Abstract
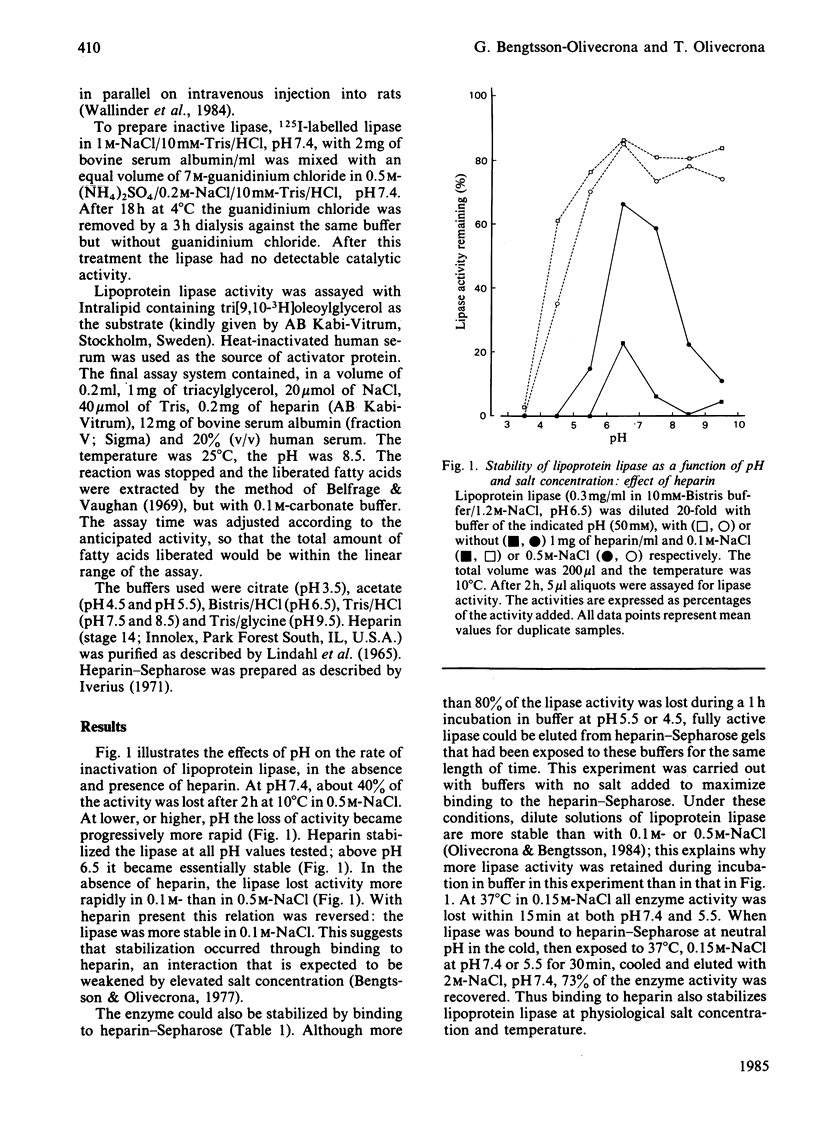
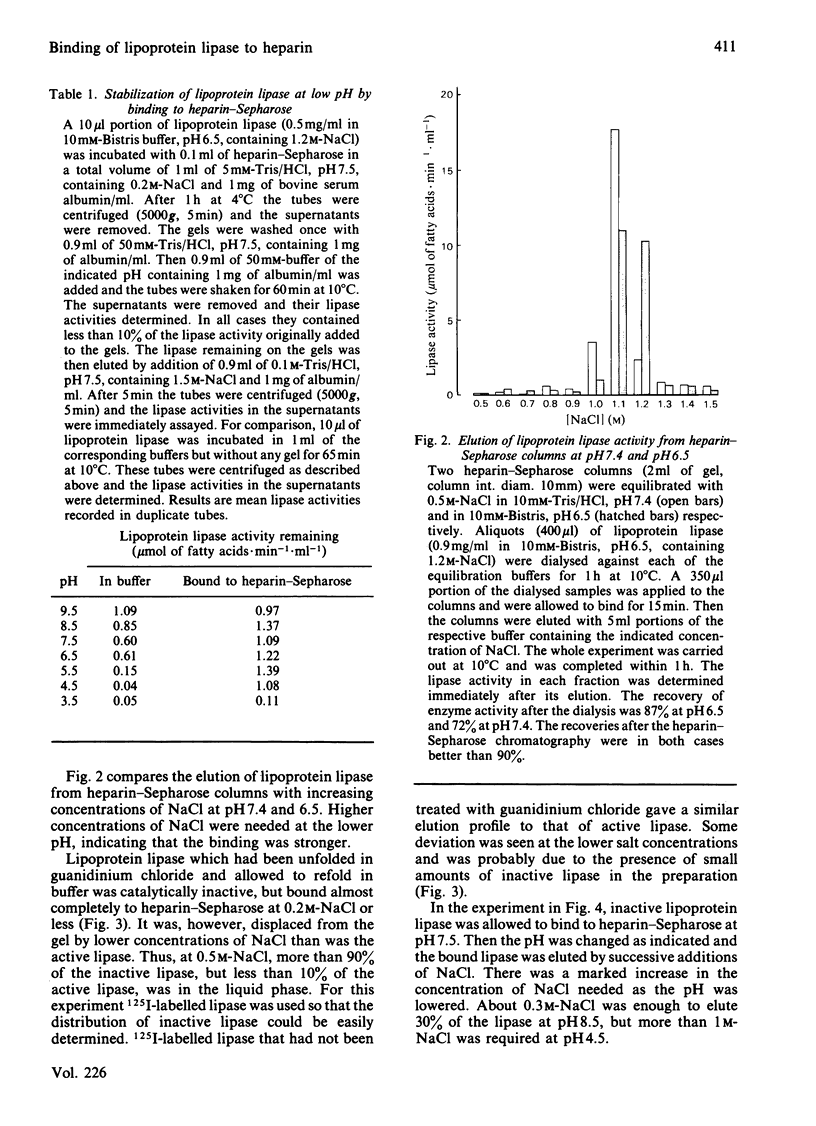
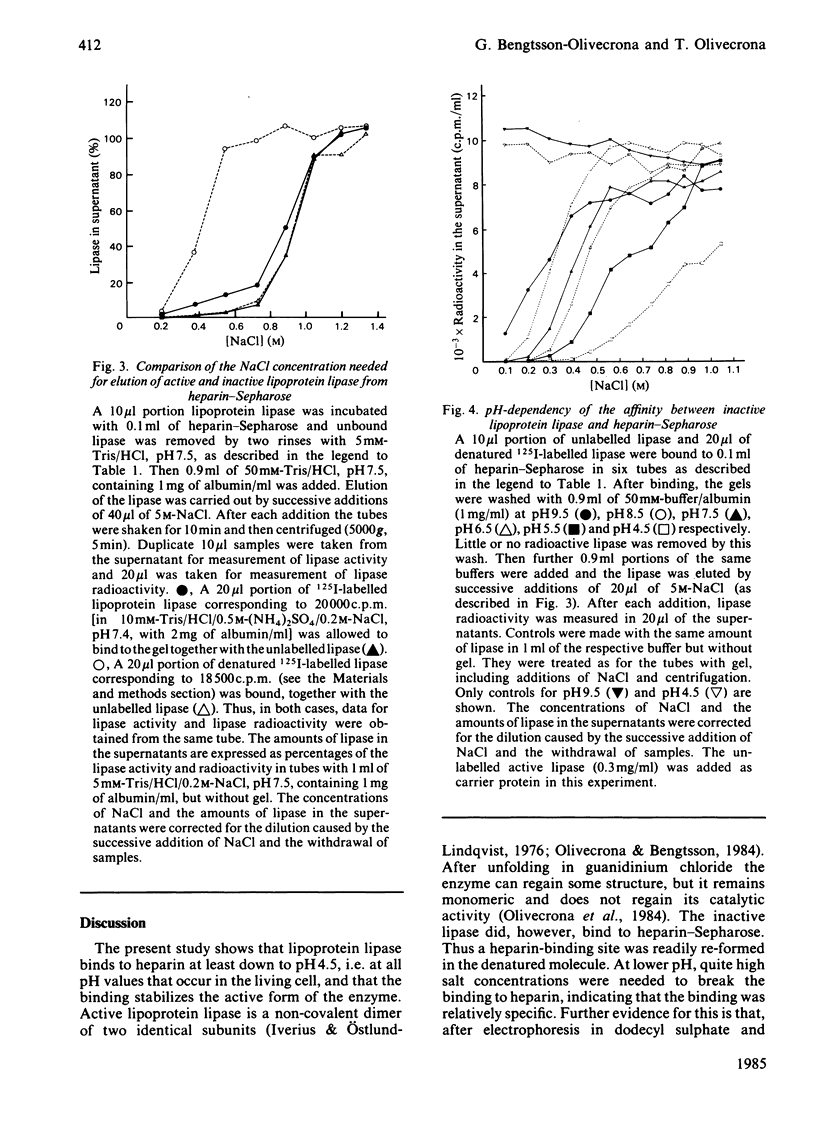
Lipoprotein lipase has been shown to bind to, be internalized by, and perhaps be transferred through, a variety of cells. These processes may involve a heparin-like cell-surface receptor and passage through acidified cell compartments. We have therefore studied effects of low pH on the binding of the lipase to heparin and on its catalytic activity. The rate of inactivation of the lipase in solution was found to increase as the pH was lowered. Addition of heparin stabilized the enzyme. Binding of active lipoprotein lipase to heparin-Sepharose could be demonstrated at pH down to 6.5. At pH below 6, binding could not be studied directly because the lipase was too unstable in solution. Lipase bound to heparin-Sepharose could, however, be exposed to pH 4.5 at 10 degrees C with little loss of activity. Binding to heparin-Sepharose also stabilized under physiological conditions (37 degrees C, 0.15 M-NaCl, pH 5.5-7.4). Catalytically inactive lipoprotein lipase retained the ability to bind to heparin-Sepharose. Higher concentrations of salt were needed to displace both active and inactive lipase from heparin-Sepharose at lower pH, indicating that the affinity increased as pH was lowered. The inactive lipase was, however, displaced by lower concentrations of salt than was active lipase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belfrage P., Vaughan M. Simple liquid-liquid partition system for isolation of labeled oleic acid from mixtures with glycerides. J Lipid Res. 1969 May;10(3):341–344. [PubMed] [Google Scholar]
- Bengtsson G., Olivecrona T. Interaction of lipoprotein lipase with heparin-Sepharose. Evaluation of conditions for affinity binding. Biochem J. 1977 Oct 1;167(1):109–119. doi: 10.1042/bj1670109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson G., Olivecrona T. Lipoprotein lipase: modification of its kinetic properties by mild tryptic digestion. Eur J Biochem. 1981 Jan;113(3):547–554. doi: 10.1111/j.1432-1033.1981.tb05097.x. [DOI] [PubMed] [Google Scholar]
- Bengtsson G., Olivecrona T. Lipoprotein lipase: some effects of activator proteins. Eur J Biochem. 1980 May;106(2):549–555. doi: 10.1111/j.1432-1033.1980.tb04602.x. [DOI] [PubMed] [Google Scholar]
- Cheng C. F., Oosta G. M., Bensadoun A., Rosenberg R. D. Binding of lipoprotein lipase to endothelial cells in culture. J Biol Chem. 1981 Dec 25;256(24):12893–12898. [PubMed] [Google Scholar]
- Cryer A. Tissue lipoprotein lipase activity and its action in lipoprotein metabolism. Int J Biochem. 1981;13(5):525–541. doi: 10.1016/0020-711x(81)90177-4. [DOI] [PubMed] [Google Scholar]
- Friedman G., Chajek-Shaul T., Olivecrona T., Stein O., Stein Y. Fate of milk 125I-labelled lipoprotein lipase in cells in culture. Comparison of lipoprotein lipase- and non-lipoprotein lipase-synthesizing cells. Biochim Biophys Acta. 1982 Apr 15;711(1):114–122. doi: 10.1016/0005-2760(82)90016-9. [DOI] [PubMed] [Google Scholar]
- Iverius P. H. Coupling of glycosaminoglycans to agarose beads (sepharose 4B). Biochem J. 1971 Oct;124(4):677–683. doi: 10.1042/bj1240677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverius P. H., Ostlund-Lindqvist A. M. Lipoprotein lipase from bovine milk. Isolation procedure, chemical characterization, and molecular weight analysis. J Biol Chem. 1976 Dec 25;251(24):7791–7795. [PubMed] [Google Scholar]
- LINDAHL U., CIFONELLI J. A., LINDAHL B., RODEN L. THE ROLE OF SERINE IN THE LINKAGE OF HEPARIN TO PROTEIN. J Biol Chem. 1965 Jul;240:2817–2820. [PubMed] [Google Scholar]
- Nilsson-Ehle P., Garfinkel A. S., Schotz M. C. Lipolytic enzymes and plasma lipoprotein metabolism. Annu Rev Biochem. 1980;49:667–693. doi: 10.1146/annurev.bi.49.070180.003315. [DOI] [PubMed] [Google Scholar]
- Olivecrona T., Bengtsson G., Marklund S. E., Lindahl U., Hök M. Heparin-lipoprotein lipase interactions. Fed Proc. 1977 Jan;36(1):60–65. [PubMed] [Google Scholar]
- Olivecrona T., Egelrud T., Iverius P. H., Lindahl U. Evidence for an ionic binding of lipoprotein lipase to heparin. Biochem Biophys Res Commun. 1971 May 7;43(3):524–529. doi: 10.1016/0006-291x(71)90645-0. [DOI] [PubMed] [Google Scholar]
- Quinn D., Shirai K., Jackson R. L. Lipoprotein lipase: mechanism of action and role in lipoprotein metabolism. Prog Lipid Res. 1983;22(1):35–78. doi: 10.1016/0163-7827(83)90003-6. [DOI] [PubMed] [Google Scholar]
- Shimada K., Gill P. J., Silbert J. E., Douglas W. H., Fanburg B. L. Involvement of cell surface heparin sulfate in the binding of lipoprotein lipase to cultured bovine endothelial cells. J Clin Invest. 1981 Oct;68(4):995–1002. doi: 10.1172/JCI110354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallinder L., Peterson J., Olivecrona T., Bengtsson-Olivecrona G. Hepatic and extrahepatic uptake of intravenously injected lipoprotein lipase. Biochim Biophys Acta. 1984 Oct 4;795(3):513–524. doi: 10.1016/0005-2760(84)90181-4. [DOI] [PubMed] [Google Scholar]


