Abstract
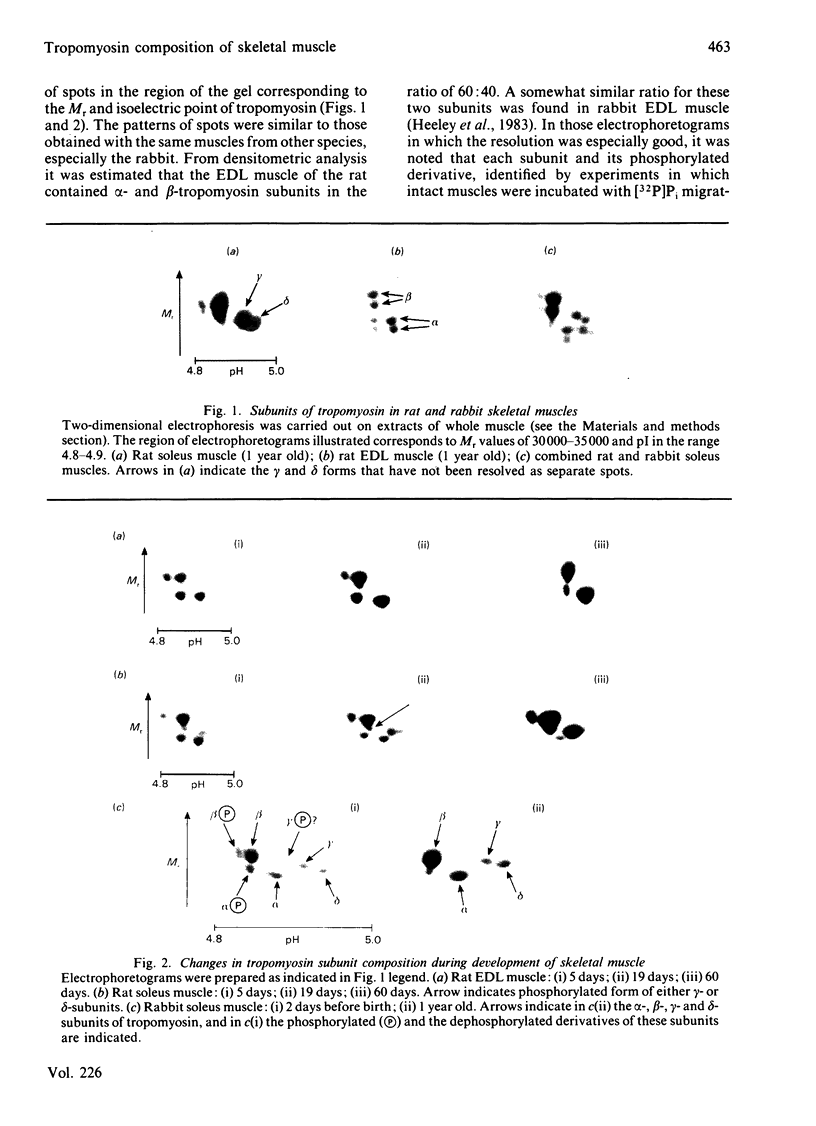
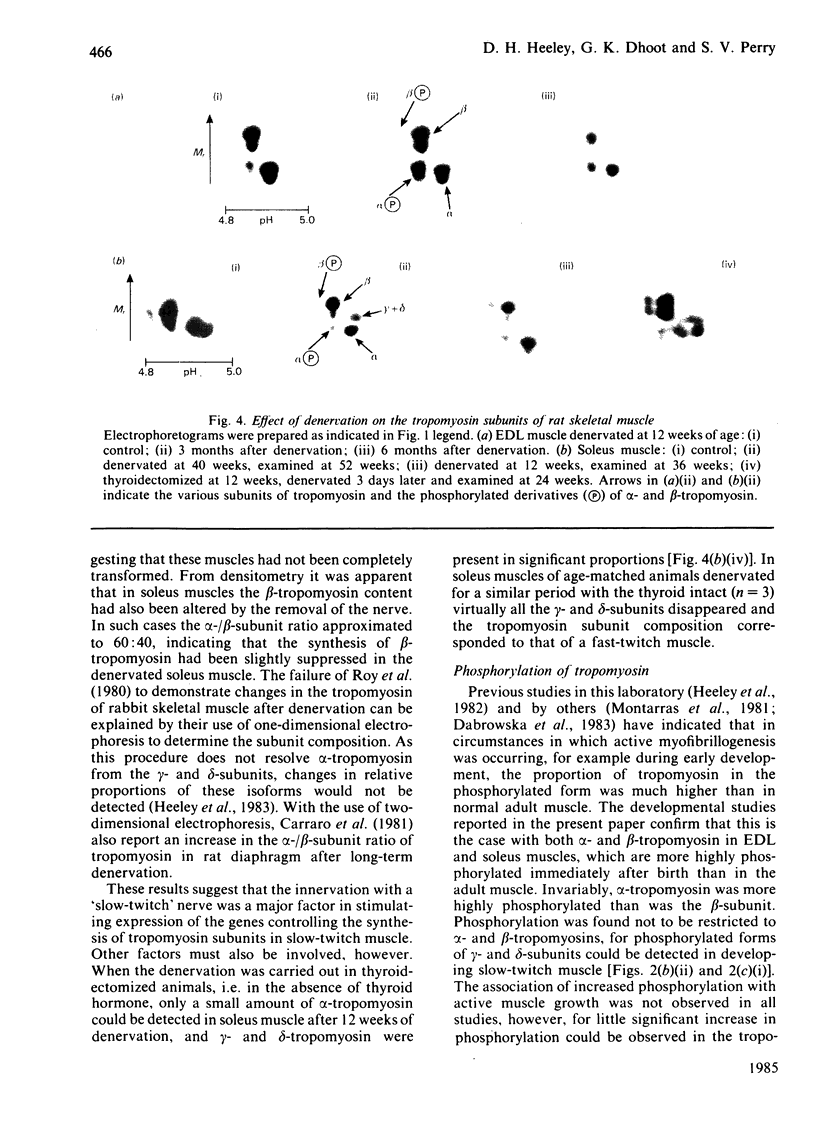
Adult rat fast-twitch skeletal muscle such as extensor digitorum longus contains alpha- and beta-tropomyosin subunits, as is the case in the corresponding muscles of rabbit. Adult rat soleus muscle contains beta-, gamma- and delta-tropomyosins, but no significant amounts of alpha-tropomyosin. Evidence for the presence of phosphorylated forms of at least three of the four tropomyosin subunit isoforms was obtained, particularly in developing muscle. Immediately after birth alpha- and beta-tropomyosins were the major components of skeletal muscle, in both fast-twitch and slow-twitch muscles. Differentiation into slow-twitch skeletal muscles was accompanied by a fall in the amount of alpha-tropomyosin subunit and its replacement with gamma- and delta-subunits. After denervation and during regeneration after injury, the tropomyosin composition of slow-twitch skeletal muscle changed to that associated with fast-twitch muscle. Thyroidectomy slowed down the changes in tropomyosin composition resulting from the denervation of soleus muscle. The results suggest that the 'ground state' of tropomyosin-gene expression in the skeletal muscle gives rise to alpha- and beta-tropomyosin subunits. Innervation by a 'slow-twitch' nerve is essential for the expression of the genes controlling gamma- and delta-subunits. There appears to be reciprocal relationship between expression of the gene controlling the synthesis of alpha-tropomyosin and those controlling the synthesis of gamma- and delta-tropomyosin subunits.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad Z., DePaoli-Roach A. A., Roach P. J. Purification and characterization of a rabbit liver calmodulin-dependent protein kinase able to phosphorylate glycogen synthase. J Biol Chem. 1982 Jul 25;257(14):8348–8355. [PubMed] [Google Scholar]
- Amphlett G. W., Syska H., Perry S. V. The polymorphic forms of tropomyosin and troponin I in developing rabbit skeletal muscle. FEBS Lett. 1976 Mar 15;63(1):22–26. doi: 10.1016/0014-5793(76)80186-x. [DOI] [PubMed] [Google Scholar]
- Anderson N. G., Anderson N. L. Analytical techniques for cell fractions. XXI. Two-dimensional analysis of serum and tissue proteins: multiple isoelectric focusing. Anal Biochem. 1978 Apr;85(2):331–340. doi: 10.1016/0003-2697(78)90229-4. [DOI] [PubMed] [Google Scholar]
- Anderson N. L., Anderson N. G. Analytical techniques for cell fractions. XXII. Two-dimensional analysis of serum and tissue proteins: multiple gradient-slab gel electrophoresis. Anal Biochem. 1978 Apr;85(2):341–354. doi: 10.1016/0003-2697(78)90230-0. [DOI] [PubMed] [Google Scholar]
- Billeter R., Heizmann C. W., Reist U., Howald H., Jenny E. alpha- and beta-tropomyosin in typed single fibers of human skeletal muscle. FEBS Lett. 1981 Sep 14;132(1):133–136. doi: 10.1016/0014-5793(81)80446-2. [DOI] [PubMed] [Google Scholar]
- Carraro U., Catani C., Dalla Libera L., Vascon M., Zanella G. Differential distribution of tropomyosin subunits in fast and slow rat muscles and its change in long-term denervated hemidiaphragm. FEBS Lett. 1981 Jun 15;128(2):233–236. doi: 10.1016/0014-5793(81)80088-9. [DOI] [PubMed] [Google Scholar]
- Cummins P., Perry S. V. Chemical and immunochemical characteristics of tropomyosins from striated and smooth muscle. Biochem J. 1974 Jul;141(1):43–49. doi: 10.1042/bj1410043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins P., Perry S. V. The subunits and biological activity of polymorphic forms of tropomyosin. Biochem J. 1973 Aug;133(4):765–777. doi: 10.1042/bj1330765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska R., Sosiński J., Drabikowski W. Dimerization of the polypeptide chains of skeletal muscle tropomyosin. Biochim Biophys Acta. 1983 Mar 30;743(3):331–337. doi: 10.1016/0167-4838(83)90390-4. [DOI] [PubMed] [Google Scholar]
- Dhoot G. K., Gell P. G., Perry S. V. The localization of the different forms of troponin I in skeletal and cardiac muscle cells. Exp Cell Res. 1978 Dec;117(2):357–370. doi: 10.1016/0014-4827(78)90149-0. [DOI] [PubMed] [Google Scholar]
- Dhoot G. K., Perry S. V. Effect of denervation at birth on the development of skeletal muscle cell types in the rat. Exp Neurol. 1983 Oct;82(1):131–142. doi: 10.1016/0014-4886(83)90248-0. [DOI] [PubMed] [Google Scholar]
- Dhoot G. K., Perry S. V. Effect of thyroidectomy on the distribution of the fast and slow forms of troponin I in rat soleus muscle. FEBS Lett. 1981 Oct 26;133(2):225–229. doi: 10.1016/0014-5793(81)80511-x. [DOI] [PubMed] [Google Scholar]
- Dhoot G. K., Perry S. V. The components of the troponin complex and development in skeletal muscle. Exp Cell Res. 1980 May;127(1):75–87. doi: 10.1016/0014-4827(80)90416-4. [DOI] [PubMed] [Google Scholar]
- Dhoot G. K., Perry S. V., Vrbova G. Changes in the distribution of the components of the troponin complex in muscle fibers after cross-innervation. Exp Neurol. 1981 Jun;72(3):513–530. doi: 10.1016/0014-4886(81)90001-7. [DOI] [PubMed] [Google Scholar]
- Heeley D. H., Dhoot G. K., Frearson N., Perry S. V., Vrbova G. The effect of cross-innervation on the tropomyosin composition of rabbit skeletal muscle. FEBS Lett. 1983 Feb 21;152(2):282–286. doi: 10.1016/0014-5793(83)80396-2. [DOI] [PubMed] [Google Scholar]
- Heeley D. H., Heeley D. A., Moir A. J., Perry S. V. Phosphorylation of tropomyosin during development in mammalian striated muscle. FEBS Lett. 1982 Sep 6;146(1):115–118. doi: 10.1016/0014-5793(82)80716-3. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Beavo J. A., Bechtel P. J., Krebs E. G. Comparison of adenosine 3':5'-monophosphate-dependent protein kinases from rabbit skeletal and bovine heart muscle. J Biol Chem. 1975 Oct 10;250(19):7795–7801. [PubMed] [Google Scholar]
- Kardami E., Montarras D., Fiszman M. Fast and slow chicken skeletal muscles contain different alpha and beta tropomyosins. Biochem Biophys Res Commun. 1983 Jan 14;110(1):147–154. doi: 10.1016/0006-291x(83)91272-x. [DOI] [PubMed] [Google Scholar]
- Matsuda R., Spector D. H., Strohman R. C. Regenerating adult chicken skeletal muscle and satellite cell cultures express embryonic patterns of myosin and tropomyosin isoforms. Dev Biol. 1983 Dec;100(2):478–488. doi: 10.1016/0012-1606(83)90240-3. [DOI] [PubMed] [Google Scholar]
- Montarras D., Fiszman M. Y., Gros F. Changes in tropomyosin during development of chick embryonic skeletal muscles in vivo and during differentiation of chick muscle cells in vitro. J Biol Chem. 1982 Jan 10;257(1):545–548. [PubMed] [Google Scholar]
- Montgomery K., Mak A. S. In vitro phosphorylation of tropomyosin by a kinase from chicken embryo. J Biol Chem. 1984 May 10;259(9):5555–5560. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- PRICE H. M., HOWES E. L., Jr, BLUMBERG J. M. ULTRASTRUCTURAL ALTERATIONS IN SKELETAL MUSCLE FIBERS INJURED BY COLD. I. THE ACUTE DEGENERATIVE CHANGES. Lab Invest. 1964 Oct;13:1264–1278. [PubMed] [Google Scholar]
- Perry S. V., Dhoot G. K., Heeley D. H. Muscle protein isoforms and physiological function: role of nerve in gene expression. Biochem Soc Symp. 1984;49:137–147. [PubMed] [Google Scholar]
- Roy R. K., Sreter F. A., Sarkar S. Changes in tropomyosin subunits and myosin light chains during development of chicken and rabbit striated muscles. Dev Biol. 1979 Mar;69(1):15–30. doi: 10.1016/0012-1606(79)90271-9. [DOI] [PubMed] [Google Scholar]
- Rubinstein N. A., Kelly A. M. Myogenic and neurogenic contributions to the development of fast and slow twitch muscles in rat. Dev Biol. 1978 Feb;62(2):473–485. doi: 10.1016/0012-1606(78)90229-4. [DOI] [PubMed] [Google Scholar]
- Salviati G., Betto R., Danieli Betto D. Polymorphism of myofibrillar proteins of rabbit skeletal-muscle fibres. An electrophoretic study of single fibres. Biochem J. 1982 Nov 1;207(2):261–272. doi: 10.1042/bj2070261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O., Young H. A., Scholnick E. M. Identification of a sarcoma virus-coded phosphoprotein in nonproducer cells transformed by Kirsten or Harvey murine sarcoma virus. Virology. 1979 Jul 15;96(1):64–79. doi: 10.1016/0042-6822(79)90173-9. [DOI] [PubMed] [Google Scholar]
- Stadel J. M., Nambi P., Shorr R. G., Sawyer D. F., Caron M. G., Lefkowitz R. J. Catecholamine-induced desensitization of turkey erythrocyte adenylate cyclase is associated with phosphorylation of the beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3173–3177. doi: 10.1073/pnas.80.11.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach J. H., Schubert D., Eldridge L. Changes in cat muscle contractile proteins after prolonged muscle inactivity. Exp Neurol. 1980 Mar;67(3):655–669. doi: 10.1016/0014-4886(80)90134-x. [DOI] [PubMed] [Google Scholar]
- Wegener A. D., Jones L. R. Phosphorylation-induced mobility shift in phospholamban in sodium dodecyl sulfate-polyacrylamide gels. Evidence for a protein structure consisting of multiple identical phosphorylatable subunits. J Biol Chem. 1984 Feb 10;259(3):1834–1841. [PubMed] [Google Scholar]
- Zoller M. J., Kerlavage A. R., Taylor S. S. Structural comparisons of cAMP-dependent protein kinases I and II from porcine skeletal muscle. J Biol Chem. 1979 Apr 10;254(7):2408–2412. [PubMed] [Google Scholar]






