Abstract
Background
Lenvatinib, programmed cell death 1 (PD-1) antibodies, and gemcitabine and oxaliplatin (GEMOX) chemotherapy have shown significant antitumor activity as first-line therapy against biliary tract cancer. This study evaluated their efficacy and safety as non-first-line therapy in advanced gallbladder cancer (GBC).
Methods
Patients with advanced GBC who received lenvatinib combined with anti-PD-1 antibodies and GEMOX chemotherapy as a non-first-line therapy were retrospectively analyzed. The primary endpoints were overall survival (OS) and progression-free survival (PFS), and the secondary endpoints were objective response rate (ORR) and safety.
Results
A total of 36 patients with advanced GBC were included in this study. The median follow-up time was 11.53 (95% confidence interval (CI): 2.2–20.9) months, and the ORR was 36.1%. The median OS and PFS were 15.1 (95% CI: 3.2–26.9) and 6.1 (95% CI: 4.9–7.2) months, respectively. The disease control rate (DCR) and clinical benefit rate (CBR) were 75% and 61.1%, respectively. Subgroup analysis demonstrated that patients with programmed cell death-ligand 1 (PD-L1) expression had significantly longer PFS and OS than those without PD-L1 expression. Additionally, patients with a neutrophil–lymphocyte ratio (NLR) < 5.57 had a longer OS than those with an NLR ≥ 5.57. All patients experienced adverse events (AEs), with 61.1% experiencing grade 3 or 4 AEs, including myelosuppression (13.9%) and fatigue (13.3%), alanine transaminase or aspartate transaminase levels (8.3%), and diarrhea (8.3%). No grade 5 AEs were reported.
Conclusion
Anti-PD-1 antibodies combined with lenvatinib and GEMOX chemotherapy are effective and well-tolerated as a non-first-line therapy in advanced GBC. PD-L1 expression and baseline NLR may potentially predict treatment efficacy.
Keywords: Gallbladder cancer, Lenvatinib, PD-1, GEMOX, Chemotherapy, Systemic therapy
Introduction
Gallbladder cancer (GBC), a common subtype of biliary tract cancer (BTC), is associated with hidden onset, high malignancy, and poor prognosis [1]. According to global cancer statistics, in 2020, there were 115,949 new cases of GBC and 84,695 related deaths worldwide, making it the sixth among most common digestive system tumors [2]. Most patients with GBC are diagnosed at an advanced stage, where curative surgery is not feasible, resulting in poor prognosis. Systemic therapy remains the main treatment for advanced GBC, although it is mostly based on BTC research data from the limited trials specifically on GBC. Studies on drug therapies for BTC usually include GBC, allowing their results to be directly applicable to GBC.
The ABC-002 study established gemcitabine and cisplatin (GC) as the standard first-line treatment for BTC [3], although it has suboptimal efficacy. When first-line therapy fails, second-line treatment options are scarce. The folinic acid, fluorouracil, and oxaliplatin (FOLFOX) regimen may be an alternative based on a phase 3 ABC-06 study. However, the benefit of best supportive care is marginal (OS: 6.2 vs. 5.3 months), and its side effects are significant [4]. Additionally, the liposomal irinotecan, fluorouracil, and leucovorin regimen (FOLFIRI) can be used as a second-line option after progression following GC chemotherapy, though has limited efficacy. The median progression-free survival (PFS) was prolonged (7.1 vs. 1.4 months) but was accompanied by a higher incidence of adverse events (AE) (42% vs. 24%) compared with those in patients treated only with fluorouracil and leucovorin [5]. Thus, when first-line treatment for advanced GBC, even BTC, fails, second-line treatment options remain limited and suboptimal.
Over the past decade, significant efforts have been made to enhance the efficacy of standard chemotherapy, with immune checkpoint inhibitors (ICIs) significantly transforming treatment paradigms for various solid tumors [6]. Ongoing research on ICIs, targeted therapy, chemotherapy, and related combination therapies has expanded treament options for advanced GBC [7, 8]. Following the TOPAZ-1 and Keynote-966 trial, durvalumab/pembrolizumab (PD-L1/PD-1 inhibitors) plus GC chemotherapy has been recommended as the preferred first-line therapy for advanced BTC. However, addition of ICIs to chemotherapy may only extend survival by approximately 1 month [9, 15]. Recently, a phase II trial demonstrated that tislelizumab (a PD-1 inhibitor) plus lenvatinib and gemcitabine and oxaliplatin (GEMOX) showed promising efficacy in locally advanced BTC, achieving an objective response rate (ORR) of 56% and a conversion surgical resection rate of 52% [10]. Additionally, a study observed that the treatment was more effective for GBC and intrahepatic cholangiocarcinoma (ICC) compared with extrahepatic cholangiocarcinoma (ECC) [10]. Another phase II trial suggested that toripalimab (a PD-1 inhibitor) plus lenvatinib and GEMOX chemotherapy showed good efficacy in advanced ICC as first-line therapy, with an ORR of 80% and a median OS of 22.5 months [11]. Our team has confirmed the efficacy of a triplet regimen incorporating four different ICIs, including toripalimab, in the treatment of advanced ICC [12]. We also assessed triple therapy as first-line and non-first-line treatment for advanced BTC, with an ORR of 43.9% [13]. However, the effectiveness of triple therapy as a non-first-line treatment after the failure of first-line therapy for advanced GBC remains unclear owing to the small number of patients with GBC in studies.
Toripalimab, pembrolizumab, and tislelizumab are anti-PD-1 antibodies approved for clinical trials by the Unites States Food and Drug Administration and China’s National Medical Products Administration [14–16]. Lenvatinib, a multikinase inhibitor, has demonstrated efficacy in GBC when combined with PD-1 therapy [17]. Additionally, the combination of lenvatinib and chemotherapy regimens can significantly upregulate PD-L1 expression, and co-administration with anti-PD-1 significantly enhance its effect [18]. We posit that PD-1 inhibitors plus lenvatinib and GEMOX chemotherapy may be a promising therapeutic regimen for patients with advanced GBC after first-line treatment failures. However, data on the safety of the triplet therapy are limited. Given the diverse nature of immune-related AEs [19, 20], further assessment of the incidence of AEs associated with these regimens is essential. Recently, peripheral blood-based biomarkers have emerged as significant indirect indicators of host immune status [21]. To support clinical decision-making, we also investigated several commonly used biomarkers, including carbohydrate antigen 19–9 (CA19-9), PD-L1, and neutrophil–lymphocyte ratio (NLR) [15, 22].
Based on these results, we conducted a retrospective study to assess the safety and efficacy of lenvatinib combined with anti-PD-1 antibodies and GEMOX as a non-first-line systemic therapy in patients with advanced GBC.
Materials and methods
Study population
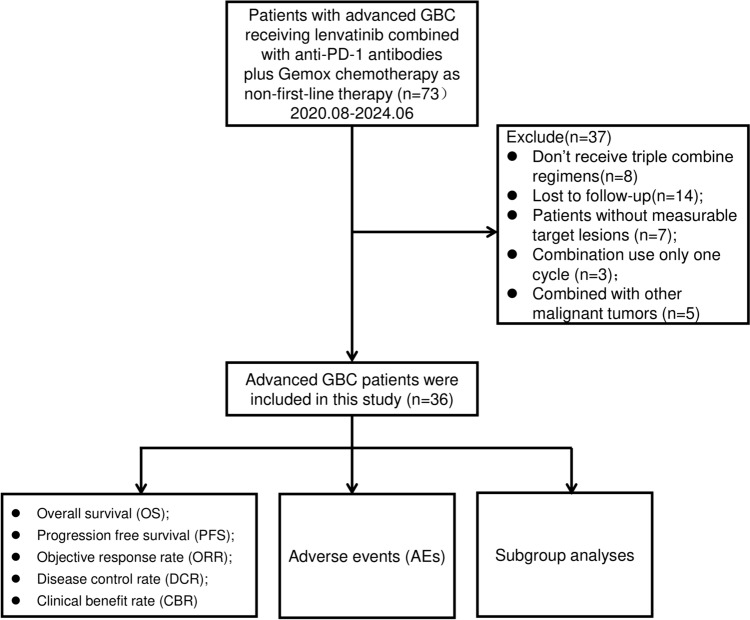
Between August 2020 and June 2024, 73 patients with advanced GBC who received lenvatinib combined with anti-PD-1 antibodies and chemotherapy as non-first-line therapy at the Peking Union Medical College Hospital (PUMCH) were enrolled in this study. Non-first-line therapy refers to the treatment administered after the failure of first-line treatment. The inclusion criteria were pathologically confirmed adenocarcinoma and assessable tumor lesions according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 [23]. Among the initial 73 patients, eight did not receive triple combination regimens, three received only one cycle of the regimen, five had other additional malignant tumors, 14 were lost to follow-up, and seven patients had non-measurable target lesions (Fig. 1). Consequently, 36 patients were enrolled for final analysis. Data on age, sex, Eastern Cooperative Oncology Group (ECOG) performance, Child–Pugh score, CA19-9 level, hepatitis B virus (HBV) infection, differentiated histology, tumor node metastasis classification (TNM) stage, site of metastases, PD-L1 expression, previous treatment regimens, baseline NLR level, and types of PD-1 inhibitors were collected (Table 1). This study was approved by the Institutional Review Board and Ethics Committee of PUMCH (IRB No. JS-1391).
Fig. 1.
Flow diagram of study population
Table 1.
Baseline characteristics of the study population
| Parameters | Total (n = 36) |
|---|---|
| Age, years (median, IQR) | 59 (53.25–64.25) |
| ≥ 60 | 16 [44.4%] |
| < 60 | 20 [55.6%] |
| Sex, n [%] | |
| Female | 15 [41.7] |
| Male | 21 [58.3] |
| ECOG performance, n [%] | |
| 0 | 20 [55.6] |
| 1 | 13 [36.1] |
| 2 | 3 [8.3] |
| Child–Pugh score, n [%] | |
| A | 27 [75] |
| B* | 9 [25] |
| CA19-9, U/mL (median, IQR) | 107.1 (21.6–421.5) |
| ≥ 200 | 21 [58.3] |
| < 200 | 15 [41.7] |
| HBV infection, n [%] | 4 [11.1] |
| Differentiated histology, n [%] | |
| Poor | 16 [44.4] |
| Moderate | 13 [36.1] |
| Well | 7 [19.0] |
| TNM stage, n [%] | |
| III | 8 [22.2] |
| IV | 28 [77.8] |
| Site of metastases, n [%] | |
| Liver | 28 [77.8] |
| Direct invasion | 5 [13.8] |
| Intrahepatic | 23 [68.9] |
| Lymph nodes | 30 [83.3] |
| Lung | 6 [16.7] |
| Bone | 4 [11.1] |
| Others | 4 [11.1] |
| PD-L1 expression, n [%] | |
| Positive | 12 [33.3] |
| Negative | 24 [66.7] |
| Previous treatment regimens, n [%] | |
| Systemic chemotherapy | |
| Capecitabine | 14 [38.9] |
| Gemcitabine + capecitabine | 9 [25] |
| Gemcitabine + cisplatin | 5 [13.9] |
| Gemcitabine + S-1 | 2 [5.6] |
| Durvalumab + gemcitabine + cisplatin | 2 [5.6] |
| Pembrolizumab + gemcitabine + cisplatin | 4 [11.1] |
| Targeted therapy | |
| Lenvatinb | 9 [25] |
| Transarterial chemoembolization | 6 [16.7] |
| Radical surgery resection | 30 [83.3] |
| Palliative surgical resection | 6 [16.7] |
| Regional radiotherapy or ablation | 8 [22.2] |
| Type of anti-PD-1 antibodies, n [%] | |
| Toripalimab | 17 [47.2] |
| Pembrolizumab | 13 [36.1] |
| Tislelizumab | 6 [16.7] |
| Parameters | Total (n = 36) |
|---|---|
| Age, years (median, IQR) | 59 (53.25–64.25) |
| ≥ 60 | 16 [44.4%] |
| < 60 | 20 [55.6%] |
| Sex, n [%] | |
| Female | 15 [41.7] |
| Male | 21 [58.3] |
| ECOG performance, n [%] | |
| 0 | 20 [55.6] |
| 1 | 13 [36.1] |
| 2 | 3 [8.3] |
| Child–Pugh score, n [%] | |
| A | 27 [75] |
| B* | 9 [25] |
| CA19-9, U/mL (median, IQR) | 107.1 (21.6–421.5) |
| ≥ 200 | 21 [58.3] |
| < 200 | 15 [41.7] |
| NLR, (median, IQR) | 3.3 (2.2–7.4) |
| ≥ 5.57 | 12 [33.3] |
| < 5.57 | 21 [58.3] |
| NA | 3 [8.3] |
| HBV infection, n [%] | 4 [11.1] |
| Differentiated histology, n [%] | |
| Poor | 16 [44.4] |
| Moderate | 13 [36.1] |
| Well | 7 [19.0] |
| TNM stage, n [%] | |
| III | 8 [22.2] |
| IV | 28 [77.8] |
| Site of metastases, n [%] | |
| Liver | 28 [77.8] |
| Direct invasion | 5 [13.8] |
| Intrahepatic | 23 [68.9] |
| Lymph nodes | 30 [83.3] |
| Lung | 6 [16.7] |
| Bone | 4 [11.1] |
| Others | 4 [11.1] |
| PD-L1 expression, n [%] | |
| Positive | 12 [33.3] |
| Negative | 24 [66.7] |
| Previous treatment regimens, n [%] | |
| Systemic chemotherapy | |
| Capecitabine | 14 [38.9] |
| Gemcitabine + capecitabine | 9 [25] |
| Gemcitabine + cisplatin | 5 [13.9] |
| Gemcitabine + S-1 | 2 [5.6] |
| Durvalumab + gemcitabine + cisplatin | 2 [5.6] |
| Pembrolizumab + gemcitabine + cisplatin | 4 [11.1] |
| Targeted therapy | |
| Lenvatinb | 9 [25] |
| Transarterial chemoembolization | 6 [16.7] |
| Radical surgery resection | 30 [83.3] |
| Palliative surgical resection | 6 [16.7] |
| Regional radiotherapy or ablation | 8 [22.2] |
| Type of anti-PD-1 antibodies, n [%] | |
| Toripalimab | 17 [47.2] |
| Pembrolizumab | 13 [36.1] |
| Tislelizumab | 6 [16.7] |
*The Child–Pugh score was 7
IQR Interquartile range, ECOG Eastern Cooperative Oncology Group, CA19-9 carbohydrate antigen 19–9, HBV hepatitis type B virus, and TNM tumor node metastasis classification
Treatment
Lenvatinib was administered orally at doses of 12 mg (body weight ≥ 60 kg) or 8 mg (body weight < 60 kg) once daily. Similarly, the dose of anti-PD-1 antibodies was 200 mg (240 mg for toripalimab) or 3 mg/kg body weight every 3 weeks. The GEMOX chemotherapy regimen was administered as 1000 mg/m2 gemcitabine on days 1 and 8, and 100 mg/m2 oxaliplatin on day 1, and Q3W was administered by intravenous injection for six cycles.
Outcome assessment
The clinical objective response was assessed using the RECIST v1.1 [23]. Radiologists at PUMCH independently assessed treatment responses based on changes in tumor size using computed tomography, magnetic resonance imaging, or positron emission tomography. The primary endpoints of the study were OS and PFS, whereas the secondary endpoints were ORR and safety. The OS, PFS, ORR, DCR, and CBR were used to assess treatment efficacy. CBR was defined as the proportion of patients with a radiologically confirmed objective response (complete response, CR or partial response, PR) or stable disease (SD) for more than 6 months [24]. Safety assessments and grading were recorded through physical examination, laboratory evaluation, and electronic medical records, with data collected by the investigators using the Common Terminology Criteria for Adverse Events (version 5.0).
Evaluation of biomarkers
Whole-section immunohistochemical staining was performed on formalin-fixed, paraffin-embedded tumor specimens. For each tissue slice, 5-μm-thick sections were selected and mounted on glass slides. Anti-PD-L1 was used as the primary antibody, followed by the addition of secondary antibodies to all sections, including negative control slides. PD-L1 expression was evaluated by independent pathologists, who were blinded to the clinicopathological data, including the therapeutic response and survival time. PD-L1 positivity or overexpression was defined as > 1% of the tumor area and/or immune cells with PD-L1 staining at any intensity [15, 16]. Baseline NLR and CA19-9 level, obtained from electronic medical records, served as predictors of treatment efficacy and outcomes. According to prior literatures, an NLR cutoff value of 5.57 [22] and a CA19-9 cutoff level of 200 U/mL are considered optimal [12].
Statistical analysis
June 10, 2024, was the data cutoff point for analysis, with baseline characteristics, treatment effects, and AEs summarized accordingly. Survival curves were estimated using the Kaplan–Meier method, and group comparisons were analyzed using the log-rank test. The hazard ratios (HRS) of each clinicopathological feature for PFS and OS were estimated using the Cox proportional hazards model. To compare individual variables, the t-test, Mann–Whitney U-test, χ2 test, and Fisher’s exact test were appropriately performed. Results with two-tailed p-values < 0.05 were considered statistically significant. Statistical analyses were performed using R version 4.3.3 and SPSS (version 29.0) software.
Results
Baseline characteristics
We screened 73 patients with advanced GBC treated at PUMCH between August 2020 and June 2024, and excluded 37 patients from the study (Fig. 1). We finally included 36 patients with advanced GBC who received lenvatinib combined with anti-PD-1 antibodies and GEMOX chemotherapy as non-first-line treatment. The demographic and baseline characteristics of the 36 patients are summarized in Table 1. At the time of the initial treatment, the median age of the patients was 59 years, with 44.4% aged over 60 years and 41.7% being women. We observed that 33 (91.7%) patients had an ECOG performance status of 0–1, and 27 (75%) patients had a Child–Pugh score A. At baseline, the median CA19-9 level was 107.1 U/ml, with 58.3% of patients having a level > 200 U/ml. The median baseline NLR level was 3.3, and the NLRs of 21 (58.3%) patients were < 5.57. Four (11.1%) patients had a history of HBV infection. In total, 16 (44.4%) patients had poorly differentiated histology, and 12 (33.3%) had positive PD-L1 expression. We further observed that before treatment, most patients had metastatic tumor in the liver (28/36, 77.8%), lymph nodes (30/36, 83.3%), lungs (6/36, 16.7%), and bones (4/36, 11.1%); 28 patients (77.9%) had TNM stage IV disease. Prior to treatment with lenvatinib, anti-PD-1 antibodies, and GEMOX chemotherapy as a non-first-line treatment option, 14 (38.9%) patients have received capecitabine; 9 (25%) patients had received gemcitabine + capecitabine; 5 (13.9%) patients had received gemcitabine + cisplatin; 2 (5.6%) had received gemcitabine + S-1; 2 (5.6%) had received with durvalumab + gemcitabine + cisplatin, 4 (11.1%) had received with pembrolizumab + gemcitabine + cisplatin; and 5 (13.9%) had received targeted therapy, who failed first-line chemotherapy, with lenvatinib; 6 (16.7%) had received transarterial chemoembolization; 6 (16.7%) had received regional radiotherapy or ablation; 30 (83.3%) had received radical surgical resection; and 6 (16.7%) had received palliative surgical resection. Additionally, among the 36 patients, 17 (47.2%) were treated with toripalimab regimen, 13 (36.1%) with pembrolizumab regimen, and 6 (16.7%) with tislelizumab regimen.
Treatment and efficacy
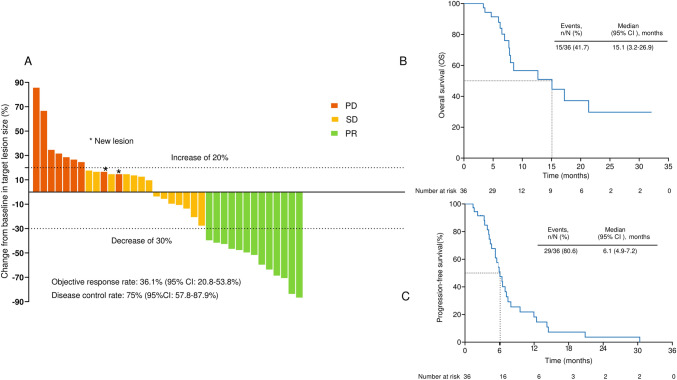
The median duration of treatment of lenvatinib combined with anti-PD-1 antibodies and GEMOX chemotherapy was 5.4 (interquartile range: 3.1–7.4) months. The median duration of follow-up was 11.53 (95% CI:2.2–20.9) months for all participants in our cohort. All patients underwent a complete radiological evaluation. Overall, 20 (55.6%) patients had a decrease in tumor size from baseline (Fig. 2A), with 13 (36.1%) patients achieving an objective response. Although all 13 (36.1%) patients achieved PR, none achieved CR. We observed that 14 (38.9%) patients exhibited SD, whereas 9 (25%) exhibited PD. Although the growth of the target lesion did not exceed 20% of the baseline size, two patients developed new metastatic lesions in the lungs after two cycles of continuous treatment, and the other developed new retroperitoneal lymph node metastases after six cycles of continuous treatment. The overall radiologically confirmed ORR was 36.1% (95% CI: 20.8–53.8%), and the DCR was 75% (95% CI: 57.8–87.9%) (Fig. 2A and Table 2).
Fig. 2.
Therapeutic efficacy of lenvatinib combined with anti-PD-1 antibodies plus GEMOX chemotherapy in patients with advanced gallbladder cancer. Maximum percentage change in the sum of diameters of target lesions from baseline (A). Kaplan–Meier estimation of overall survival (B) and progression-free survival (C) of the entire cohort
Table 2.
Therapeutic efficacy of response and survival outcomes
| Therapeutic response assessment | n = 36 |
|---|---|
| Objective response rate (ORR, n, %, 95% CI) | 13, 36.1% (20.8–53.8) |
| Partial response (PR, n, %) | 13 (36.1%) |
| Stable disease (SD, n, %) | 14 (38.9%) |
| Progressive disease (PD, n, %) | 9 (25%) |
| Disease control rate (DCR, n, %, 95% CI) | 27, 75% (57.8–87.9) |
| Clinical benefit rate (CBR, n, %, 95% CI) | 22, 61.1% (43.5–76.8) |
| Median progression-free survival (mPFS, months, 95% CI) | 6.1 (4.9–7.2) |
| Median overall survival (mOS, months, 95% CI) | 15.1 (3.2–26.9) |
We investigated the survival outcomes of the enrolled patients. For the entire cohort, we observed a median OS of 15.1 (95% CI: 3.2–26.9) months and a median PFS of 6.1 (95% CI: 4.9–7.2) months (Fig. 2B, C). We determined the CBR in all 36 patients and found it to be 61.1% (95% CI: 43.5–76.8%) (Table 2), with one patient’s successful conversion to surgery.
Subgroup analyses
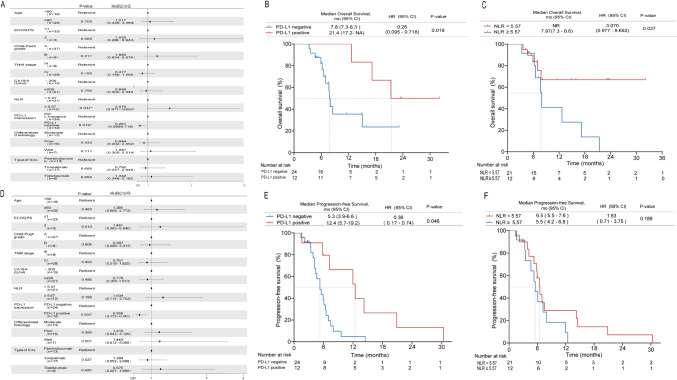
Post hoc subgroup analyses of prespecified baseline factors, such as age, TNM stage, differentiated histology, ECOG performance, Child–Pugh score, CA19-9 level, baseline NLR, PD-L1 expression, and PD-1 inhibitor regimen, are presented in a forest plot in Fig. 3A, D. No significant differences in treatment effects were observed between the subgroups, except for PD-L1 expression and baseline NLR. When patients were stratified according to PD-L1 expression and baseline NLR, Kaplan–Meier survival curve and log-rank test analysis demonstrated that patients expressing PD-L1 (positive PD-L1 expression) had a longer median PFS (12.4 vs. 5.3 months, P = 0.046; Fig. 3E) and a longer median OS (21.4 vs.7.8 months, P = 0.019; Fig. 3B) than did those not expressing PD-L1 (negative). In the group with a NLR < 5.57, the median PFS was 6.5 months compared with 5.5 months in the NLR ≥ 5.57 group, with a p-value of 0.189, indicating no significant difference (Fig. 3F). Conversely, the median OS was extended in the NLR < 5.57 group. Although the median OS was not yet reached, the difference was significant (p = 0.037) (Fig. 3C). Variables with p < 0.1 in the univariate analyses were included in the multivariate analyses. Our findings indicated that PD-L1 expression was associated with an OS HR of 0.19 (95% CI: 0.05–0.76; p = 0.019). Conversely, in patients with a NLR < 5.57, the HR for OS was 3.49 (95% CI, 1.13–10.73; p = 0.030).
Fig. 3.
Subgroup analyses. Subgroup analyses of progression-free survival (PFS) and overall survival (OS) in the entire cohort (A, D). Kaplan–Meier plot for PFS (E) and OS (B) based on the expression of programmed cell death-ligand 1 (PD-L1). PFS (F) and OS (C) based on the baseline level of neutrophil–lymphocyte ratio (NLR)
Safety
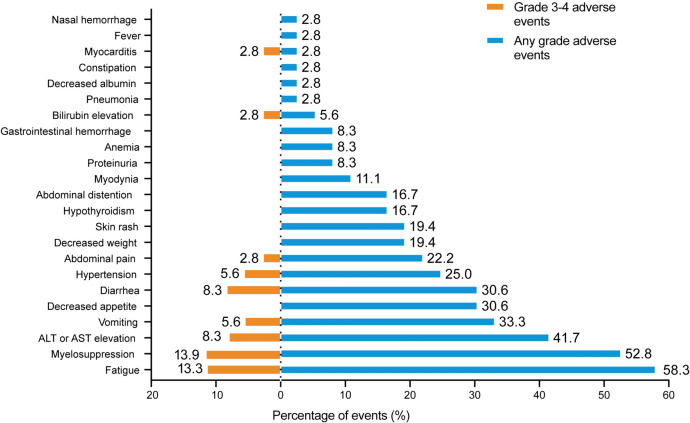
AEs were reported in all 36 (100%) patients throughout the study. No grade 5 AEs were detected. Additionally, we found that only 5.6% (2/36) of the patients experienced grade 4 AEs (diarrhea and elevated of bilirubin levels). Regarding severe AEs (SAEs), we noticed that 61.1% (22/36) of patients had ≥ grade 3 AEs (Table 3 and Fig. 4). Notably, the most common AEs (of any grade) were fatigue (21/36, 58.3%), myelosuppression (19/36, 52.8%), and elevated ALT or AST levels (15/36, 41.7%). Most AEs were manageable, treatable, and tolerable. Particularly, the most common > grade 3 SAEs were myelosuppression (5/36, 13.9%), elevation in fatigue (4/36, 13.3%), elevated ALT or AST levels (3/36, 8.3%), and diarrhea (3/36, 8.3%). After careful treatment, all the observed AEs could be controlled.
Table 3.
Commonly observed adverse events
| Adverse events (AEs) | Any grade | Grade 1–2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Fatigue | 21 (58.3%) | 17 (47.2%) | 4 (13.3%) | 0 |
| Myelosuppression | 19 (52.8%) | 14 (38.9%) | 5 (13.9%) | 0 |
| ALT or AST elevation | 15 (41.7%) | 12 (33.3%) | 3 (8.3%) | 0 |
| Vomiting | 12 (33.3%) | 10 (27.8%) | 2 (5.6%) | 0 |
| Decreased appetite | 11 (30.6%) | 11 (30.6%) | 0 | 0 |
| Diarrhea | 11 (30.6%) | 8 (22.2%) | 2 (5.6%) | 1 (2.8%) |
| Hypertension | 9 (25.0%) | 7 (19.4%) | 2 (5.6%) | 0 |
| Abdominal pain | 8 (22.2%) | 7 (19.4%) | 1 (2.8%) | 0 |
| Decreased weight | 7 (19.4%) | 7 (19.4%) | 0 | 0 |
| Skin rash | 7 (19.4%) | 7 (19.4%) | 0 | 0 |
| Hypothyroidism | 6 (16.7%) | 6 (16.7%) | 0 | 0 |
| Abdominal distention | 6 (16.7%) | 6 (16.7%) | 0 | 0 |
| Myodynia | 4 (11.1%) | 4 (11.1%) | 0 | 0 |
| Proteinuria | 3 (8.3%) | 3 (8.3%) | 0 | 0 |
| Anemia | 3 (8.3%) | 3 (8.3%) | 0 | 0 |
| Gastrointestinal hemorrhage | 3 (8.3%) | 3 (8.3%) | 0 | 0 |
| Bilirubin elevation | 2 (5.6%) | 1 (2.8%) | 0 | 1 (2.8%) |
| Pneumonia | 1 (2.8%) | 1 (2.8%) | 0 | 0 |
| Decreased albumin | 1 (2.8%) | 1 (2.8%) | 0 | 0 |
| Constipation | 1 (2.8%) | 1 (2.8%) | 0 | 0 |
| Myocarditis | 1 (2.8%) | 0 | 1 (2.8%) | 0 |
| Fever | 1 (2.8%) | 1 (2.8%) | 0 | 0 |
| Nasal hemorrhage | 1 (2.8%) | 1 (2.8%) | 0 | 0 |
ALT alanine aminotransferase and AST aspatate aminotransferase
Fig. 4.
Adverse events during lenvatinib combined with anti-PD-1 antibodies plus GEMOX chemotherapy treatment in patients with advanced gallbladder cancer
Discussion
To our knowledge, this is the first study to investigate the use of PD-1 inhibitors and lenvatinib with GEMOX as non-first-line treatment options for GBC. In this study, the triple regimen of drugs showed good efficacy with tolerable AEs, resulting in a median OS of 15.1 months, median PFS of 6.1 months, and an ORR of 36.1%. These results surpass those achieved with chemotherapy-based second-line therapy obtained for advanced GBC [4, 5]. The incidence of grade 3 and 4 AEs was 61.1% (22/36), which is considered acceptable and within the control range. Additionally, subgroup analysis confirmed that this regimen may be more effective in patients with high PD-L1 expression.
With the application of ICIs, particularly PD-1 inhibitors, in the treatment of BTCS, increasing evidence supports their effectiveness of PD-1 inhibitors in the treatment of advanced GBC [25–29]. In a study evaluating camrelizumab-based regimens for advanced GBC, the median OS was 12 months, median PFS was 7 months, and ORR was 30.2%, which were superior to those of camrelizumab-based monotherapy [25]. Another study involving PD-1 inhibitor plus lenvatinib as a first-line treatment for unresectable BTC in 13 patients with GBC, reported an ORR of 42.1% [26]. Additionally, immunotherapy combined with chemotherapy has shown promising outcomes in the first-line treatment for advanced biliary tract tumors, such as nivolumab combined with gemcitabine and tegafur chemotherapy, achieving an ORR of 41.7% [27]. These findings suggest that combining different treatment regimens may have incremental efficacy in the overall therapy. Various studies have reported that targeted therapy and chemotherapy can enhance the effectiveness of immunotherapy against tumors [30, 31]. Chemotherapy promotes the activation of tumor-targeted immune responses by increasing the immunogenicity of tumor cells or inhibiting immunosuppressive circuits [30]. These results suggest that PD-1 inhibitors, combined with targeted agents and chemotherapy, may have unexpected effects in treating BTC. Shi et al. and Li further confirmed the efficacy of PD-1 inhibitors combined with lenvatinib and GEMOX chemotherapy in BTC, suggesting that this approach may be effective for GBC [10, 11]. Both discussed studies focused on these regimens as a first-line treatment for advanced BTC, leaving the efficacy of such regimens after the failure of first-line treatment largely unexplored, especially in GBC. Given the promising ORR of triple therapy in the first-line treatment of BTC, it is expect that triple therapy would also be effective in non-first-line treatments. In our study, PD-1 inhibitors combined with lenvatinib and GEMOX chemotherapy as a non-first-line treatment for advanced GBC achieved good results, significantly prolonging the survival of patients.
In this study, 33.3% (12/36) of patients exhibited positive PD-L1 expression, and these patients showed improved survival, compared with those without PD-L1 expression, suggesting that PD-L1 can be used as a marker for evaluating the efficacy of treatment. Furthermore, baseline analysis of NLR revealed that patients with an NLR ≥ 5.57 had significantly worse OS, compared with those with an NLR < 5.57, suggesting that the NLR could be an effective prognostic predictor. Numerous studies have consistently reported that PD-L1 expression may serve as potential markers for predicting immunotherapy effectiveness [32–34]. The role of NLR as a predictor in malignant tumor immunotherapy has garnered increasing attention [22]. Additionally, emerging biomarkers, such as gut microbiota and DNA damage repair mechanisms, are showing promising potential for predicting the efficacy of ICIs in BTC [35, 36]. Although further validation and refinement are required, the Royal Marsden Hospital (RMH) score, which relies on routine blood tests and clinical features, represents a promising avenue for prognostic research in cancer patients [37].
One patient underwent conversion surgery following clinical evaluation of PR, GEMOX chemotherapy was discontinued after surgery, whereas long-term maintenance with pembrolizumab and lenvatinib was continued. No disease progression was observed until the last follow-up date.
In this study, the triple treatments resulted in tolerable and manageable AEs. Dose modification was observed in three patients and medication delay in six patients due to AEs. However, no discontinuation or death was attributed to treatment-related AEs. Myelosuppression, a common side effect of chemotherapy, occurred in 52.8% of patients (19/36), higher than reported in other studies, likely due to the chemotherapy regimen included in the study protocol [38]. Nonetheless, the AE incidence in this study was not significantly higher than that in other studies involving chemotherapy regimens [3, 39]. The rate of SAE in a study on FOLFIRI as a second-line treatment for BTC was 42%, which was comparable to the 42.9% observed in the current study, suggesting that PD-1 inhibitors combined with lenvatinib and chemotherapy do not trigger more AEs [5]. The chemotherapy regimen used in this study was GEMOX; however, GC chemotherapy was used as the first-line standard regimen for advanced GBC. Further studies are required to determine whether chemotherapy regimens have varying sensitizing effects on immunotherapy. Additionally, the increasing use of immunotherapy has been associated with specific immune-related AEs, such as neuropathy and headaches, which require further investigation and close monitoring [40].
This study has certain limitations. First, it was a retrospective, single-center, real-world analysis with a limited sample size owing to the low incidence of the disease and specific treatment regimens employed. Additionally, only relatively young patients (median 59 years, range 40–80 years) with good performance status were included, which may have introduced selection bias and influenced the estimation of ORR, PFS, and OS. A more rigorous prospective study with a larger sample size or a multicenter approach is needed to validate these findings. Second, various PD-1 inhibitors were used in this study. Although the subgroup analysis showed no significant differences, any variations in the efficacy of different types of ICIs on GBC remain unclear. Future studies that evaluate the use of single-class ICIs are required to address this issue. Finally, although the efficacy of non-first-line treatment regimens was good, this study lacked a direct comparison with the standard second-line chemotherapy regimen for advanced GBC. Thus, further research using the second-line chemotherapy regimen as the control group is needed. Despite these limitations, this study’s results provide additional non-first-line therapy options for patients with advanced GBC when first-line therapy fails. Additionally, this study can be used as a reference for future clinical study designs and selection of treatment strategies.
Conclusions
Anti-PD-1 antibodies combined with lenvatinib and GEMOX chemotherapy are effective and well-tolerated as non-first-line therapies for advanced GBC. This regimen represents a viable subsequent-line therapeutic option for advanced GBC, with PD-L1 expression and baseline NLR potentially predicting treatment efficacy. Thus, further large-scale prospective studies are required to validate these findings.
Abbreviations
- AEs
Adverse events
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- BTC
Biliary tract cancer
- CA19-9
Carbohydrate antigen 19–9
- CBR
Clinical benefit rate
- CT
Computed tomography
- DCR
Disease control rate
- ECC
Extrahepatic cholangiocarcinoma
- ECOG
Eastern Cooperative Oncology Group
- FOLFOX
Folinic acid, fluorouracil, and oxaliplatin chemotherapy
- FOLFIRI
Liposomal irinotecan, fluorouracil, and leucovorin regimen
- GBC
Gallbladder cancer
- GC
Gemcitabine and cisplatin
- GEMCIS
Gemcitabine and cisplatin
- GEMOX
Oxaliplatin and gemcitabine
- HBV
Hepatitis type B virus
- ICC
Intrahepatic cholangiocarcinoma
- ICIs
Immune checkpoint inhibitors
- IQR
Interquartile range
- IV
Intravenously
- NLR
Neutrophil–lymphocyte ratio
- ORR
Objective response rate
- OS
Overall survival
- PFS
Progression-free survival
- PUMCH
Peking Union Medical College Hospital
- PD-L1
Programmed death-ligand 1
- SAEs
Severe AEs
- TNM
Tumor node metastasis classification
Authors contributions
All authors contributed to the study conception, design, and data collection. Material preparation and analysis was performed by YT, KL, CPZ, XBY, and SSW. YT, KL, and CPZ wrote the first draft of the manuscript, and all authors commented on the subsequent versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by National High Level Hospital Clinical Research Funding [2022-PUMCH-B-128], CAMS Innovation Fund for Medical Sciences (CIFMS) [2022-I2M-C&T-A-003] [2021-I2M-1–061] [2021-I2M-1–003], CSCO-hengrui Cancer Research Fund [Y-HR2019-0239] [Y-HR2020QN-0414], CSCO-MSD Cancer Research Fund [Y-MSDZD2021-0213] and National Ten-thousand Talent Program, the National Natural Science Foundation of China (NSFC) (No. 81960125), and the Department of Science and Technology of Guizhou Province (No. Qiankehe Foundation [2020] 1Y302).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
The protocol was approved by the Institutional Review Board (IRB) and Ethics Committee (EC) of PUMCH (IRB No. JS-1391) and conformed to Good Clinical Practice guidelines and Declaration of Helsinki principles.
Informed consent
The informed consent was waived by the Ethics Committee (EC) of PUMCH.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yang Tan, Kai Liu, Chengpei Zhu, and Shanshan Wang have contributed equally to this work and should be considered as co-first authors.
Contributor Information
Xiaobo Yang, Email: yangxiaobo67@pumch.cn.
Daobing Zeng, Email: dao_zeng@aliyun.com.
Lijin Zhao, Email: lijin.zhao@zmu.edu.cn.
Haitao Zhao, Email: zhaoht@pumch.cn.
References
- 1.Valle JW, Kelley RK, Nervi B et al (2021) Biliary tract cancer. Lancet 397:428–444. 10.1016/S0140-6736(21)00153-7 [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 3.Valle J, Wasan H, Palmer DH et al (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362:1273–1281. 10.1056/NEJMoa0908721 [DOI] [PubMed] [Google Scholar]
- 4.Lamarca A, Palmer DH, Wasan HS et al (2021) Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol 22:690–701. 10.1016/S1470-2045(21)00027-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoo C, Kim K-P, Jeong JH et al (2021) Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): a multicentre, open-label, randomised, phase 2b study. Lancet Oncol 22:1560–1572. 10.1016/S1470-2045(21)00486-1 [DOI] [PubMed] [Google Scholar]
- 6.Rizzo A, Brandi G (2021) First-line chemotherapy in advanced biliary tract cancer ten years after the ABC-02 Trial: “and yet it moves!” Cancer Treat Res Commun 27:100335. 10.1016/j.ctarc.2021.100335 [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Wu X, Wu H et al (2020) Camrelizumab plus gemcitabine and oxaliplatin (GEMOX) in patients with advanced biliary tract cancer: a single-arm, open-label, phase II trial. J Immunother Cancer 8:e001240. 10.1136/jitc-2020-001240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng K, Liu Y, Zhao Y et al (2020) Efficacy and biomarker analysis of nivolumab plus gemcitabine and cisplatin in patients with unresectable or metastatic biliary tract cancers: results from a phase II study. J Immunother Cancer 8:e000367. 10.1136/jitc-2019-000367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh D-Y, Ruth He A, Qin S et al (2022) Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 10.1056/EVIDoa2200015 [DOI] [PubMed] [Google Scholar]
- 10.Li H (2022) A single-arm, open-label, phase II study of tislelizumab combined with lenvatinib and Gemox regimen for conversion therapy of potentially resectable locally advanced biliary tract cancers. Ann Oncol 33:S570. 10.1016/j.annonc.2022.07.093 [Google Scholar]
- 11.Shi G-M, Huang X-Y, Wu D et al (2023) Toripalimab combined with lenvatinib and GEMOX is a promising regimen as first-line treatment for advanced intrahepatic cholangiocarcinoma: a single-center, single-arm, phase 2 study. Signal Transduct Target Ther 8:106. 10.1038/s41392-023-01317-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu C, Li H, Yang X et al (2023) Efficacy, safety, and prognostic factors of PD-1 inhibitors combined with lenvatinib and Gemox chemotherapy as first-line treatment in advanced intrahepatic cholangiocarcinoma: a multicenter real-world study. Cancer Immunol Immunother 72:2949–2960. 10.1007/s00262-023-03466-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu C, Xue J, Wang Y et al (2023) Efficacy and safety of lenvatinib combined with PD-1/PD-L1 inhibitors plus Gemox chemotherapy in advanced biliary tract cancer. Front Immunol 14:1109292. 10.3389/fimmu.2023.1109292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu J, Wang F, Dong L-H et al (2017) Preclinical evaluation of the efficacy, pharmacokinetics and immunogenicity of JS-001, a programmed cell death protein-1 (PD-1) monoclonal antibody. Acta Pharmacol Sin 38:710–718. 10.1038/aps.2016.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelley RK, Ueno M, Yoo C et al (2023) Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 401:1853–1865. 10.1016/S0140-6736(23)00727-4 [DOI] [PubMed] [Google Scholar]
- 16.Qin S, Kudo M, Meyer T et al (2023) Tislelizumab vs sorafenib as first-line treatment for unresectable hepatocellular carcinoma: a phase 3 randomized clinical trial. JAMA Oncol 9:1651–1659. 10.1001/jamaoncol.2023.4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuo B, Yang X, Yang X et al (2022) A real-world study of the efficacy and safety of anti-PD-1 antibodies plus lenvatinib in patients with advanced gallbladder cancer. Cancer Immunol Immunother 71:1889–1896. 10.1007/s00262-021-03121-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu M-J, He X-X, Bi N-R et al (2018) Effects of liver-targeted drugs on expression of immune-related proteins in hepatocellular carcinoma cells. Clin Chim Acta 485:103–105. 10.1016/j.cca.2018.06.032 [DOI] [PubMed] [Google Scholar]
- 19.Rizzo A, Mollica V, Tateo V et al (2023) Hypertransaminasemia in cancer patients receiving immunotherapy and immune-based combinations: the MOUSEION-05 study. Cancer Immunol Immunother 72:1381–1394. 10.1007/s00262-023-03366-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guven DC, Erul E, Kaygusuz Y et al (2023) Immune checkpoint inhibitor-related hearing loss: a systematic review and analysis of individual patient data. Support Care Cancer 31:624. 10.1007/s00520-023-08083-w [DOI] [PubMed] [Google Scholar]
- 21.Guven DC, Sahin TK, Erul E et al (2022) The association between albumin levels and survival in patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Front Mol Biosci 9:1039121. 10.3389/fmolb.2022.1039121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mallardo D, Fordellone M, White A et al (2023) CD39 and LDHA affects the prognostic role of NLR in metastatic melanoma patients treated with immunotherapy. J Transl Med 21:610. 10.1186/s12967-023-04419-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz LH, Seymour L, Litière S et al (2016) RECIST 1.1—standardisation and disease-specific adaptations: perspectives from the RECIST working group. Eur J Cancer 62:138–145. 10.1016/j.ejca.2016.03.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quispel-Janssen J, van der Noort V, de Vries JF et al (2018) Programmed death 1 blockade with nivolumab in patients with recurrent malignant pleural mesothelioma. J Thorac Oncol 13:1569–1576. 10.1016/j.jtho.2018.05.038 [DOI] [PubMed] [Google Scholar]
- 25.Zheng Q, Wu C, Ye H et al (2021) Analysis of the efficacy and prognostic factors of PD-1 inhibitors in advanced gallbladder cancer. Ann Transl Med 9:1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Q, Liu X, Wei S et al (2021) Lenvatinib plus PD-1 inhibitors as first-line treatment in patients with unresectable biliary tract cancer: a single-arm, open-label. Phase II Study Front Oncol 11:751391. 10.3389/fonc.2021.751391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiang N-J, Bai L-Y, Huang C-J et al (2021) 49P A phase II trial of nivolumab and gemcitabine and S-1 as the first-line treatment in patients with advanced biliary tract cancer. Ann Oncol 32:S377. 10.1016/j.annonc.2021.08.328 [Google Scholar]
- 28.Jin S, Zhao R, Zhou C et al (2023) Feasibility and tolerability of sintilimab plus anlotinib as the second-line therapy for patients with advanced biliary tract cancers: An open-label, single-arm, phase II clinical trial. Int J Cancer 152:1648–1658. 10.1002/ijc.34372 [DOI] [PubMed] [Google Scholar]
- 29.Villanueva L, Lwin Z, Chung HCC et al (2021) Lenvatinib plus pembrolizumab for patients with previously treated biliary tract cancers in the multicohort phase 2 LEAP-005 study. JCO 39:4080–4080. 10.1200/JCO.2021.39.15_suppl.4080 [Google Scholar]
- 30.Galluzzi L, Buqué A, Kepp O et al (2015) Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 28:690–714. 10.1016/j.ccell.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 31.Sun W, Patel A, Normolle D et al (2019) A phase 2 trial of regorafenib as a single agent in patients with chemotherapy-refractory, advanced, and metastatic biliary tract adenocarcinoma. Cancer 125:902–909. 10.1002/cncr.31872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis AA, Patel VG (2019) The role of PD-L1 expression as a predictive biomarker: an analysis of all US food and drug administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer 7:278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gibney GT, Weiner LM, Atkins MB (2016) Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 17:e542–e551. 10.1016/S1470-2045(16)30406-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tray N, Weber JS, Adams S (2018) Predictive biomarkers for checkpoint immunotherapy: current status and challenges for clinical application. Cancer Immunol Res 6:1122–1128. 10.1158/2326-6066.CIR-18-0214 [DOI] [PubMed] [Google Scholar]
- 35.Zhu C, Wang Y, Zhu R et al (2024) Gut microbiota and metabolites signatures of clinical response in anti-PD-1/PD-L1 based immunotherapy of biliary tract cancer. Biomark Res 12:56. 10.1186/s40364-024-00607-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ricci AD, Rizzo A, Brandi G (2020) The DNA damage repair (DDR) pathway in biliary tract cancer (BTC): a new Pandora’s box? ESMO Open 5:e001042. 10.1136/esmoopen-2020-001042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sahin TK, Rizzo A, Aksoy S, Guven DC (2024) Prognostic significance of the royal marsden hospital (RMH) score in patients with cancer: a systematic review and meta-analysis. Cancers 16:1835. 10.3390/cancers16101835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin J, Yang X, Long J et al (2020) Pembrolizumab combined with lenvatinib as non-first-line therapy in patients with refractory biliary tract carcinoma. Hepatobiliary Surg Nutr 9:414–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitsudomi T, Morita S, Yatabe Y et al (2010) Gefitinib versus cisplatin plus ocetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11:121–128. 10.1016/S1470-2045(09)70364-X [DOI] [PubMed] [Google Scholar]
- 40.Rizzo A, Santoni M, Mollica V et al (2021) Peripheral neuropathy and headache in cancer patients treated with immunotherapy and immuno-oncology combinations: the MOUSEION-02 study. Expert Opin Drug Metab Toxicol 17:1455–1466. 10.1080/17425255.2021.2029405 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.