Abstract
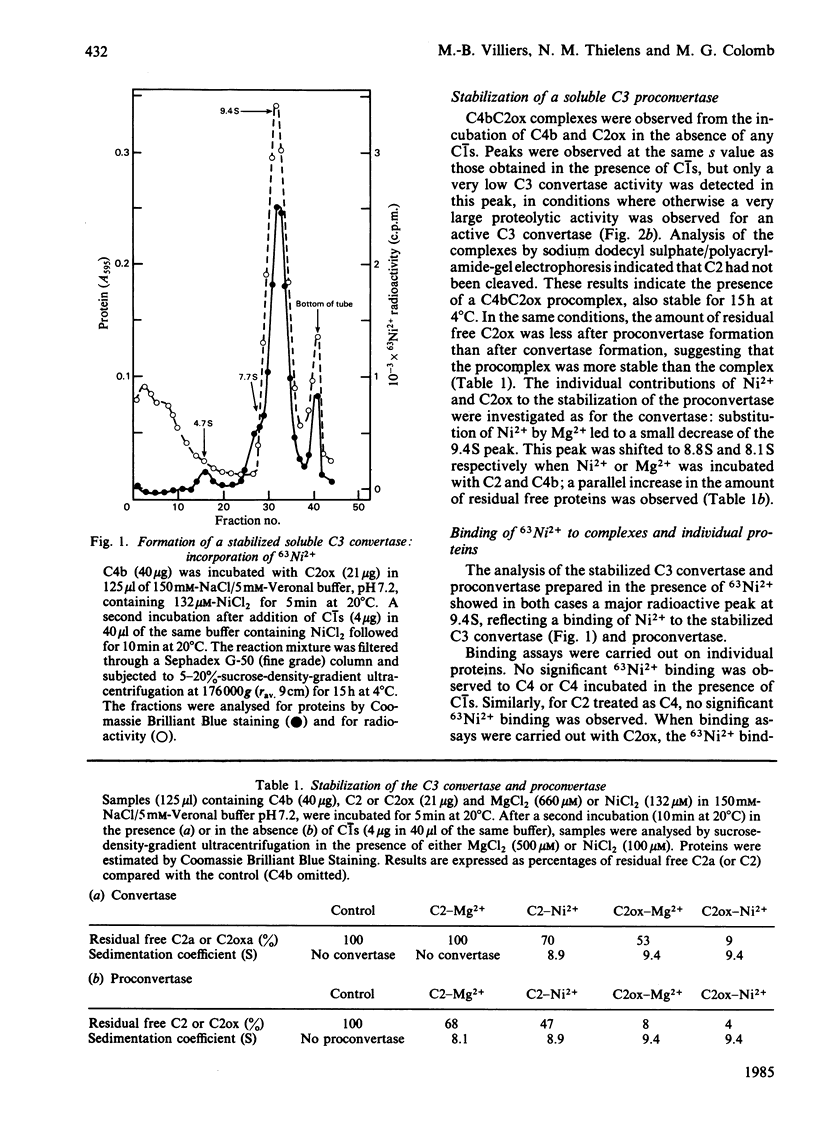
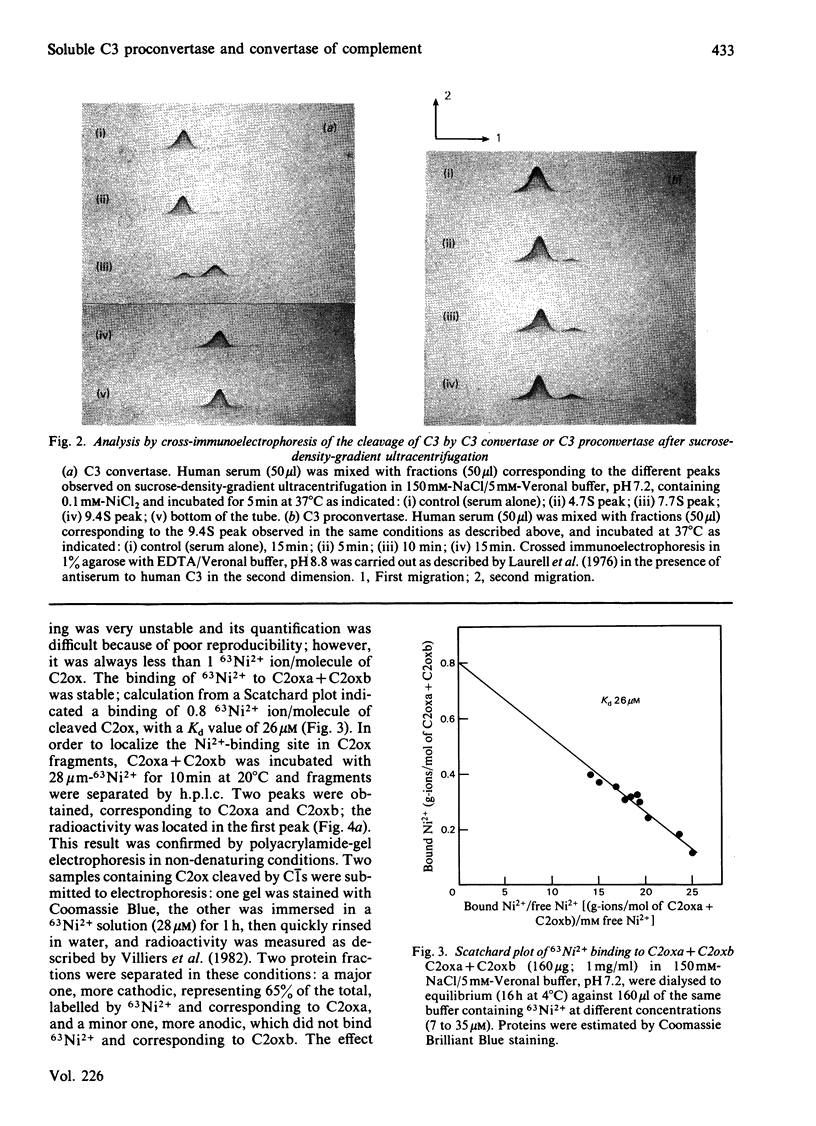
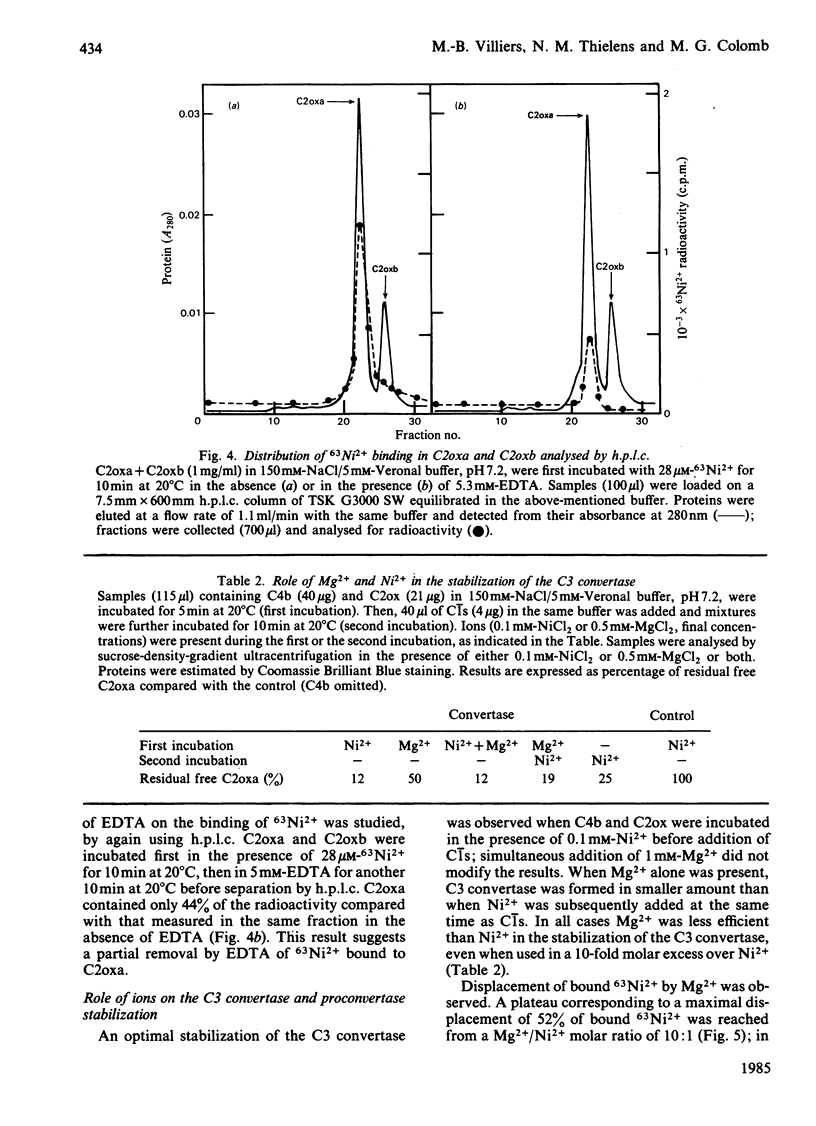
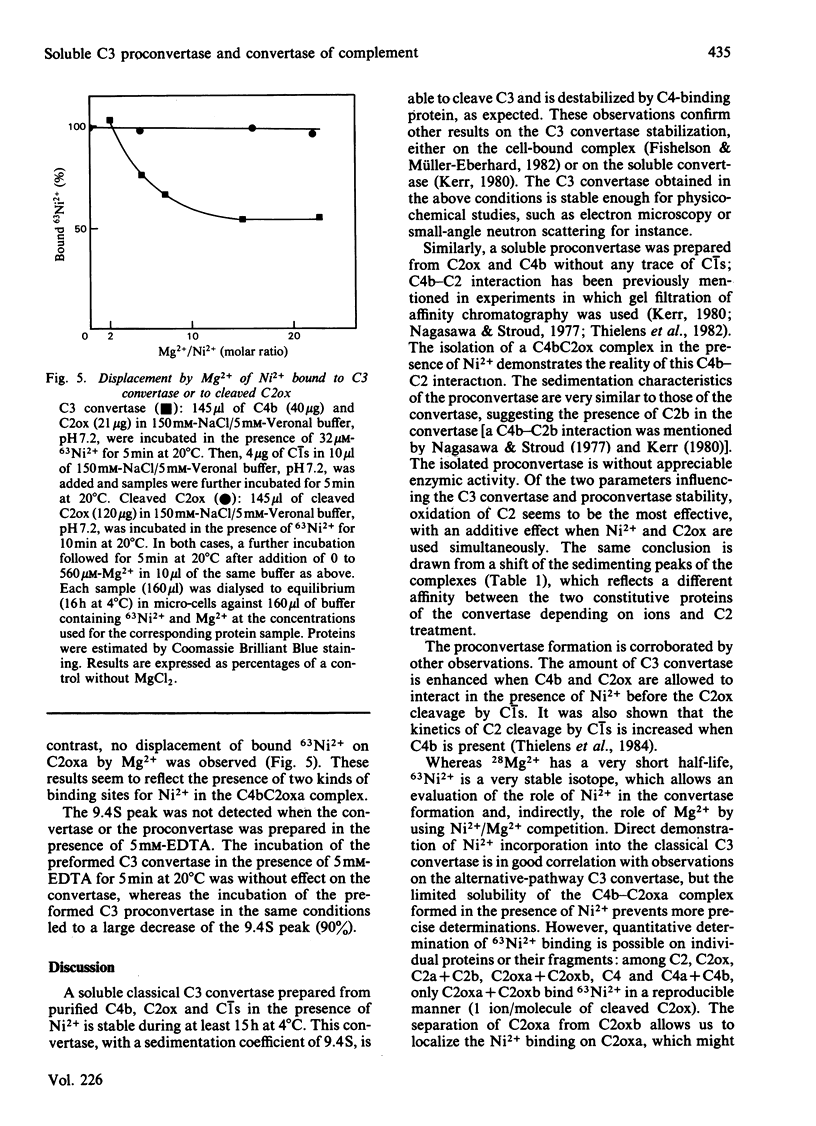
Soluble classical-pathway C3 convertase and proconvertase were prepared from purified C4b-C2ox complex in the presence of Ni2+; the two complexes, stable for at least 15 h at 4 degrees C, were isolated by sucrose-density-gradient ultracentrifugation. The C3 convertase alone was able to cleave C3, and its decay was accelerated in the presence of C4-binding protein. The individual roles of Ni2+ and I2 treatment of C2 in the stabilization of the complexes seemed to be different and additive. 63Ni2+ binding coupled to h.p.l.c. analysis showed that 63Ni2+ bound only to the C2ox proteolytic fragment a (1 mol/mol) with a Kd of 26 microM. Competition studies between Ni2+ and Mg2+ indicated that only half of the Ni2+ bound to the C3 convertase was removed by Mg2+, whereas, in the same conditions, Ni2+ bound to C2ox proteolytic fragment a was not displaced, suggesting the presence of two sets of sites on the convertase. EDTA prevented the formation of both C3 convertase and proconvertase; EDTA had no effect on the preformed C3 convertase, whereas it dissociated the preformed proconvertase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arlaud G. J., Reboul A., Meyer C. M., Colomb M. G. Purification of proenzymic and activated human C1s free ofC1r. Effect of calcium and ionic strength on activated C1s. Biochim Biophys Acta. 1977 Nov 23;485(1):215–225. doi: 10.1016/0005-2744(77)90208-x. [DOI] [PubMed] [Google Scholar]
- Arlaud G. J., Sim R. B., Duplaa A. M., Colomb M. G. Differential elution of Clq, Clr and Cls from human Cl bound to immune aggregates. Use in the rapid purification of Cl subcomponents. Mol Immunol. 1979 Jul;16(7):445–450. doi: 10.1016/0161-5890(79)90069-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Campbell R. D., Dodds A. W., Porter R. R. The binding of human complement component C4 to antibody-antigen aggregates. Biochem J. 1980 Jul 1;189(1):67–80. doi: 10.1042/bj1890067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R. D., Gagnon J., Porter R. R. Amino acid sequence around the thiol and reactive acyl groups of human complement component C4. Biochem J. 1981 Nov 1;199(2):359–370. doi: 10.1042/bj1990359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Daha M. R., van Es L. A. Relative resistance of the F-42-stabilized classical pathway C3 convertase to inactivation by C4-binding protein. J Immunol. 1980 Nov;125(5):2051–2054. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fishelson Z., Müller-Eberhard H. J. C3 convertase of human complement: enhanced formation and stability of the enzyme generated with nickel instead of magnesium. J Immunol. 1982 Dec;129(6):2603–2607. [PubMed] [Google Scholar]
- Fishelson Z., Müller-Eberhard H. J. The C3/C5 convertase of the alternative pathway of complement: stabilization and restriction of control by lanthanide ions. Mol Immunol. 1983 Mar;20(3):309–315. doi: 10.1016/0161-5890(83)90070-6. [DOI] [PubMed] [Google Scholar]
- Fishelson Z., Pangburn M. K., Müller-Eberhard H. J. C3 convertase of the alternative complement pathway. Demonstration of an active, stable C3b, Bb (Ni) complex. J Biol Chem. 1983 Jun 25;258(12):7411–7415. [PubMed] [Google Scholar]
- Gigli I., Fujita T., Nussenzweig V. Modulation of the classical pathway C3 convertase by plasma proteins C4 binding protein and C3b inactivator. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6596–6600. doi: 10.1073/pnas.76.12.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigli I., Sorvillo J., Mecarelli-Halbwachs L., Leibowitch J. Mechanism of action of the C4 nephritic factor. Deregulation of the classical pathway of C3 convertase. J Exp Med. 1981 Jul 1;154(1):1–12. doi: 10.1084/jem.154.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydey R. P., Patarroyo de Rojas M., Gigli I. A newly described control mechanism of complement activation in patients with mixed cryoglobulinemia (cryoglobulins and complement). J Invest Dermatol. 1980 May;74(5):328–332. doi: 10.1111/1523-1747.ep12543575. [DOI] [PubMed] [Google Scholar]
- Iida K., Nussenzweig V. Complement receptor is an inhibitor of the complement cascade. J Exp Med. 1981 May 1;153(5):1138–1150. doi: 10.1084/jem.153.5.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenman D. E., Kells D. I. Conformational and functional changes in the fourth component of human complement produced by nucleophilic modification and by proteolysis with C1s-. Biochemistry. 1982 Mar 16;21(6):1109–1117. doi: 10.1021/bi00535a001. [DOI] [PubMed] [Google Scholar]
- Kerr M. A. The human complement system: assembly of the classical pathway C3 convertase. Biochem J. 1980 Jul 1;189(1):173–181. doi: 10.1042/bj1890173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurell A. B., Mårtensson U., Sjöholm A. G. C1 subcomponent conplexes in normal and pathological sera studied by crossed immunoelectrophoresis. Acta Pathol Microbiol Scand C. 1976 Dec;84C(6):455–464. doi: 10.1111/j.1699-0463.1976.tb00055.x. [DOI] [PubMed] [Google Scholar]
- Law S. K., Lichtenberg N. A., Holcombe F. H., Levine R. P. Interaction between the labile binding sites of the fourth (C4) and fifth (C5) human complement proteins and erythrocyte cell membranes. J Immunol. 1980 Aug;125(2):634–639. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Polley M. J., Calcott M. A. Formation and functional significance of a molecular complex derived from the second and the fourth component of human complement. J Exp Med. 1967 Feb 1;125(2):359–380. doi: 10.1084/jem.125.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Parkes C., Gagnon J., Kerr M. A. The reaction of iodine and thiol-blocking reagents with human complement components C2 and factor B. Purification and N-terminal amino acid sequence of a peptide from C2a containing a free thiol group. Biochem J. 1983 Jul 1;213(1):201–209. doi: 10.1042/bj2130201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Reboul A., Thielens N., Villiers M. B., Colomb M. G. Purification of human complement subcomponent C4. C4 cleavage by C1s. FEBS Lett. 1979 Jul 1;103(1):156–161. doi: 10.1016/0014-5793(79)81271-5. [DOI] [PubMed] [Google Scholar]
- Reboul A., Thielens N., Villiers M. B., Colomb M. G. Structural changes in C4 produced by cleavage with C1-s. FEBS Lett. 1980 Jun 16;115(1):118–122. doi: 10.1016/0014-5793(80)80739-3. [DOI] [PubMed] [Google Scholar]
- Reboul A., Villiers M. B., Thielens N. M., Colomb M. G. C4 binding to artificial systems. FEBS Lett. 1981 Oct 12;133(1):151–156. doi: 10.1016/0014-5793(81)80493-0. [DOI] [PubMed] [Google Scholar]
- Reid K. B. Proteins involved in the activation and control of the two pathways of human complement. Biochem Soc Trans. 1983 Jan;11(1):1–12. doi: 10.1042/bst0110001. [DOI] [PubMed] [Google Scholar]
- Sim R. B., Porter R. R., Reid K. B., Gigli I. The structure and enzymic activities of the C1r and C1s subcomponents of C1, the first component of human serum complement. Biochem J. 1977 May 1;163(2):219–227. doi: 10.1042/bj1630219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim R. B., Twose T. M., Paterson D. S., Sim E. The covalent-binding reaction of complement component C3. Biochem J. 1981 Jan 1;193(1):115–127. doi: 10.1042/bj1930115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielens N. M., Villiers C. L., Villiers M. B., Colomb M. G. Comparative study of the fluid-phase proteolytic cleavage of human complement subcomponents C4 and C2 by C1s and C1r2-C1s2. FEBS Lett. 1984 Jan 2;165(1):111–116. doi: 10.1016/0014-5793(84)80025-3. [DOI] [PubMed] [Google Scholar]
- Thielens N. M., Villiers M. B., Reboul A., Villiers C. L., Colomb M. G. Human complement subcomponent C2: purification and proteolytic cleavage in fluid phase by C1s, C1r2-C1s2 and C1. FEBS Lett. 1982 May 3;141(1):19–24. doi: 10.1016/0014-5793(82)80006-9. [DOI] [PubMed] [Google Scholar]
- Villiers C. L., Duplaa A. M., Arlaud G. J., Colomb M. G. Fluid phase activation of proenzymic C1r purified by affinity chromatography. Biochim Biophys Acta. 1982 Jan 4;700(1):118–126. doi: 10.1016/0167-4838(82)90299-0. [DOI] [PubMed] [Google Scholar]
- Villiers M. B., Reboul A., Thielens N. M., Colomb M. G. Purification and characterization of C4-binding protein from human serum. FEBS Lett. 1981 Sep 14;132(1):49–54. doi: 10.1016/0014-5793(81)80425-5. [DOI] [PubMed] [Google Scholar]
- Vogt W., Hinsch B., Schmidt G., von Zabern I. Function of the activated fourth component of complement (C4b) in activation of C2. FEBS Lett. 1982 Aug 2;144(2):195–198. doi: 10.1016/0014-5793(82)80636-4. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Ziccardi R. J. Activation of the early components of the classical complement pathway under physiologic conditions. J Immunol. 1981 May;126(5):1769–1773. [PubMed] [Google Scholar]



