into microtubules, since when the capability of tubulin to incorporate into microtubules was diminished by binding of colchicine a concomitant decrease in the rate of release of tyrosine was observed. When detyrosination was accelerated by increasing the concentration of TTCPase relative to the microtubule protein concentration, microtubules were found to be detyrosinated faster than was non-assembled tubulin. Using exogenous TTCPase in an incubation system in which the formation of microtubules was not allowed, tubulinyl[14C]tyrosine and tubulinyl[14C]tyrosine-colchicine complex were shown to have similar capabilities to act as substrates for this enzyme. Free colchicine was shown not to affect the activity of TTCPase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arce C. A., Barra H. S. Association of tubulinyl-tyrosine carboxypeptidase with microtubules. FEBS Lett. 1983 Jun 27;157(1):75–78. doi: 10.1016/0014-5793(83)81119-3. [DOI] [PubMed] [Google Scholar]
- Arce C. A., Hallak M. E., Rodriguez J. A., Barra H. S., Caputto R. Capability of tubulin and microtubules to incorporate and to release tyrosine and phenylalanine and the effect of the incorporation of these amino acids on tubulin assembly. J Neurochem. 1978 Jul;31(1):205–210. doi: 10.1111/j.1471-4159.1978.tb12449.x. [DOI] [PubMed] [Google Scholar]
- Arce C. A., Rodriguez J. A., Barra H. S., Caputo R. Incorporation of L-tyrosine, L-phenylalanine and L-3,4-dihydroxyphenylalanine as single units into rat brain tubulin. Eur J Biochem. 1975 Nov 1;59(1):145–149. doi: 10.1111/j.1432-1033.1975.tb02435.x. [DOI] [PubMed] [Google Scholar]
- Argarana C. E., Barra H. S., Caputto R. Tubulinyl-tyrosine carboxypeptidase from chicken brain: properties and partial purification. J Neurochem. 1980 Jan;34(1):114–118. doi: 10.1111/j.1471-4159.1980.tb04628.x. [DOI] [PubMed] [Google Scholar]
- Barra H. S., Arce C. A., Rodríguez J. A., Caputto R. Some common properties of the protein that incorporates tyrosine as a single unit and the microtubule proteins. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1384–1390. doi: 10.1016/0006-291x(74)90351-9. [DOI] [PubMed] [Google Scholar]
- Barra H. S., Rodriguez J. A., Arce C. A., Caputto R. A soluble preparation from rat brain that incorporates into its own proteins ( 14 C)arginine by a ribonuclease-sensitive system and ( 14 C)tyrosine by a ribonuclease-insensitive system. J Neurochem. 1973 Jan;20(1):97–108. doi: 10.1111/j.1471-4159.1973.tb12108.x. [DOI] [PubMed] [Google Scholar]
- Dahl J. L., Weibel V. J. Changes in tubulin heterogeneity during postnatal development of rat brain. Biochem Biophys Res Commun. 1979 Feb 14;86(3):822–828. doi: 10.1016/0006-291x(79)91786-8. [DOI] [PubMed] [Google Scholar]
- Deanin G. G., Preston S. F., Hanson R. K., Gordon M. W. On the mechanism of turnover of the carboxy-terminal tyrosine of the alpha chain of tubulin. Eur J Biochem. 1980 Aug;109(1):207–216. doi: 10.1111/j.1432-1033.1980.tb04786.x. [DOI] [PubMed] [Google Scholar]
- Gozes I., Littauer U. Z. Tubulin microheterogeneity increases with rat brain maturation. Nature. 1978 Nov 23;276(5686):411–413. doi: 10.1038/276411a0. [DOI] [PubMed] [Google Scholar]
- Hallak M. E., Rodriguez J. A., Barra H. S., Caputto R. Release of tyrosine from tyrosinated tubulin. Some common factors that affect this process and the assembly of tubulin. FEBS Lett. 1977 Feb 1;73(2):147–150. doi: 10.1016/0014-5793(77)80968-x. [DOI] [PubMed] [Google Scholar]
- Kumar N., Flavin M. Modulation of some parameters of assembly of microtubules in vitro by tyrosinolation of tubulin. Eur J Biochem. 1982 Nov;128(1):215–222. doi: 10.1111/j.1432-1033.1982.tb06954.x. [DOI] [PubMed] [Google Scholar]
- Kumar N., Flavin M. Preferential action of a brain detyrosinolating carboxypeptidase on polymerized tubulin. J Biol Chem. 1981 Jul 25;256(14):7678–7686. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marotta C. A., Harris J. L., Gilbert J. M. Characterization of multiple forms of brain tubulin subunits. J Neurochem. 1978 Jun;30(6):1431–1440. doi: 10.1111/j.1471-4159.1978.tb10475.x. [DOI] [PubMed] [Google Scholar]
- Raybin D., Flavin M. Enzyme which specifically adds tyrosine to the alpha chain of tubulin. Biochemistry. 1977 May 17;16(10):2189–2194. doi: 10.1021/bi00629a023. [DOI] [PubMed] [Google Scholar]
- Rodriguez J. A., Borisy G. G. Tyrosination state of free tubulin subunits and tubulin disassembled from microtubules of rat brain tissue. Biochem Biophys Res Commun. 1979 Aug 13;89(3):893–899. doi: 10.1016/0006-291x(79)91862-x. [DOI] [PubMed] [Google Scholar]
- Sloboda R. D., Rosenbaum J. L. Purification and assay of microtubule-associated proteins (MAPs). Methods Enzymol. 1982;85(Pt B):409–416. doi: 10.1016/0076-6879(82)85041-6. [DOI] [PubMed] [Google Scholar]
- Thompson W. C., Deanin G. G., Gordon M. W. Intact microtubules are required for rapid turnover of carboxyl-terminal tyrosine of alpha-tubulin in cell cultures. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1318–1322. doi: 10.1073/pnas.76.3.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. C. Post-translational addition of tyrosine to alpha tubulin in vivo in intact brain and in myogenic cells in culture. FEBS Lett. 1977 Aug 1;80(1):9–13. doi: 10.1016/0014-5793(77)80395-5. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


