Abstract
Background
Treatment options for advanced intrahepatic cholangiocarcinoma (ICC) are currently limited. Chemo-containing regimens are the mainstay treatments but associated with notable toxicity, poor tolerance, and reduced compliance, necessitating exploration of alternative therapies. Lenvatinib plus PD-1 inhibitors has shown substantial clinical activity in preliminary studies. This study aimed to assess the effectiveness and safety of lenvatinib plus toripalimab (a novel PD-1 antibody) as chemo-free therapy in advanced ICC.
Methods
This retrospective study included consecutive advanced ICC patients receiving lenvatinib plus toripalimab between February 2019 and December 2023. The main outcomes were overall survival (OS), progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), and safety. Prognostic factors and exploratory analyses for genetic alternations were also conducted.
Results
A total of 78 patients were included, with a median follow-up of 25.9 months. Median OS and PFS were 11.3 (95% CI: 9.5–13.1) and 5.4 (95% CI: 3.8–7.0) months, respectively. ORR was 19.2% and DCR was 75.6%. The incidence of grade 3 or 4 adverse events (AEs) was 50.0%, with no grade 5 AEs reported. Patients with normal baseline CA19-9 levels exhibited a higher ORR (p = 0.011), longer PFS (11.5 versus 4.6 months; HR 0.47; p=0.005), and OS (21.0 versus 9.7 months; HR 0.43; p=0.003). The presence of IDH1 mutations correlated with increased ORR (60.0% versus 8.9%, p=0.016).
Conclusion
Lenvatinib plus toripalimab represents an effective and well-tolerated chemo-free therapeutic option for advanced ICC. Baseline CA19-9 levels and IDH1 mutations may serve as predictive treatment-related biomarkers.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-024-03841-z.
Keywords: Cholangiocarcinoma, Lenvatinib, PD-1 inhibitor, PD-L1, IDH1, CA19-9
Introduction
Intrahepatic cholangiocarcinoma (ICC), the second most common primary liver cancer globally, is an aggressive and lethal malignancy [1]. While curative surgery is effective, approximately 80% patients present with advanced-stage disease, leading to a discouraging 5-year overall survival rate of less than 10% [1].
Current therapeutic approaches primarily rely on chemo-containing regimens for both first and subsequent lines of treatment in advanced ICC [2–5]. However, chemo-containing regimens are marred by substantial toxicity, poor tolerability, and decreased patient compliance [2, 5]. In real-world scenarios, a significant proportion of patients exhibit poor performance status (PS), refuse chemotherapy, or develop intolerance, with only 15–25% eligible for second-line chemotherapy following disease progression due to deteriorating PS [6].
Given these challenges, there is an urgent need to explore novel therapeutic strategies, particularly those that offer chemotherapy-free alternatives. The combination of anti-angiogenic therapy with immune checkpoint inhibitors (ICIs) has demonstrated synergistic effects and promising antitumor activity across various malignancies [7]. Lenvatinib, a small-molecule tyrosine kinase inhibitor of vascular endothelial growth factor receptors (VEGFR) 1-3, fibroblast growth factor receptors (FGFR) 1-4, and platelet-derived growth factor receptor alpha (PDGFR-α), has shown favorable antitumor efficacy and safety profiles in combination with ICIs in advanced biliary tract cancer (BTC) [7–9]. Initial evidence from phase 2 trials suggests that this combination may serve as a viable chemo-free treatment option for advanced ICC [10–12]. However, due to the rarity of ICC, previous studies always pooled different subtypes of BTC or utilized various PD-1 inhibitors, resulting in significant heterogeneity. Consequently, there remains a critical gap in knowledge regarding the efficacy of lenvatinib combined with a uniform ICI regimen specifically for advanced ICC.
Toripalimab, a humanized anti-PD-1 IgG4 monoclonal antibody, has received approvals from both the US Food and Drug Administration (FDA) and the China National Medical Products Administration (NMPA) for multiple malignancies, and it is endorsed in the treatment guidelines (Biliary Tract Carcinoma, version 2023) for advanced BTC by the Chinese Society of Clinical Oncology (CSCO) [9, 13]. However, to date, there are no published data regarding lenvatinib plus toripalimab in advanced ICC. Herein, we conducted a retrospective cohort study to evaluate the effectiveness and safety of the lenvatinib plus toripalimab in advanced ICC, alongside with exploring treatment-related predictive biomarkers.
Methods
Study design and population
This was a retrospective cohort study of patients with histologically confirmed advanced ICC who received lenvatinib plus toripalimab between March 2019 and December 2023, at Peking Union Medical College Hospital (PUMCH). This study was conducted according to the Declaration of Helsinki and approved by the PUMCH Institutional Review Board and Ethics Committee (No. JS-1391). Informed consent was waived because this retrospective review of medical records was considered minimal risk. The study was registered at ClinicalTrials.gov (NCT03892577). We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies (Supplementary STROBE checklist).
Inclusion criteria included: (1) aged 18 years or older; (2) patients receiving at least 2 cycles of the combination; (3) with at least one measurable lesion per response evaluation criteria in solid tumors (RECIST) version 1.1; and (4) eastern cooperative oncology group performance status (ECOG-PS) score 0-2. Patients were ineligible if they received other systemic therapy concurrently or adjuvant therapy or with insufficient clinical information.
Treatment
Toripalimab was administered intravenously at a dose of 240 mg every three weeks (one cycle) [10, 13, 14]. Lenvatinib was given orally 12 mg/day (body weight ≥ 60 kg) or 8 mg/day (body weight < 60 kg) [8]. Dose interruption and reduction of lenvatinib were permitted for drug-related adverse events. The decision to receive lenvatinib plus toripalimab is guided by patient autonomy. This choice was made in alignment with patient preferences, following a comprehensive discussion of the latest efficacy and safety data, treatment cycles, and associated costs.
Data collection and outcome assessment
Demographic and clinicopathological data, treatment therapy, adverse events (AEs), prior treatments and radiological evaluation data were collected from medical records and databases. Radiological assessments were conducted every 6–9 weeks using computed tomography (CT) or magnetic resonance imaging (MRI) scans according to RECIST v1.1, as follows: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Patient follow-up data were collected by outpatient service, telephone calls or WeChat interviews. Patients lost to follow-up were censored at the last visit.
Outcomes and definitions
Effectiveness outcomes included progression-free survival (PFS; the time interval from toripalimab initiation to progression, last follow-up, or death), overall survival (OS; the time interval from toripalimab initiation to death or last follow-up), objective response rate (ORR; the proportion of patients with complete response [CR] or partial response [PR]), disease control rate (DCR; the proportion of CR, PR, or stable disease [SD]). Additional outcomes included safety.
Biomarker analysis
Biomarker analyses were performed to identify predictors associated with tumor response and prognosis. We investigated the potential roles of carbohydrate antigen 19-9 (CA19-9) level [15], programmed death ligand 1 (PD-L1) expression [16], and tumor genetic alterations [17] as treatment-related biomarkers. Baseline serum CA19-9 levels were categorized as ≤ 37 U/ml (normal level) and > 37 U/ml. PD-L1 expression was assessed via immunohistochemistry using formalin-fixed, paraffin-embedded tumor specimens and the PD-L1 IHC 22C3 pharmDx kit (Agilent Technologies, USA) [18]. PD-L1 positivity was defined as a tumor proportion score (TPS) ≥ 1% [19, 20]. Tumor genetic alterations were identified through targeted panel sequencing of tumor DNA utilizing next-generation sequencing (NGS) platforms provided by Genecast (China) [21], 3D Medicines (China) [22], and OrigiMed (China) [23]. Genes demonstrating mutation frequencies exceeding 10% within our cohort were selected for exploratory biomarker analysis. Additionally, the TCGA pathway analysis method was employed to further investigate the relationship between gene-associated pathway alterations and treatment efficacy [24]. Fifteen cancer pathways were analyzed, including phosphoinositide 3-kinase (PI3K), homologous recombination repair deficiency (HRD), wingless/integrated (WNT), fibroblast growth factor (FGF), cell cycle (CellCycle), switch/sucrose nonfermentable (SWI/SNF), base excision repair (BER), homologous recombination repair (HRR), mismatch repair (MMR), nucleotide excision repair (NER), nonhomologous end joining (NHEJ), Fanconi anemia (FA), checkpoint factor (CPF), translesion synthesis (TLS), and DNA damage response (DDR). For details, see Supplementary Table S1 [24, 25].
Comparison with prior studies
We conducted a comprehensive review of the literature, focusing on four large phase 3 randomized controlled trials (ABC-02, ABC-06, TOPAZ-1, and KEYNOTE-966), to contextualize our findings with existing evidence on lenvatinib plus ICIs and standard therapy for advanced BTC. A simplified comparison with our real-world cohorts was performed to provide insights into the similarities and differences in baseline characteristics and clinical outcomes, which may also highlight the potential benefits of utilizing lenvatinib plus toripalimab in treating ICC.
Statistical analysis
Continuous variables were shown as median and range or interquartile range (IQR) and compared using the unpaired t test or Mann–Whitney U test, as appropriate. Categorical variables were presented as numbers (%) and compared using χ2 test or Fisher’s exact test, as appropriate. Kaplan–Meier method was applied and using log-rank test to assess survival outcomes. Treatment differences were calculated with a stratified Cox proportional hazards model. To capture variables that may exhibit weaker associations yet remain clinically significant, factors with a P value < 0.10 in univariate analysis were incorporated into the multivariate Cox regression model. A two-sided P value < 0.05 was deemed significant. Statistical analyses were performed using SPSS version 27.
Results
Patients characteristics
A total of 78 patients (median [IQR] age, 59.5 [51.8–64.3] years; 51 [65.4%] men) were included (Supplementary Fig. S1), of whom 42 (53.8%) had an ECOG-PS of 1 or 2 and 71 (91.0%) were categorized as Child–Pugh class A. Fifty-five (70.5%) presented metastatic disease, with the liver (79.5%) and lymph nodes (76.9%) being the most common metastatic sites. Fifty patients (64.1%) received lenvatinib plus toripalimab as first-line therapy. Twenty-eight patients (35.9%) had experienced prior systemic treatments, 19 (67.9%) of them received chemotherapy and 9 (11.5%) were treated with PD-1/L1 inhibitors. Specifically, among the 19 patients who received prior chemotherapy, 3 were treated with the gemcitabine + albumin-bound paclitaxel regimen, 6 with the gemcitabine + cisplatin regimen, and 10 with the gemcitabine + oxaliplatin regimen. Patient demographics and baseline characteristics are presented in Table 1.
Table 1.
Baseline Characteristics
| Characteristic | No. (%) | ||
|---|---|---|---|
| Total (N = 78) | 1st line (n = 50) | ≥ 2nd line (n = 28) | |
| Age, years, median (IQR) | 59.5 (51.8–64.3) | 60.5 (53.3–64.3) | 57.5 (50.5–64.8) |
| Female sex | 27 (34.6) | 18 (36.0) | 9 (32.1) |
| ECOG-PS | |||
| 0 | 36 (46.2) | 27 (54.0) | 9 (32.1) |
| 1 | 36 (46.2) | 19 (38.0) | 17 (60.7) |
| 22 | 6 (7.6) | 4 (8.0) | 2 (7.1) |
| Child–Pugh grade | |||
| A | 71 (91.0) | 47 (94.0) | 24 (85.7) |
| B | 7 (9.0) | 3 (6.0) | 4 (14.3) |
| CA19-9 level, U/ml | |||
| ≤ 37 | 31 (39.8) | 24 (48.0) | 7 (25.0) |
| >37 | 47 (60.2) | 26 (52.0) | 21 (75.0) |
| Extent of disease | |||
| Locally advanced | 23 (29.5) | 17 (34.0) | 6 (21.4) |
| Metastatic | 55 (70.5) | 33 (66.0) | 22 (78.6) |
| Treatment line | |||
| 1 | 50 (64.1) | 50 (100) | – |
| 2 | 26 (33.3) | – | 26 (92.9) |
| ≥ 3 | 2 (2.6) | – | 2 (7.1) |
| Metastatic site | |||
| Lymph nodes | 60 (76.9) | 39 (78.0) | 21 (75.0) |
| Liver | 62 (79.5) | 41 (82.0) | 21 (75.0) |
| Lung | 12 (15.2) | 3 (6.0) | 9 (32.1) |
| Bone | 11 (14.1) | 6 (1.2) | 5 (17.9) |
| Previous treatment | |||
| Surgery | 23 (29.5) | 11 (22.0) | 12 (42.9) |
| Local–regional therapy | 23 (29.5) | 9 (18.0) | 14 (50.0) |
| Systemic chemotherapy† | 19 (24.4) | – | 19 (67.9) |
| Immunotherapy‡ | 9 (11.5) | – | 9 (32.1) |
Percentages may not total 100 because of rounding.
IQR, Interquartile range; ECOG-PS, eastern cooperative oncology group performance status; CA19-9, carbohydrate antigen 19-9.
†Systemic chemotherapy included gemcitabine + cisplatin, gemcitabine + oxaliplatin, gemcitabine + albumin-bound paclitaxel.
‡Immunotherapy refers to PD-1 and PD-L1 inhibitors.
Effectiveness
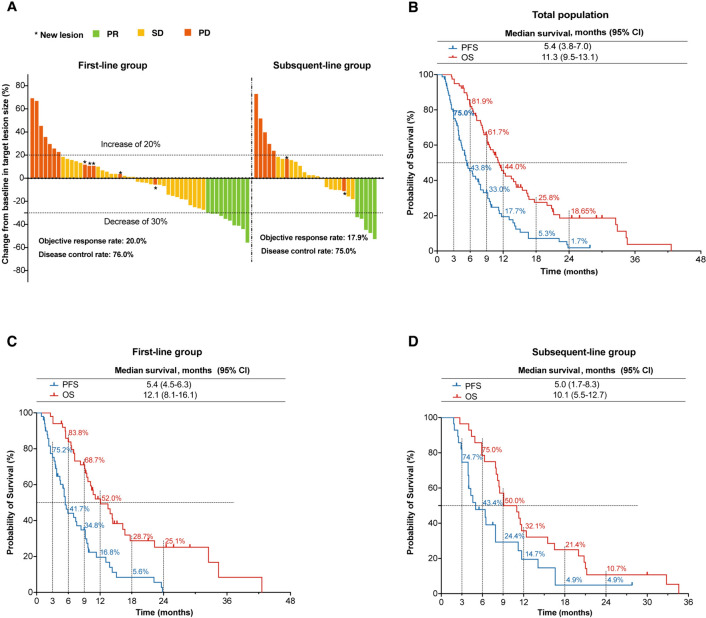
As of June 13, 2024, the median follow-up time was 25.9 (range, 2.6–42.6) months. Among the entire cohort, 39 (50.0%) exhibited a decrease in tumor size from baseline (Fig. 1A), no patients achieved CR, 15 (19.2%) had PR, 44 (56.0%) exhibited SD, 19 (24.4%) experienced PD, and the corresponding ORR was 19.2% (Table 2). The median PFS (mPFS) and median OS (mOS) were 5.4 (95% CI, 3.8–7.0) months and 11.3 (95% CI, 9.5–13.1) months, respectively (Fig. 1B).
Fig. 1.
Efficacy outcomes. A Best response for target lesions by patient. Kaplan–Meier estimates of overall survival and progression-free survival in the total population (B), first-line group (C) and subsequent-line group (D). ORR, objective response rate; PFS, progression-free survival; OS, overall survival
Table 2.
Summary of response and survival outcomes
| No. (%) | |||
|---|---|---|---|
| Parameter | Total (N = 78) |
1st line (n = 50) |
≥ 2nd line (n = 28) |
| Complete response (CR) | 0 (0) | 0 (0) | 0 (0) |
| Partial response (PR) | 15 (19.2) | 10 (20.0) | 5 (17.9) |
| Stable disease (SD) | 44 (56.0) | 28 (56.0) | 16 (57.1) |
| Objective response rate (ORR) | 15 (19.2) | 10 (20.0) | 5 (17.9) |
| Progressive disease (PD) | 19 (24.4) | 12 (24.0) | 7 (25.0) |
| Disease control rate (DCR) | 59 (75.6) | 38 (76.0) | 21 (75.0) |
| Progression-free survival (PFS), months, median, (95% CI) | 5.4 (3.8–7.0) | 5.4 (4.5–6.3) | 5.0 (1.7–8.3) |
|
Overall survival (OS) months, median, (95% CI) |
11.3 (9.5–13.1) | 12.1 (8.1–16.1) | 10.1 (5.5–12.7) |
ORR, objective response rate; PFS, progression-free survival; DCR, disease control rate; CBR, clinical benefit rate; SD, stable disease; PD, progressive disease; CR, complete response; PR, partial response, CI, confidence interval.
In the first-line group, ORR and DCR were 20.0% (10/50) and 76.0% (38/50), respectively (Table 2). The mPFS was 5.4 months (95% CI, 4.5–6.3), and the mOS was 12.1 months (95% CI, 8.1–16.1). The 6- and 12-month PFS rates were 41.7% and 16.8%, respectively. The 6-, 12-, and 24-month OS rates were 83.8%, 52.0%, and 25.1%, respectively (Fig. 1C).
In subsequent-line group, ORR and DCR were 17.9% (5/28) and 75.0% (21/28), respectively (Table 2). The mPFS was 5.0 (95% CI, 1.7–8.3) months and mOS 10.1 (95% CI, 5.5–12.7) months, respectively. The 6- and 12-month PFS rates were 43.4% and 14.7%, respectively. The 6-, 12-, and 24-month OS rates were 75.0%, 32.1%, and 10.7%, respectively (Fig. 1D).
Safety outcomes analyses
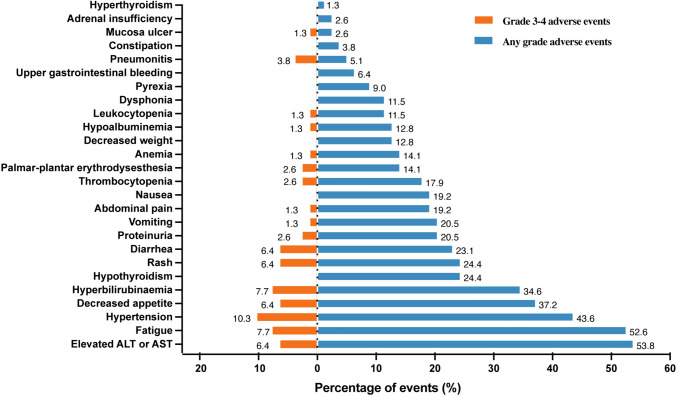
All patients had AEs of various grades (summarized in Fig. 2). The most frequent treatment-related AEs were transaminase elevation (53.8%), fatigue (52.6%), and hypertension (43.6%). Decreased appetite (37.2%) and bilirubin level elevation (34.6%) were also commonly observed. Grade 3 or 4 AEs occurred in 50.0% (39/78) patients. The most frequent grade 3 or 4 events were hypertension (10.3%), fatigue (7.7%), and elevated bilirubin (7.7%). Nine (11.5%) discontinued treatment due to AEs. Most AEs were safe, well-tolerated, and no treatment-related deaths occurred.
Fig. 2.
Adverse events. Abbreviations: ALT, alanine aminotransferase; AST, aspartate alanine aminotransferase
Biomarkers analysis
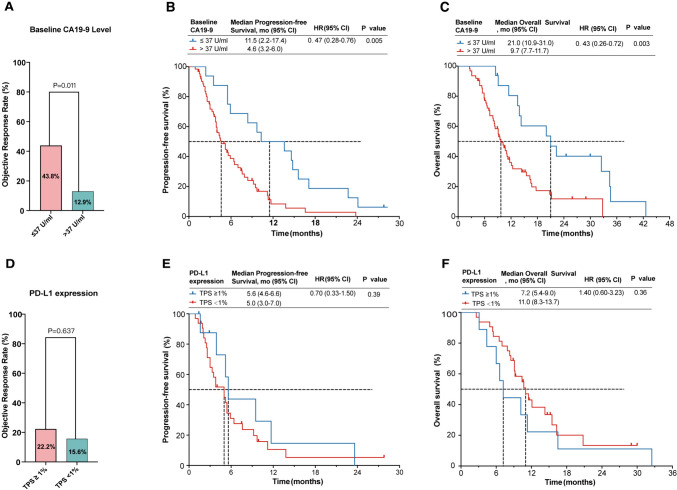
All 78 patients had recorded baseline CA19-9 levels. Patients with normal baseline CA19-9 levels exhibited a higher ORR (43.8% versus 12.9%, p = 0.011; Fig. 3A), longer PFS (11.5 versus 4.6 months; HR=0.47; p= 0.005; Fig. 3B) and OS (21.0 versus 9.7 months; HR=0.43; p = 0.003; Fig. 3C) than those with CA19-9 > 37 U/ml. Evaluation of PD-L1 expression was available for 41 (52.5%) patients, with 9 (22.0%) showing positive expression. Patients with positive PD-L1 expression tended to exhibit higher ORR than those with negative expression (22.2% versus 15.6%), but the difference did not reach statistical significance (p = 0.637). Similarly, there were no significant differences observed in PFS (p = 0.39) or OS (p = 0.36) between these two subgroups (Fig. 3E, F).
Fig. 3.
Biomarkers analysis for response and prognosis. ORR (A), PFS (B), and OS (C) stratified by CA19-9 level (> 37 U/ml and ≤ 37 U/ml). ORR (D), PFS (E), and OS (F) stratified by PD-L1 expression (TPS ≥ 1% and < 1%)
Multivariable Cox regression analysis was performed on the entire cohort to identify prognostic factors. Univariate analyses indicated that CA19-9 level (> 37 vs. ≤ 37 U/ml) significantly correlated with both PFS and OS (P < 0.05). Multivariate analyses confirmed that CA19-9 > 37 U/ml independently predicted shorter PFS (HR=2.20, 95% CI 1.20-4.10; p = 0.015) and OS (HR=2.97, 95% CI 1.47-6.03; p = 0.003) (Supplemental Table S2).
Exploratory analysis on genetic alterations
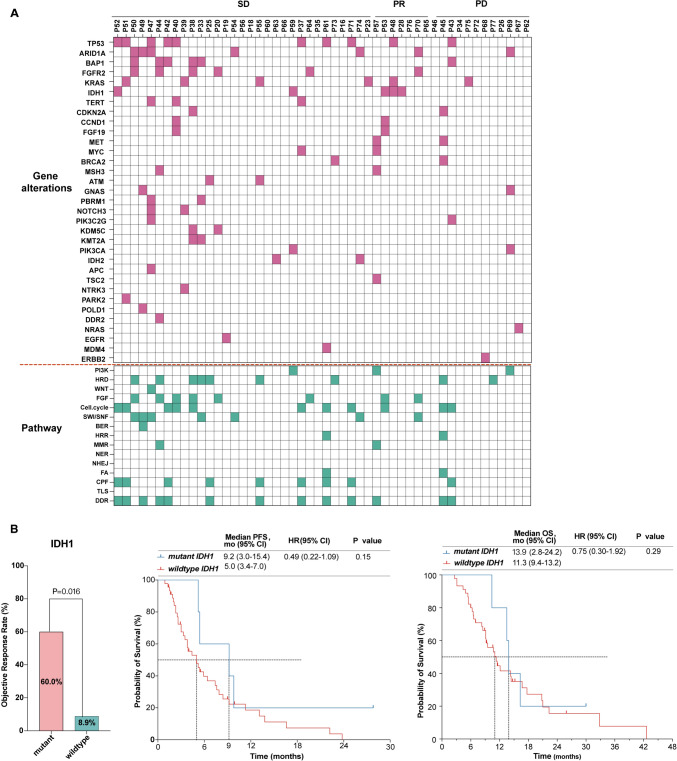
Genetic alterations profiles were available for 50 (64.1%) patients (Fig. 4A). Demographics and disease characteristics between patients with and without genetic profiles (Supplementary Table S2) were generally balanced between the two groups. The most frequently altered genes included TP53 (20.0%), ARID1A (14.0%), BAP1 (12.0%), FGFR2 (12.0%), KRAS (12.0%), and IDH1 (10.0%). Additionally, out of 15 cancer pathways examined, only two—nucleotide excision repair (NER) and nonhomologous end joining (NHEJ)—did not show alterations (Fig. 4A). The DNA damage response (DDR) pathway was notably affected, with an alteration rate of 26% (13/50). These mutations affect critical DDR pathways, including mismatch repair (MMR), base excision repair (BER), homologous recombination repair (HRR), translesion synthesis (TLS), checkpoint factors (CPF), and Fanconi anemia (FA). Notably, nearly half of the patients with PR had IDH1 mutations, while patients with SD commonly exhibited TP53, ARID1A, BAP1, and FGFR2 alterations. Herein, biomarker analysis was conducted for the top six altered genes and DDR-related pathway alternations.
Fig. 4.
Exploratory analysis for genetic alternations. A Profiles of genetic alternations and pathways. ORR, PFS, and OS stratified by mutation status for IDH1 (B). Abbreviations: PD progressive disease, PR partial response, SD stable disease, CA19-9 carbohydrate antigen 19-9, DDR DNA damage response. PI3K phosphoinositide 3-kinase, HRD homologous recombination repair deficiency, WNT wingless/integrated, FGF fibroblast growth factor, CellCycle cell cycle, SWI/SNF switch/sucrose nonfermentable, HRR homologous recombination repair, BER base excision repair, MMR mismatch repair, NER nucleotide excision repair, NHEJ, nonhomologous end joining, CPF checkpoint factor; FA Fanconi anemia, TLS, translesion synthesis
IDH1 mutations were associated with a significantly increased ORR (60.0% versus 8.9%, p = 0.016; Fig. 4B). Furthermore, patients with IDH1 mutations tended to demonstrate prolonged survival compared with the wildtype (PFS: 9.2 versus 5.0 months, p = 0.15; OS: 13.9 vs. 11.3 months, p = 0.29), although the difference did not reach statistical significance (Fig. 4B). Patients with TP53, FGFR2, or ARID1A alterations exhibited higher ORR without statistical significance. Survival outcomes did not significantly differ between patients with mutated versus wildtype genes among the six top genes (Supplementary Fig. S2 A–E). Similarly, no significant differences in ORR and survival were observed between patients with DDR pathway alterations and those without (Supplementary Fig. S2 F).
Comparison with prior studies and standard therapy for advanced BTC
Table 3 summarizes studies evaluating lenvatinib plus ICIs, including ABC-02, ABC-06, TOPAZ-1, and KEYNOTE-966, for advanced BTC.
Table 3.
Summary of studies evaluating lenvatinib plus PD-1 inhibitors compared with ABC-02, ABC-06, TOPAZ-1, and KEYNOTE-996 study in advanced BTC
| Study (year) | Country | Design | Patients | ICC (n, %) | Therapy | ECOG-PS2 (%) | Metastatic (%) | PD-L1 positive (%) |
Follow-up (mo) | PFS/OS (mo) |
ORR/DCR (%) |
≥ Grade 3 AEs (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lin et al. (2020) [8] | China | Single-arm retrospective |
≥2nd line BTC |
32 (50.0) |
Len + Pembrolizumab |
13 | NA | 34.4 | 9.5 |
PFS 4.9 OS 11.0 |
ORR 25.0 DCR 78.1 |
62.4 |
| LEAP-005 (2021) [7] | Global | Basket trial, multicenter phase 2 |
2nd line BTC |
31 (NA) |
Len + Pembrolizumab |
0 | NA | NA | NA |
PFS 6.1 OS 8.6 |
ORR 10.0 DCR 68.0 |
48.0 |
| Ding et al. (2022) [20] | China |
Single-arm, multicenter phase 2 |
2nd line ICC |
41 (100) |
Len + Sintilimab |
4.9 | 80.5 | 80.5 | 12.1 |
PFS 8.0 OS 16.6 |
ORR 46.3 DCR 70.3 |
37.8 |
| ABC-06 (2021) [2] | UK | Multicenter, phase 3 RCT |
2nd line BTC |
162 (44.4) |
ASC+FOLFOX vs. ASC |
0 |
83.0 vs. 81.0 |
NA | 21.7 |
PFS 4.0 vs. NA; OS 6.2 vs. 5.3 |
ORR 3.0 vs. NA DCR 24.0 vs. NA |
69.0 vs. 52.0 |
| Zhang et al. (2021) [12] | China | Single-arm, phase 2 |
1st line BTC |
38 (52.6) |
Len +PD-1 inhibitors* |
0 | 36.8 | 61.1 | 13.7 |
PFS 8.0 OS 17.7 |
ORR 42.1 DCR 76.3 |
34.2 |
| Shi et al. (2022) [11] | China | China |
≥1st line BTC |
74 (47.3) |
Len +PD-1 inhibitors** |
2.7 | 59.5 | 36.4 | 15 |
PFS 4.0 OS 9.5 |
ORR 20.27 DCR 71.62 |
52.7 |
| Zhou et al. (2021) [10] ‡ | China | Single-arm, phase 2 |
1st line ICC |
31 (100) |
Len + Toripalimab |
0 | NA | NA | 6.9 |
PFS NR OS NR 6-month OS% 87.1 |
ORR 32.3 DCR 74.2 |
32.3 |
| ABC-02 (2010) [5] | UK | Multicenter, phase 3 RCT |
1st line BTC |
410 (19.5) |
GC vs. Gemcitabine |
13.2 | 73.0 | NA | 8.2 |
PFS 8.0 vs. 5.0; OS 11.7 vs. 8.1 |
ORR 19.0 vs. 11.7 DCR 81.4 vs. 71.8 |
70.7 vs. 68.8 |
| TOPAZ-1 (2022) [3] | Global | Multicenter, phase 3 RCT |
1st line BTC |
685 (55.9) |
GC+ Durvalumab vs. GC |
0 |
88.9 vs. 83.1 |
57.8 vs. 59.6 |
23.4 vs. 22.4 |
PFS 7.2 vs. 5.7; OS 12.8 vs. 11.5 |
ORR 26.7 vs. 18.7 DCR 85.3 vs. 82.6 |
62.7 vs. 64.9 |
| KEYNOTE-966 (2023) [4] | Global | Multicenter, phase 3 RCT |
1st line BTC |
1069 (59.2) |
GC+ Pembrolizumab vs. GC |
0 |
89.0 vs. 88.0 |
68.0 vs. 68.0 |
25.6 |
PFS 6.5 vs. 5.6 OS 12.7 vs. 10.9 |
ORR 29.0 vs.29.0 DCR 75.0 vs. 76.0 |
70.0 vs. 69.0 |
| Present | China | Single-arm, retrospective |
1st line ICC ≥2nd line ICC |
78 (100) |
Len + Toripalimab |
1st 8.0 |
1st 66.0 |
20.0 | 25.9 |
1st PFS 5.4 1st OS 12.1 |
1st ORR 20.0 1st DCR 76.0 |
50.0% |
|
≥2nd 7.1 |
≥2nd 78.6 |
27.3 |
≥2nd PFS 5.0 ≥2nd OS 10.1 |
2nd ORR 17.9 2nd DCR 75.0 |
‡Unpublished data, initial results showed at 2021 ASCO annual meeting.
*Nivolumab, sintilimab, toripalimab, and tislelizumab.
**Pembrolizumab, tislelizumab, sintilimab, camrelizumab and toripalimab.
BTCs, biliary tract cancers; ICC, intrahepatic cholangiocarcinoma; GC, gemcitabine and cisplatin; FOLFOX, fluorouracil, folinic acid, and oxaliplatin; PD-L1, programmed cell death ligand 1; PD-1, programmed cell death protein 1; ORR, objective response rate; PFS, progression-free survival; OS, overall survival; DCR, disease control rate; AEs, adverse events; ECOG-PS, eastern cooperative oncology group performance status; Len, lenvatinib; NR, not reach; NA, not available, ASC, active symptom control.
Discussion
In this study, we comprehensively evaluated the effectiveness, safety profile, and predictive treatment-related biomarkers of lenvatinib plus toripalimab for advanced ICC. Our data indicated that (1) lenvatinib plus toripalimab is an effective and safe chemo-free option for advanced ICC patients in real-world scenarios, even patients with poor performance status may benefit from this regimen; (2) baseline CA19-9 level, and specific gene alternations may predict treatment response.
Consistent with previous studies, available evidence indicates that lenvatinib plus ICIs regimen shows promising antitumor activity in advanced ICC (Table 3). In 2020, Lin et al. reported lenvatinib plus pembrolizumab achieving an ORR of 25%, with a mPFS of 4.9 months and a mOS of 11.0 months in the non-first-line setting [8]. Conversely, the LEAP-005 trial reported a slightly reduced mOS of 8.6 months, potentially attributed to the higher dose of lenvatinib (20 mg/day) utilized employed therein, whereas our study utilized a weight-based dosing strategy, capped at 12 mg/day [7]. Furthermore, other investigations have yielded more encouraging outcomes. Preliminary results from a phase 2 clinical trial including 31 advanced ICC patients treated with lenvatinib plus toripalimab demonstrated an ORR of 32.3% and the median survival has not yet been reached during a median follow-up of 6.9 months [10]. Another smaller phase 2 study investigating lenvatinib plus PD-1 inhibitors as first-line therapy demonstrated a remarkable ORR of 42.1%, with median PFS and OS durations of 8.6 and 17.7 months, respectively, significantly surpassing prior studies [12]. Variations in baseline characteristics such as performance status, biliary tract cancer subtypes, PD-L1 expression, and radiotherapy utilization within the study cohorts may account for the divergent results. Overall, while the available evidence remains inconclusive, our study contributes valuable insights into the potential of lenvatinib plus toripalimab as a promising therapeutic strategy for advanced ICC. To validate our findings, further well-designed trials are imperative. An ongoing phase 2 trial (NCT04361331) is expected to yield more robust evidence.
Safety is a critical concern in oncology, particularly given the demanding nature of chemotherapy which may not be suitable for patients with poor performance status (PS). Previous well-known phase III trials (TOPAZ-1, KEYNOTE-966, and ABC-06) explicitly omitted these patients, while only 10% of participants in the ABC-02 trial had an ECOG-PS score of 2 [3, 5, 18, 26]. Consequently, the findings from these studies may not directly guide treatment decisions for ICC patients with poor PS. However, a substantial proportion of patients in real-world clinical practice present with poor PS, often attributed to disease progression, comorbidities, or the effects of prior front-line therapies. These patients urgently require tolerable and effective treatment options. In present study, the incidence of grade 3-4 AEs was 50%, with most AEs being manageable and well-tolerated. These findings align with prior reports on the use of lenvatinib plus ICIs in advanced BTC (Table 3). Patients with poor PS generally derive less benefit from combination chemotherapy regimens. Conversely, anti-PD-1 antibodies and TKIs are generally better tolerated than chemotherapy and some research demonstrated that salvage immunotherapy can induce potentially long‐lasting “Lazarus responses” [27, 28]. Similarly, we observed some patients with ECOG-PS of 2 benefited effectively and safely from lenvatinib plus toripalimab. Importantly, we prioritize combination regimens over chemotherapy when PS decline is cancer-related rather than due to irreversible comorbidities. Nonetheless, this inclusion of both good and poor PS patients mirrors the complexities of clinical practice in ICC, thereby enhancing the generalizability of our findings to a broader patient population.
Identifying biomarkers to predict which patients would benefit most from immunotherapy is crucial. PD-L1 expression is widely used to predict immunotherapy efficacy, typically correlating with improved response rates [18]. However, its predictive value in BTC remains elusive [16]. Lin et al. reported that positive PD-L1 expression indicated better survival [8]. In contrast, in this study, PD-L1 expression was not associated with treatment efficacy, consistent with a previous phase 2 trial reports [29]. Instead, we found baseline CA19-9 levels and specific gene alterations to be potentially valuable predictors. Patients with CA19-9 > 37U/ml exhibited significantly decreased ORR and reduced survival, possibly due to CA19-9 association with disease severity [30]. Additionally, evidence regarding the predictive utility of genetic alterations in BTCs remains limited [31]. IDH mutations in ICC are characteristic, and we found that patients with IDH1 mutations exhibited higher ORR. This favorable outcome may be attributed to mutant IDH represents an attractive therapeutic neoantigen [32], as ICC with IDH mutation often exhibits distinct features such as DNA hypermethylation and unique drug sensitivity profiles [33]. However, the predictive role of IDH1 mutations in different tumors was controversial [34–36], possibly due to varying mutational clusters demonstrating diverse response patterns [37]. Overall, the underlying mechanisms of this phenomenon remain poorly defined. Additionally, prior research suggested that certain gene mutations, such as BAP1 and CDKN2A mutations, may correlate with aggressive disease behavior and poorer response to standard therapies in BTC [17, 38]. However, due to our study’s design and limited sample size, we could not conclusively establish the predictive significance of these mutations. Nevertheless, our findings suggest that genetic alterations hold promise as potential predictive markers for therapeutic response in combined therapies for ICC. Further research is warranted to clarify this association.
This study has several limitations. First, due to its retrospective nature, lacking a standard therapy control and a relatively small sample; thus, it is hypothesis-generating rather than confirmatory. Nevertheless, given the urgent clinical need in advanced ICC, initial exploration of the feasibility, effectiveness, and safety of chemo-free regimen is crucial. Owing to the rarity and heterogeneity of ICC, multicenter or worldwide prospective studies are needed to confirm these findings. Second, the decision to administer lenvatinib plus toripalimab instead of chemotherapy plus durvalumab or pembrolizumab as a first-line treatment was guided by patient consent and adherence to ethical and compassionate use guidelines. Although this approach offers valuable insights, it introduces inherent biases related to treatment choices and patient preferences. Third, as an investigator-initiated study, our research is susceptible to selection and participant biases. Finally, the absence of PD-L1 expression data and incomplete whole genome sequencing in nearly half of the patients due to inadequate tissue samples limited the accuracy of our biomarker analysis.
Conclusions
Lenvatinib plus toripalimab demonstrates encouraging activity and manageable toxicity in advanced ICC patients, offering a promising chemo-free treatment option. Baseline CA19-9 levels might predict treatment response and prognosis. Patients harboring IDH1 mutations could potentially benefit more from this regimen. Future prospective registry studies are warranted to confirm these findings.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 1. Fig. S1Flowchart.Abbreviations: ICC, intrahepatic cholangiocarcinoma; PDL1, programmed cell deathligand 1; ORR, objective response rate; PFS, progression free survival; OS, overallsurvival; DCR, disease control rate; AEs, adverse events; ECOG-PS, easterncooperative oncology group performance status; CA19-9, carbohydrate antigen 19-9
Supplementary file 2. Fig. S2 Exploratory analysis for genetic alternations. ORR, PFS, andOS stratified by mutation status for ARID1A A B AP1 (B), FGFR2 (C), KRAS (D),TP53 (E), and DDR (F)Abbreviations: PD progressive disease, PR partial response, SD stable disease,DDRDNA dam age response
Acknowledgements
We thank the patients participating in this study and all staff at the hospital for their contributions to this study.
Abbreviations
- AEs
Adverse events
- ASC
Active symptom control
- BER
Base excision repair
- BTC
Biliary tract cancer
- CA19-9
Carbohydrate antigen 19-9
- CellCycle
Cell cycle
- CI
Confidence interval
- CPF
Checkpoint factor
- CR
Complete response
- CT
Computed tomography
- CTCAE
Common Terminology Criteria for Adverse Events
- DCR
Disease control rate
- DDR
DNA damage response
- ECOG-PS
Eastern cooperative oncology group performance status
- FA
Fanconi anemia
- FGF
Fibroblast growth factor
- FOLFOX
Fluorouracil, folinic acid, and oxaliplatin
- GC
Gemcitabine and cisplatin
- HR
Hazard rate
- HRD
Homologous recombination repair deficiency
- HRR
Homologous recombination repair
- ICC
Intrahepatic cholangiocarcinoma
- ICIs
Immune checkpoint inhibitors
- IQR
Interquartile range
- Len
Lenvatinib
- MMR
Mismatch repair
- MRI
Magnetic resonance imaging
- NA
Not available
- NER
Nucleotide excision repair
- NGS
Next-generation sequencing
- NHEJ
Nonhomologous end joining
- NR
not reach
- ORR
Objective response rate
- OS
Overall survival
- PI3K
Phosphoinositide 3-kinase
- PD
Progressive disease
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed cell death ligand 1
- PFS
Progression-free survival
- PR
Partial response
- RECIST
Response evaluation criteria in solid tumors
- SD
Stable disease
- SWI/SNF
Switch/sucrose nonfermentable
- TKI
Tyrosine kinase inhibitors
- TLS
Translesion synthesis
- TPS
Combined positive score
- WNT
Wingless/integrated
Author contributions
H.Z., W.D., and X.Y. were involved in conception/design. S.W., J.C., H.W. and S.L. helped in data analysis and interpretation, manuscript writing. All authors contributed to collection and/or assembly of data. All authors helped in final approval of manuscript.
Funding
This research was funded by National High Level Hospital Clinical Research Funding [2022-PUMCH-B-128], CAMS Innovation Fund for Medical Sciences (CIFMS) ([2022-I2M-C&T-A-003], [2021-I2M-1-061], [2021-I2M-1-003]), CSCO-hengrui Cancer Research Fund ([Y-HR2019-0239], [Y-HR2020MS-0415], [Y-HR2020QN-0414]), CSCO-MSD Cancer Re-search Fund [Y-MSDZD2021-0213], and National Ten-thousand Talent Program.
Data availability
All data supporting the results of the study can be found in the article and online supplementary material files. Researchers can contact the corresponding author of this article by email and indicate the required research materials and purpose. We will be glad to provide relevant materials for this study after approval and discussion.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (IRB) and Ethics Committee (EC) of PUMCH (No. JS-1391). Informed consent was provided by patients or waived by the ethical review committee.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shanshan Wang, Jiashuo Chao, Hao Wang, and Shuofeng Li have contributed equally to this work.
Contributor Information
Xiaobo Yang, Email: yangxiaobo67@pumch.cn.
Weidong Duan, Email: duanwd301@163.com.
Haitao Zhao, Email: zhaoht@pumch.cn.
References
- 1.Sposito C, Ratti F, Cucchetti A, Ardito F, Ruzzenente A, Di Sandro S, Maspero M, Ercolani G, Di Benedetto F, Guglielmi A, Giuliante F, Aldrighetti L, Mazzaferro V (2023) Survival benefit of adequate lymphadenectomy in patients undergoing liver resection for clinically node-negative intrahepatic cholangiocarcinoma. J Hepatol 78(2):356–363. 10.1016/j.jhep.2022.10.021 [DOI] [PubMed] [Google Scholar]
- 2.Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, Falk S, Gillmore R, Wadsley J, Patel K, Anthoney A, Maraveyas A, Iveson T, Waters JS, Hobbs C, Barber S, Ryder WD, Ramage J, Davies LM, Bridgewater JA, Valle JW, Advanced Biliary Cancer Working G (2021) Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol 22(5):690–701. 10.1016/S1470-2045(21)00027-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh D-Y, Ruth He A, Qin S, Chen L-T, Okusaka T, Vogel A, Kim JW, Suksombooncharoen T, Ah Lee M, Kitano M (2022) Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM evidence. 10.1056/EVIDoa2200015 [DOI] [PubMed] [Google Scholar]
- 4.Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, Yau T, Klumpen HJ, Chan SL, Ozaka M, Verslype C, Bouattour M, Park JO, Barajas O, Pelzer U, Valle JW, Yu L, Malhotra U, Siegel AB, Edeline J, Vogel A, Investigators K (2023) Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 401(10391):1853–1865. 10.1016/S0140-6736(23)00727-4 [DOI] [PubMed] [Google Scholar]
- 5.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J, Investigators ABCT (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362(14):1273–1281. 10.1056/NEJMoa0908721 [DOI] [PubMed] [Google Scholar]
- 6.Tella SH, Kommalapati A, Borad MJ, Mahipal A (2020) Second-line therapies in advanced biliary tract cancers. Lancet Oncol 21(1):e29–e41. 10.1016/S1470-2045(19)30733-8 [DOI] [PubMed] [Google Scholar]
- 7.Lwin Z, Gomez-Roca C, Saada-Bouzid E, Yanez E, Muñoz FL, Im SA, Castanon E, Senellart H, Graham D, Voss M, Doherty M, Lopez J, Ghori R, Kubiak P, Jin F, Norwood K, Chung HC (2020) LBA41 LEAP-005: Phase II study of lenvatinib (len) plus pembrolizumab (pembro) in patients (pts) with previously treated advanced solid tumours. Ann Oncol 31:S1170. 10.1016/j.annonc.2020.08.2271 [Google Scholar]
- 8.Lin J, Yang X, Long J, Zhao S, Mao J, Wang D, Bai Y, Bian J, Zhang L, Yang X, Wang A, Xie F, Shi W, Yang H, Pan J, Hu K, Guan M, Zhao L, Huo L, Mao Y, Sang X, Wang K, Zhao H (2020) Pembrolizumab combined with lenvatinib as non-first-line therapy in patients with refractory biliary tract carcinoma. Hepatobiliary Surg Nutr 9(4):414–424. 10.21037/hbsn-20-338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi GM, Huang XY, Wu D, Sun HC, Liang F, Ji Y, Chen Y, Yang GH, Lu JC, Meng XL, Wang XY, Sun L, Ge NL, Huang XW, Qiu SJ, Yang XR, Gao Q, He YF, Xu Y, Sun J, Ren ZG, Fan J, Zhou J (2023) Toripalimab combined with lenvatinib and GEMOX is a promising regimen as first-line treatment for advanced intrahepatic cholangiocarcinoma: a single-center, single-arm, phase 2 study. Signal Transduct Target Ther 8(1):106. 10.1038/s41392-023-01317-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jian Z, Fan J, Shi G-M, Huang X-Y, Wu D, Liang F, Yang G-H, Lu J-C, Chen Y, Ge N-L, Ji Y, Hou YY, Sun H-C, Qiu S-J, Ye Q-H, Huang X-W, Shi Y-H, Gao Q, Yang X-R, Wang X-Y (2021) Lenvatinib plus toripalimab as first-line treatment for advanced intrahepatic cholangiocarcinoma: A single-arm, phase 2 trial. J Clin Oncol 39(15(_suppl)):4099–4099. 10.1200/JCO.2021.39.15_suppl.4099 [Google Scholar]
- 11.Shi C, Li Y, Yang C, Qiao L, Tang L, Zheng Y, Chen X, Qian Y, Yang J, Wu D, Xie F (2022) Lenvatinib plus programmed cell death protein-1 inhibitor beyond first-line systemic therapy in refractory advanced biliary tract cancer: a real-world retrospective study in China. Front Immunol 13:946861. 10.3389/fimmu.2022.946861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Q, Liu X, Wei S, Zhang L, Tian Y, Gao Z, Jin M, Yan S (2021) Lenvatinib Plus PD-1 Inhibitors as First-Line Treatment in Patients With Unresectable Biliary Tract Cancer: A Single-Arm, Open-Label. Phase II Study. Front Oncol 11:751391. 10.3389/fonc.2021.751391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keam SJ (2019) Toripalimab: First Global Approval. Drugs 79(5):573–578. 10.1007/s40265-019-01076-2 [DOI] [PubMed] [Google Scholar]
- 14.Wei XL, Ren C, Wang FH, Zhang Y, Zhao HY, Zou BY, Wang ZQ, Qiu MZ, Zhang DS, Luo HY, Wang F, Yao S, Xu RH (2020) A phase I study of toripalimab, an anti-PD-1 antibody, in patients with refractory malignant solid tumors. Cancer Commun (Lond) 40(8):345–354. 10.1002/cac2.12068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogel A, Bridgewater J, Edeline J, Kelley RK, Klumpen HJ, Malka D, Primrose JN, Rimassa L, Stenzinger A, Valle JW, Ducreux M (2023) Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 34(2):127–140. 10.1016/j.annonc.2022.10.506 [DOI] [PubMed] [Google Scholar]
- 16.Davis AA, Patel VG (2019) The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer 7(1):278. 10.1186/s40425-019-0768-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon JG, Kim MH, Jang M, Kim H, Hwang HK, Kang CM, Lee WJ, Kang B, Lee CK, Lee MG, Chung HC, Choi HJ, Park YN (2021) Molecular Characterization of Biliary Tract Cancer Predicts Chemotherapy and Programmed Death 1/Programmed Death-Ligand 1 Blockade Responses. Hepatology 74(4):1914–1931. 10.1002/hep.31862 [DOI] [PubMed] [Google Scholar]
- 18.Rimm DL, Han G, Taube JM, Yi ES, Bridge JA, Flieder DB, Homer R, West WW, Wu H, Roden AC, Fujimoto J, Yu H, Anders R, Kowalewski A, Rivard C, Rehman J, Batenchuk C, Burns V, Hirsch FR, Wistuba II (2017) A Prospective, Multi-institutional, Pathologist-Based Assessment of 4 Immunohistochemistry Assays for PD-L1 Expression in Non-Small Cell Lung Cancer. JAMA Oncol 3(8):1051–1058. 10.1001/jamaoncol.2017.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dietel M, Savelov N, Salanova R, Micke P, Bigras G, Hida T, Antunez J, Guldhammer Skov B, Hutarew G, Sua LF, Akita H, Chan OSH, Piperdi B, Burke T, Khambata-Ford S, Deitz AC (2019) Real-world prevalence of programmed death ligand 1 expression in locally advanced or metastatic non-small-cell lung cancer: The global, multicenter EXPRESS study. Lung Cancer 134:174–179. 10.1016/j.lungcan.2019.06.012 [DOI] [PubMed] [Google Scholar]
- 20.Ding X, Li G, Sun W, Shen Y, Teng Y, Xu Y, Li W, Liu M, Chen J (2022) Sintilimab Combined with Lenvatinib for Advanced Intrahepatic Cholangiocarcinoma in Second-Line Setting-A Multi-Center Observational Study. Front Oncol 12:907055. 10.3389/fonc.2022.907055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Chen Y, Wang H, Xu Z, Wang Y, Li S, Liu J, Chen Y, Luo H, Wu L, Yang Y, Zhang H, Peng H (2021) Massive PD-L1 and CD8 double positive TILs characterize an immunosuppressive microenvironment with high mutational burden in lung cancer. J Immunother Cancer 9(6):e002356. 10.1136/jitc-2021-002356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao J, Li W, Huang Y, Huang M, Li S, Zhai X, Zhao J, Gao C, Xie W, Qin H, Cai S, Bai Y, Lan P, Zou Y (2021) A next-generation sequencing-based strategy combining microsatellite instability and tumor mutation burden for comprehensive molecular diagnosis of advanced colorectal cancer. BMC Cancer 21(1):282. 10.1186/s12885-021-07942-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao J, Chen L, Li H, Chen H, Yao J, Mu S, Liu W, Zhang P, Cheng Y, Liu B, Hu Z, Chen D, Kang H, Hu J, Wang A, Wang W, Yao M, Chrin G, Wang X, Zhao W, Li L, Xu L, Guo W, Jia J, Chen J, Wang K, Li G, Shi W (2019) An Accurate and Comprehensive Clinical Sequencing Assay for Cancer Targeted and Immunotherapies. Oncologist 24(12):e1294–e1302. 10.1634/theoncologist.2019-0236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez-Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC, Dimitriadoy S, Liu DL, Kantheti HS, Saghafinia S, Chakravarty D, Daian F, Gao Q, Bailey MH, Liang WW, Foltz SM, Shmulevich I, Ding L, Heins Z, Ochoa A, Gross B, Gao J, Zhang H, Kundra R, Kandoth C, Bahceci I, Dervishi L, Dogrusoz U, Zhou W, Shen H, Laird PW, Way GP, Greene CS, Liang H, Xiao Y, Wang C, Iavarone A, Berger AH, Bivona TG, Lazar AJ, Hammer GD, Giordano T, Kwong LN, McArthur G, Huang C, Tward AD, Frederick MJ, McCormick F, Meyerson M, Cancer Genome Atlas Research N, Van Allen EM, Cherniack AD, Ciriello G, Sander C and Schultz N (2018) Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell 173(2):321-337 e310. 10.1016/j.cell.2018.03.035 [DOI] [PMC free article] [PubMed]
- 25.Wang Z, Zhao J, Wang G, Zhang F, Zhang Z, Zhang F, Zhang Y, Dong H, Zhao X, Duan J, Bai H, Tian Y, Wan R, Han M, Cao Y, Xiong L, Liu L, Wang S, Cai S, Mok TSK, Wang J (2018) Comutations in DNA Damage Response Pathways Serve as Potential Biomarkers for Immune Checkpoint Blockade. Cancer Res. 78(22):6486–6496. 10.1158/0008-5472.CAN-18-1814 [DOI] [PubMed] [Google Scholar]
- 26.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Biliary Tract Cancers Version 2. 2023. Available online: https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/btc.pdf.
- 27.Su YY, Chiang NJ, Lin YJ, Shan YS, Chen LT (2019) AB060. P-31 Complete response to immunotherapy in cholangiocarcinoma with peritoneal metastases and high PD-L1 expression: a case report. Hepatobiliary Surg Nutr. 10.21037/hbsn.2019.AB060 [Google Scholar]
- 28.Walter T, Horgan AM, McNamara M, McKeever L, Min T, Hedley D, Serra S, Krzyzanowska MK, Chen E, Mackay H (2013) Feasibility and benefits of second-line chemotherapy in advanced biliary tract cancer: a large retrospective study. European J Cancer. 49(2):329–335 [DOI] [PubMed] [Google Scholar]
- 29.Wang D, Yang X, Long J, Lin J, Mao J, Xie F, Wang Y, Wang Y, Xun Z, Bai Y, Yang X, Guan M, Pan J, Seery S, Sang X, Zhao H (2021) The efficacy and safety of apatinib plus camrelizumab in patients with previously treated advanced biliary tract cancer: a prospective clinical study. Front Oncol 11:646979. 10.3389/fonc.2021.646979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rashidijahanabad Z, Ramadan S, O’Brien NA, Nakisa A, Lang S, Crawford H, Gildersleeve JC, Huang X (2023) Stereoselective Synthesis of Sialyl Lewis(a) Antigen and the Effective Anticancer Activity of Its Bacteriophage Qbeta Conjugate as an Anticancer Vaccine. Angew Chem Int Ed Engl 62(47):e202309744. 10.1002/anie.202309744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X, Wang D, Liu J, Qiu J, Zhou J, Ying J, Shi Y, Wang Z, Lou H, Cui J, Zhang J, Liu Y, Zhao F, Pan L, Zhao J, Zhu D, Chen S, Li X, Li X, Zhu L, Shao Y, Shu Y (2021) Genomic alterations in biliary tract cancer predict prognosis and immunotherapy outcomes. J Immunother Cancer 9(11):e003214. 10.1136/jitc-2021-003214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson LG, Miller JJ, Kitagawa Y, Wakimoto H, Choi BD, Curry WT (2022) Implications of IDH mutations on immunotherapeutic strategies for malignant glioma. Neurosurg Focus 52(2):E6. 10.3171/2021.11.FOCUS21604 [DOI] [PubMed] [Google Scholar]
- 33.Brandi G, Rizzo A (2022) IDH Inhibitors and Immunotherapy for Biliary Tract Cancer: A Marriage of Convenience? Int J Mol Sci 23(18):10869. 10.3390/ijms231810869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Csoma SL, Bedekovics J, Veres G, Arokszallasi A, Andras C, Mehes G, Mokanszki A (2022) Circulating cell-free DNA-based comprehensive molecular analysis of biliary tract cancers using next-generation sequencing. Cancers (Basel) 14(1):233. 10.3390/cancers14010233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang LE (2019) Friend or foe-IDH1 mutations in glioma 10 years on. Carcinogenesis 40(11):1299–1307. 10.1093/carcin/bgz134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouligny IM, Murray G, Doyel M, Patel T, Boron J, Tran V, Gor J, Hang Y, Alnimer Y, Zacholski K (2023) Venetoclax with decitabine or azacitidine in the first-line treatment of acute myeloid leukemia. eJHaem 201(4):593–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang XY, Zhu WW, Wang Z, Huang JB, Wang SH, Bai FM, Li TE, Zhu Y, Zhao J, Yang X, Lu L, Zhang JB, Jia HL, Dong QZ, Chen JH, Andersen JB, Ye D, Qin LX (2022) Driver mutations of intrahepatic cholangiocarcinoma shape clinically relevant genomic clusters with distinct molecular features and therapeutic vulnerabilities. Theranostics 12(1):260–276. 10.7150/thno.63417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Shamsi HO, Anand D, Shroff RT, Jain A, Zuo M, Conrad C, Vauthey JN, Javle MM (2016) BRCA-associated protein 1 mutant cholangiocarcinoma: an aggressive disease subtype. J Gastrointest Oncol 7(4):556–561. 10.21037/jgo.2016.03.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file 1. Fig. S1Flowchart.Abbreviations: ICC, intrahepatic cholangiocarcinoma; PDL1, programmed cell deathligand 1; ORR, objective response rate; PFS, progression free survival; OS, overallsurvival; DCR, disease control rate; AEs, adverse events; ECOG-PS, easterncooperative oncology group performance status; CA19-9, carbohydrate antigen 19-9
Supplementary file 2. Fig. S2 Exploratory analysis for genetic alternations. ORR, PFS, andOS stratified by mutation status for ARID1A A B AP1 (B), FGFR2 (C), KRAS (D),TP53 (E), and DDR (F)Abbreviations: PD progressive disease, PR partial response, SD stable disease,DDRDNA dam age response
Data Availability Statement
All data supporting the results of the study can be found in the article and online supplementary material files. Researchers can contact the corresponding author of this article by email and indicate the required research materials and purpose. We will be glad to provide relevant materials for this study after approval and discussion.