Abstract
Skin cancer, a prevalent malignancy worldwide, poses significant health concerns owing to its increasing incidence. Autophagy, a natural cellular process, is a pivotal event in skin cancer and has advantageous and detrimental effects. This duality has prompted extensive investigations into medical interventions targeting autophagy modulation for their substantial therapeutic potential. This systematic review aimed to investigate the relationship between skin cancer and autophagy and the contribution and mechanism of autophagy modulators in skin cancer. We outlined the effectiveness and safety of targeting autophagy as a promising therapeutic strategy for the treatment of skin cancer. This comprehensive review identified a diverse array of autophagy modulators with promising potential for the treatment of skin cancer. Each of these compounds demonstrates efficacy through distinct physiological mechanisms that have been elucidated in detail. Interestingly, findings from a literature search indicated that none of the natural, synthetic, or semisynthetic compounds exhibited notable adverse effects in either human or animal models. Consequently, this review offers novel mechanistic and therapeutic perspectives on the targeted modulation of autophagy in skin cancer.
Keywords: Autophagy, Skin cancer, Autophagy inducers, Autophagy inhibitors, Nature-derived compounds, Synthetic compounds
Graphical abstract
Highlights
-
•
Autophagy-targeted therapies are widely explored for skin cancer due to their great potential.
-
•
Autophagy plays a dual role in skin cancer and is responsible for both cancer progression and inhibition.
-
•
Autophagy modulators, including autophagy inducers and inhibitors from natural, semisynthetic, and synthetic sources, are effective against skin cancer.
-
•
Owing to their safety and effectiveness, autophagy modulators can be a useful addition to treat skin cancer.
Introduction
In the United States, skin cancer is a predominant type of cancer, accounting for 40–50 % of all reported cases. It can be broadly categorized into two distinct types: non-melanoma skin cancers (NMSCs), which develop from keratinocytes, and melanoma skin cancers (MSCs), which develop from skin pigment cells called melanocytes. The incidence rate of NMSCs is considerably higher than that of MSCs, but NMSCs have a remarkably low mortality rate. However, MSCs account for approximately 1–4 % of all skin cancer cases and are responsible for approximately 60 % of mortality cases.1
The probability of developing skin cancer is determined by a combination of inherent and external factors. Constitutional risk factors, including family history, red hair color, presence of melanocytic nevi, and sensitivity to sun exposure, exert an influence on susceptibility. Additionally, solar ultraviolet (UV) radiation is a widely recognized environmental risk factor for skin cancer.2 Surgical excision is the primary treatment for skin cancer, and the decision to undergo this intervention is influenced by factors such as patient health, potential tissue reactions, and ability to tolerate multiple treatment sessions.3 Other therapies, such as chemotherapy, immunotherapy, and targeted therapy, are occasionally useful but have certain limitations, such as high cost, increased rate of recurrence, and adverse toxic effects on the patient's body.1,4 Consequently, there is a need to develop new and more efficient methods for the management of skin cancer. Autophagy has recently emerged as a promising pathway that is thought to play a significant role in the progression and treatment of skin cancer.5,6
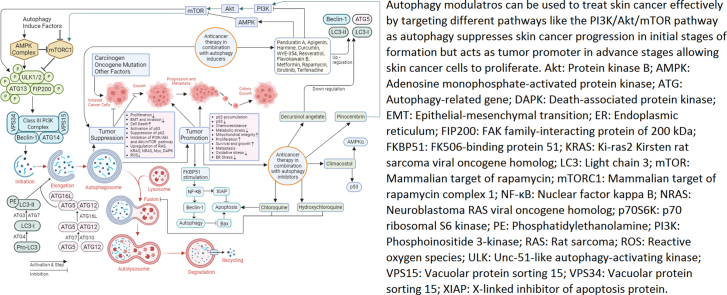
Autophagy is a conserved, intracellular, self-degradative mechanism that performs several functions in maintaining cellular homeostasis. Cellular stress induced by hunger, organelle destruction, or proteotoxic aggregates enhances autophagy, which harnesses the degradation capabilities of lysosomal enzymes to ameliorate intracellular stress.7 Autophagy performs crucial functions in cell survival and maintenance through the degradation of cytoplasmic organelles, proteins, and macromolecules and the recycling of residual products. The malfunction of this mechanism plays a role in the pathology of several human diseases, such as respiratory, neurodegenerative, and cardiovascular diseases, infection, tumors, and cancer.8,9 Owing to the instability of the genome, faulty cell development, and effects of cell stress, chronic suppression or absence of autophagy promotes cancer.10 Numerous studies have demonstrated the involvement of autophagy in cancer. However, the precise effect of autophagy on tumor development remains unclear, and its role as either a tumor suppressor or enhancer has not been fully established, which makes its role in cancer treatment controversial. However, the processes through which autophagy operates relate to cancer being involved in several alterations of autophagy-linked proteins, such as autophagy-related genes (ATGs), p53, Beclin 1, mammalian target of rapamycin (mTOR), and Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS), and autophagy pathways, such as mTOR, phosphoinositide 3-kinase (PI3K), mitogen-activated protein kinase (MAPK), reactive oxygen species (ROS), and nuclear factor kappa B (NF-κB).11
Accelerated autophagy has been demonstrated in both NMSCs and MSCs, where it plays a dual role.12,13 For example, during the initial phases of cancer development, it can inhibit tumor growth by removing dysfunctional cell components and potentially harmful substances. This process aids in halting the propagation of damage, including DNA alteration. In contrast, autophagy can assist cancer cells in surviving under stressful circumstances, including nutrition restriction, oxygen deprivation, and chemotherapeutic drug exposure, and facilitate the advancement of skin cancer by enhancing angiogenesis and the production of new blood vessels that deliver nutrition and oxygen to cancerous cells.14, 15, 16, 17 Autophagy inducers and inhibitors, collectively known as autophagy modulators, have been used to treat skin cancer and found to be comparatively effective and reliable. Although the sources of autophagy modulators are mainly plant-derived phytochemicals, known as natural compounds, a series of semisynthetic and synthetic autophagy modulators have been shown to possess anti-skin cancer activities in various laboratory experiments.
In this review, we aimed to concentrate on and asses the role of autophagy in skin cancer and its potential as an effective drug target for the treatment of skin cancer. To provide a thorough analysis of the findings on autophagy as a prospective drug target instead of conventional skin cancer treatment, we also shed light on the effects and mechanisms of autophagy modulators in various types of skin cancer.
This review focuses on the descriptive interpretation of skin cancer and autophagy and their relationship in the human body and autophagy modulators in skin cancer. Additionally, the clinical significance of certain compounds has been emphasized.
Skin cancer and its types
The skin is composed of two primary layers: the outer layer, known as the epidermis, primarily consisting of keratinocytes, and the dermis, which comprises fibroblasts, mast cells, macrophages, and Langerhans cells. Melanocytes reside within the basal layer of the epidermis and play a crucial role in supplying and transferring melanin to keratinocytes, resulting in skin pigmentation and protection against UV damage.1
Skin cancer is mainly classified into two types based on the cells from which it arises: MSCs (from melanocytes) and NMSCs (from the epidermis). These two major groups account for >90 % of skin cancer cases, whereas other skin tumor types comprise a remarkably minor portion of skin malignancies. NMSCs are divided into two groups, such as basal cell carcinoma (BSC) and squamous cell carcinoma (SCC). Although the prevalence of MSCs is remarkably lower than that of other cell types, they are responsible for more deaths compared to those with other types of skin cancer.18
Melanoma is a fatal and aggressive form of cancer and is the most predominant cancer worldwide, with approximately 325,000 newly reported cases and 57,000 fatalities in 2020.19 A buildup of genetic alterations that activate oncogenes, deactivate tumor suppressor genes, and hinder DNA repair occurs when melanocytes are exposed to UV radiation. This mechanism may result in unchecked melanocyte growth and, ultimately, malignancy.20
All non-melanoma skin malignancies affecting the skin are described using the universal term NMSCs. The term “NMSCs” originally referred to keratinocyte carcinomas, specifically BCC and SCC, considering they account for 99 % of the tumors in this group, particularly epidemiologically.21 SCC is one of the most prevalent solid malignancies and a leading cause of death worldwide. As individuals are increasingly exposed to carcinogens, such as UV radiation from sun exposure, smoking, alcohol intake, and human papillomavirus infection, the incidence rate of SCC is rapidly increasing. SCC develops from the epithelium and can be divided into stratified squamous and non-squamous epithelia, which encompass the epithelia of the skin, esophagus, oral cavity, and airways. Several types of SCCs share histological characteristics, such as the development of keratin pearls, which indicate the presence of squamous differentiation.22 BCC is the most prevalent skin neoplasm and is typically associated with chronic and regular sun exposure. It develops from pluripotent cells of the follicular epithelium, which typically carry p53 gene alterations. Occasionally, BCC is caused by PTCH1 gene mutation, which results in abnormal initiation of the sonic hedgehog signaling pathway.18 BCC is not a fast-growing tumor; however, depending on its subtype, it can be relatively aggressive. It rarely spreads to other organs, and most morbidities are caused by ignored or improperly treated lesions that have recurred. BCC typically affects individuals aged >40 years.23
Basic mechanisms and phases involved in autophagy
Autophagy involves the creation of autophagosomes, which entails enveloping a portion of the cytoplasm along with the organelles and proteins that necessitate degradation within the cells. To maintain cell homeostasis and organelle regeneration, autophagosomes combine with lysosomes to form autophagolysosomes, which break down the contents of the inclusions [Figure 1].8 This dynamic process includes autophagosome formation, autophagosome-lysosome fusion, and degradation of intra-autophagosomal contents by lysosomal hydrolases.25 The basic mechanism of autophagy is complex. The upstream signaling pathway of autophagy includes the mTOR-dependent and mTOR-independent (adenosine monophosphate-activated protein kinase [AMPK], PI3K, Rat sarcoma (Ras)-MAPK, p53, phosphatase and tensin homolog deleted on chromosome 10 [PTEN], endoplasmic reticulum [ER] stress) pathways.8 The PI3-binding proteins, PI3 phosphatases, Rab proteins, the ATG/Unc-51-like autophagy-activating kinase 1 (ULK1) protein-kinase complex, ATG9. ATG2-ATG18 complex, Vps34-ATG6/Beclin 1 class III PI3-kinase complex, and ATG12 and ATG8/light chain 3 (LC3) conjugation systems are some of the proteins and multimolecular complexes that aid the formation of autophagosomes. ATG12 and LC3 conjugations and two ubiquitin-like alterations are necessary for membrane elongation and autophagosome formation.25
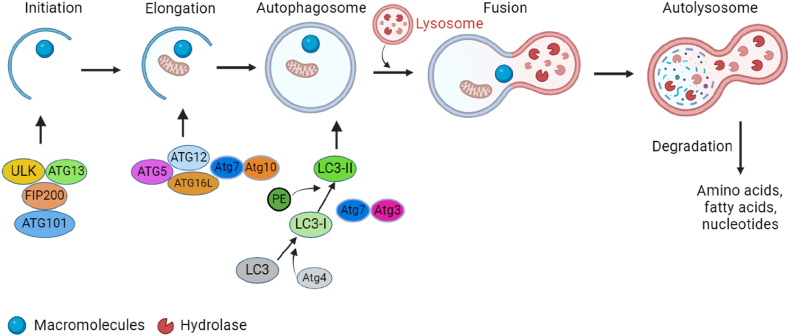
Figure 1.
The five stages of autophagy are initiation, elongation and autophagosome formation, fusion, and autolysosome formation. Targeting macromolecules to double-membrane vesicles known as autophagosomes leads to autolysosome formation by lysosome fusion. The Unc-51-like autophagy-activating kinases 1 (ULK1) complex, which includes ULK, autophagy-related gene (ATG) protein 13, FIP200, and ATG101, triggers autophagy. Two ubiquitin-like conjugation systems, such as the ATG12 and microtubule-associated protein 1A/1B-light chain 3 (LC3) systems, are involved in the elongation and maturation of autophagosomes. An autolysosome is formed by the fusion of the autophagosome with the lysosome. This autolysosome breaks down macromolecules into amino acids, fatty acids, and nucleotides.24 ATG: Autophagy-related gene; FIP200: FAK family-interacting protein of 200 kDa; LC3: Light chain 3; PE: Phosphatidylethanolamine; ULK: Unc-51-like autophagy-activating kinase; ULK1: Unc-51-like autophagy-activating kinase 1.
Autophagy involves five distinct stages: initiation, elongation, maturation, fusion, and degradation. Initially, macromolecules and organelles form cargo and are enclosed by a double-membrane vesicle that slowly expands, finally forming an autophagosome [Figure 1]. Subsequently, the autophagosome fuses with a lysosome, resulting in the emergence of an autolysosome. Within the autolysosome, lysosomal hydrolases break down the cargo, whereas lysosomal permeases recycle the resulting products back into the cytoplasm. The ULK complex, which comprises ULK1/2, ATG13, FAK family-interacting protein of 200 kDa (FIP200), and ATG101, plays a crucial role in the initiation of autophagy. Another key complex required for autophagosomal nucleation is the Beclin-Vps34-ATGq4L-p150 complex.26
Dual role of autophagy in skin cancer
Autophagy has a complex role in cancer [Figure 2] and is influenced by cancer type, stage, genetic background, and tumor microenvironment.28 Autophagy generally plays a tumor-suppressive role in normal cells and benign tumors, but it also helps established cancers survive and become treatment-resistant.29,30 Therefore, the role of autophagy in skin cancer remains controversial. Autophagy acts as a tumor suppressor by reducing the buildup of damaged organelles and regulating cell proliferation and genetic instability.31 Beclin 1+/− mice have increased susceptibility to malignancies32 and show improved angiogenesis, development, and migration of implanted melanoma tumors. Allelic33 loss of the crucial autophagy gene Beclin 1 has been found in 40–75 % of human prostate, breast, and ovarian cancers,34,35 indicating a function for autophagy in tumor suppression. Ironically, autophagy may also aid in the growth of preexisting malignancies by assisting with cellular metabolic demands.36 Knockdown of essential ATGs in tumor cells may enhance the induction of apoptosis.37
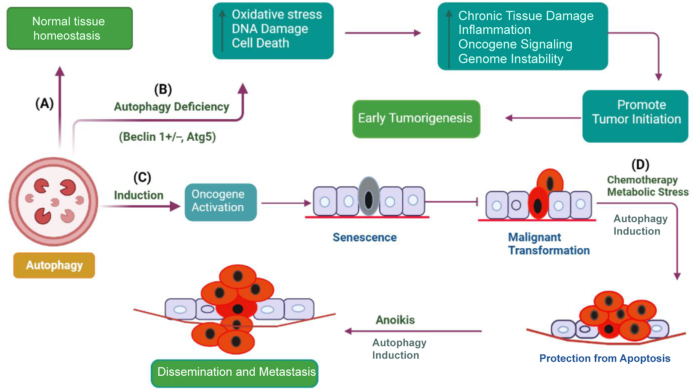
Figure 2.
Multifaceted role of autophagy in cancer progression. (A) Autophagy conducts homeostatic functions in normal tissue, such as monitoring the functionality of organelles and proteins. (B) When autophagy is inhibited in tissues, normal homeostasis is disrupted, which increases inflammation, reactive oxygen species (ROS) levels, and DNA damage (genomic instability and aneuploidy). Taken together, these modifications may encourage the development of tumors and trigger early tumorigenesis.16 (C) Autophagy accelerates oncogene-induced senescence, which inhibits malignant transformation. (D) After several stimuli, such as chemotherapy, metabolic stress, and anoikis, autophagy induction increases tumor cell survival, which may encourage drug resistance and metastasis.27 ROS: reactive oxygen species.
In 2013, Liu et al38 demonstrated that during the early stages of malignant transformation in skin cancers, such as melanoma, autophagy was diminished compared with that in normal melanocytes, correlating with downregulated ATG5. Tumor ATG5 expression was lower than that in benign nevi and normal skin cells. Their study revealed a significant association between ATG5 expression levels in melanoma samples from patients and progression-free survival, indicating that higher ATG5 levels in tumors were associated with a more favorable outcome.
Similarly, increasing ATG5 levels in cultivated tumor cells prevent their growth and lead to senescence.38 Expression of other autophagic markers, such as LC3 and Beclin 1, is also reduced in abnormal melanocytic growth, in addition to ATG5. This indicates decreased autophagy during the early stages of melanoma formation. This phenomenon was also observed by Miracco et al39 in 2010 and Hara and Nakamura40 in 2012. Furthermore, Maes et al41 reported that autophagic flux was significantly reduced in the early stages of melanoma development, with increased protein kinase B (Akt) activity. Unexpectedly, as melanoma progresses, metastatic melanoma cells regain their capacity to trigger autophagic flow, thereby promoting survival.41 This indicates that autophagy plays a tumor-suppressive role in melanocytes during the initial stages of skin cancer formation.
In contrast, skin cancer cells in advanced stages exhibit relatively higher overexpression of Beclin 1 and LC3, indicating higher autophagy compared to that in the initial primary lesions.42 Thus, mature skin cancers with elevated autophagy levels, such as melanoma and SCC, are associated with tumor aggressiveness.43, 44, 45, 46 Established melanoma cells employ autophagy as a protective mechanism to survive in harsh environments. Thus, increased autophagy aids melanoma cell survival and promotes their growth, directly contributing to cancer progression.42 In 2012, Marino et al47 found that melanoma cells, when cultured under acidic conditions, could enhance their survival by elevating autophagic activity. The survival of melanoma cells in acidic environments was reduced when autophagy was inhibited by ATG5 silencing.47 According to a recent study, the flavonoid luteolin can enhance the cell death caused by chloroquine by inhibiting autophagy in metastatic SCC cells. These findings suggest that the upregulation of autophagy acts as a cytoprotective mechanism in squamous cell cancer.48 Therefore, melanomas with high autophagy levels are more likely to withstand treatment than melanomas with low autophagy levels. According to a previous study, radiation stimulated the expression of FK506-binding protein 51 (FKBP51) in malignant melanomas. FKBP51 enhances NF-κB activation, which has two effects: it increases Beclin 1 expression, which triggers autophagy, and it stimulates X-linked inhibitor of apoptosis protein (XIAP), which suppresses apoptosis. Bax is then degraded via autophagy, preventing apoptosis. If FKBP51 treatment is stopped, cells undergo apoptosis, rather than NF-κB activation following radiation therapy.49
From the above discussion, it can be concluded that autophagy plays a tumor-suppressive role in the early stages of skin cancer formation and a tumor-promoting role in the advanced stages of skin cancer. However, how and why autophagy changes its role throughout the process remains controversial, although the autophagic process uses the same cellular machinery in both early and advanced cases of skin cancer. However, because both the induction and inhibition of autophagy are closely related to skin cancer progression and prevention, autophagy can be used as a potential therapeutic target to more effectively treat skin cancer.
Autophagy modulators against skin cancer
Both autophagy inhibitors and inducers, collectively known as autophagy modulators, are useful against skin cancer [Figure 3, Figure 4, Figure 5, Figure 6 and Table 1]. As autophagy in skin cancer aids tumor cell survival in an unfavorable environment, the inhibition of autophagy may be a useful treatment approach for skin cancer that has progressed to an advanced stage. Notably, the use of autophagy inhibitors can potentially induce localized inflammation, which in turn may promote immunogenic cell death and provide tumor-resistant cells the option for apoptosis. In contrast to its function as a defense against chemotherapy, it may also be associated with drug-induced cytotoxicity in malignancies. Several medications with the ability to kill malignant cells may also activate autophagy.1
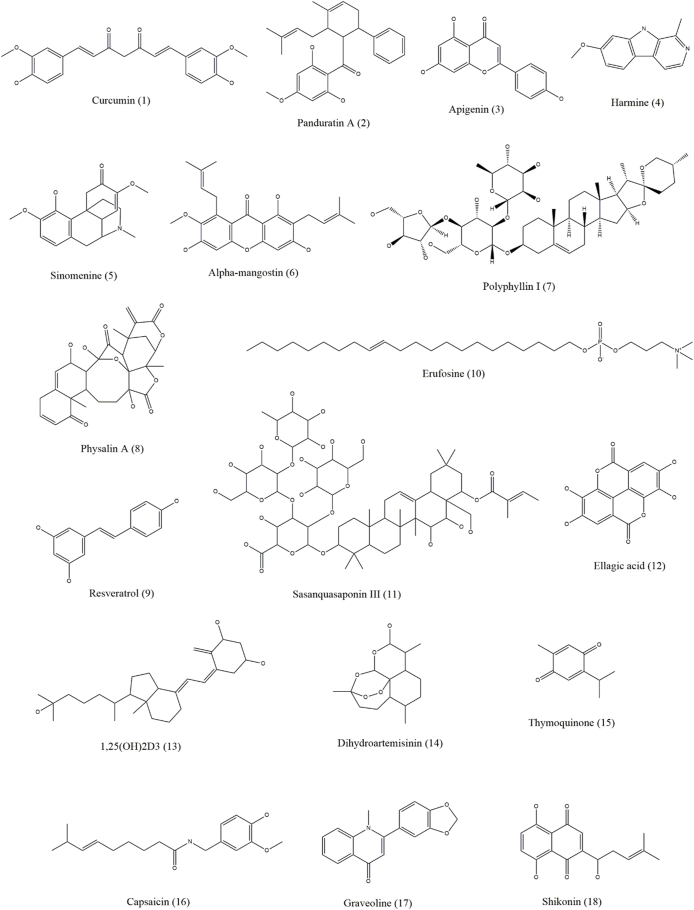
Figure 3.
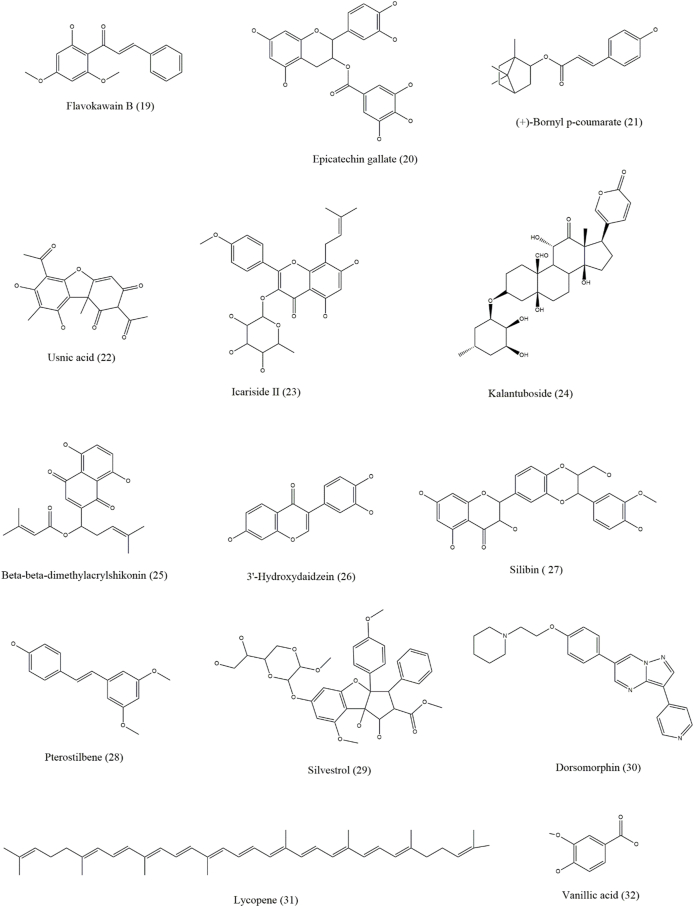
Chemical structures of compounds (1–18) that show efficacy against skin cancer by influencing autophagy.
Figure 4.
Chemical structures of compounds (19–32) that show efficacy against skin cancer by influencing autophagy.
Figure 5.
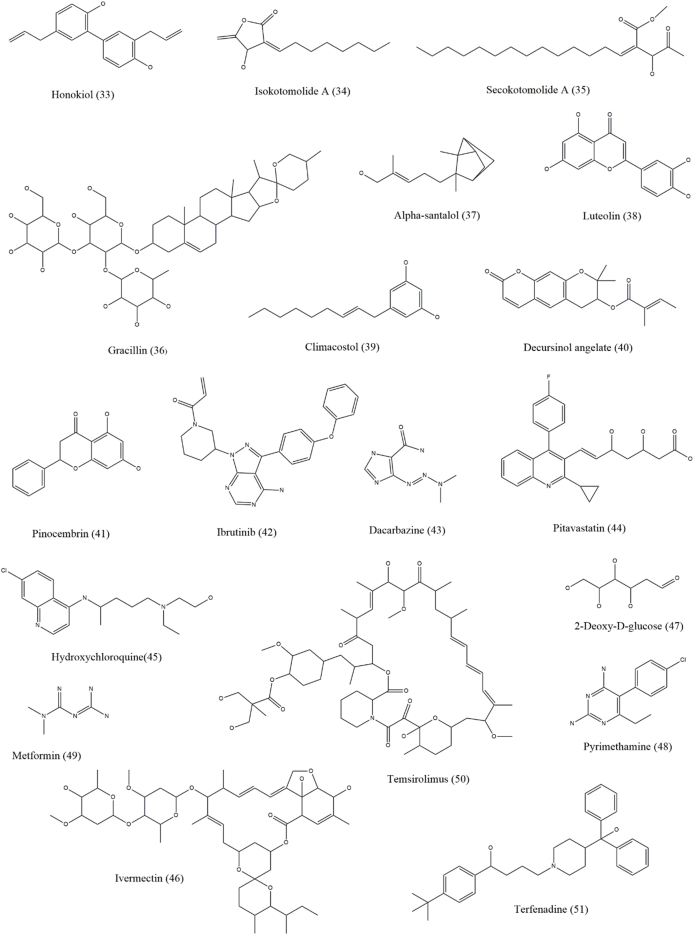
Chemical structures of compounds (33–51) that show efficacy against skin cancer by influencing autophagy.
Figure 6.
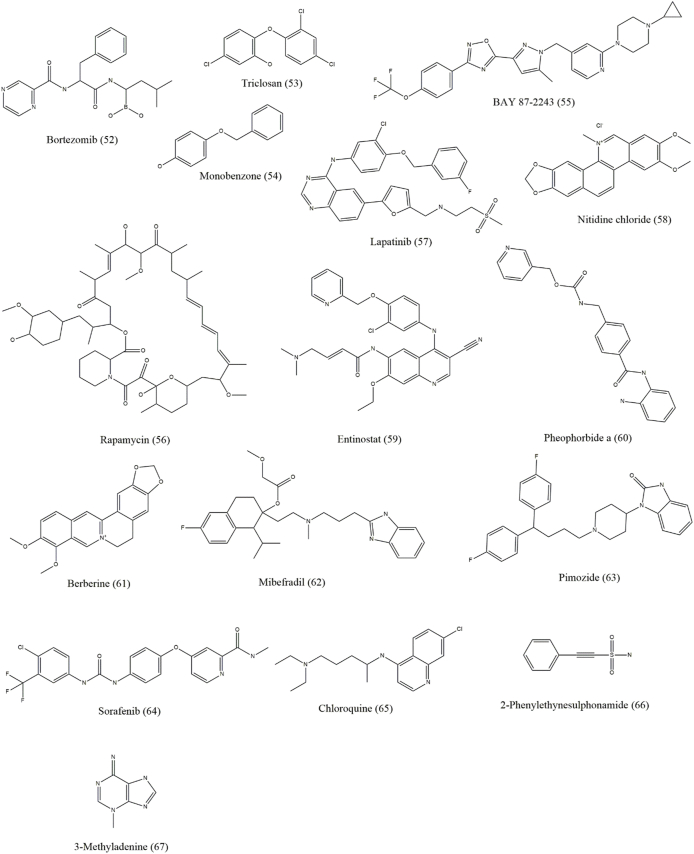
Chemical structures of compounds (53–67) that show efficacy against skin cancer by influencing autophagy.
Table 1.
Role and mechanism of autophagy modulators in various types of skin cancer.
| Compound name | Mechanism of action | Skin cancer types | References |
|---|---|---|---|
| Curcumin (diferuloylmethane) | Downregulation of the Akt/mTOR signaling pathway and ROS generation and upregulation of Beclin 1 | Melanoma | 50 |
| Panduratin A | Downregulation of the mTOR pathway and activation of the AMPK pathway | Melanoma | 51 |
| Apigenin | Downregulation of the mTOR pathway and activation of the AMPK pathway | Keratinocyte carcinoma | 52 |
| Sinomenine | Inhibition of the PI3K/Akt/mTOR pathway and increased expression of Beclin 1 and LC3-II/LC3-I | Melanoma | 53 |
| α-Mangostin | Inhibition of the PI3K/Akt/mTOR pathway and increased expression of Beclin 1, LC3-I, and LC3-II | Melanoma | 54 |
| Polyphyllin I | Inhibition of the PI3K/Akt/mTOR pathway and increased expression of Beclin 1, LC3, and LC3-II | Melanoma | 55 |
| Physalin A | Upregulation of the p38-NF-κB pathway, increased expression of Beclin 1, and regulation of LC3-II/LC3-I | Melanoma | 56 |
| Resveratrol | Modulation of Rictor and suppression of the Akt/mTOR pathway | Squamous cell carcinoma and melanoma | 57,58 |
| Erufosine | Inhibition of the Akt/mTOR pathway | Squamous cell carcinoma | 59 |
| Harmine | Suppression of the Akt/mTOR pathway | Melanoma | 60 |
| Sasanquasaponin III | Inhibition of the Akt/mTOR/p70S6K pathway, ROS accumulation, and upregulation of LC3-II expression | Melanoma | 61 |
| Ellagic acid | Inhibition of the PI3K/Akt/mTOR pathway | Melanoma | 62 |
| Dihydroartemisinin | Increased accumulation of autophagosomes | Tongue squamous cell carcinoma | 63 |
| Thymoquinone | Increased accumulation of autophagosomes | Head and neck squamous cell carcinoma | 64 |
| Protoapigenone | ROS generation and increased p53 and Beclin 1 expression | Melanoma | 65 |
| Graveoline | ROS generation and increased p53 and Beclin 1 expression | Melanoma | 66 |
| Shikonin | ROS generation, activation of the p38 pathway, and increased p-p38, LC3B-II, and Beclin 1 expression | Melanoma | 67 |
| Flavokawain B | ROS generation, mTOR inhibition, and increased LC3-II levels | Melanoma | 68 |
| Novel ECG analog 4-(S)-(2,4,6-trimethylthiobenzyl)-epigallocatechin gallate | ROS accumulation | Melanoma | 69 |
| Usnic acid | ROS generation | Melanoma | 70 |
| Icariside II | ROS generation and inhibition of the MITF, JAK-STAT3, and MAPK pathways | Melanoma | 71,72 |
| Kalantuboside B | ROS generation, p53 downregulation, and LC3-II accumulation | Melanoma | 73 |
| β-β-Dimethylacrylshikonin | ROS generation and increased LC3B-II expression | Melanoma | 74 |
| 3′-Hydroxydaidzein | Up-regulation of ATG5 | Melanoma | 75 |
| Luteolin | Activation of acidic lysosomal vacuolization and accumulation of LC3-II | Squamous cell carcinoma | 75 |
| Silibinin | Inhibition of the IGF-1R pathway and increased Beclin 1 and ATG5 expression | Epidermoid carcinoma | 76 |
| Pterostilbene | Activation of ULK1 and ATG13 by activating the AMPK pathway | Melanoma | 77 |
| Silvestrol | Autophagosome formation and accumulation of LC3-II | Melanoma | 78 |
| Dorsomorphin | ROS generation and LC3-II expression | Basal cell carcinoma | 79 |
| Polygonatum cyrtonema lectin | Regulation of the ROS-p38-p53 pathway | Melanoma | 80 |
| Capsaicin | Increasing ULK1 acetylation and promotion of ROS-dependent autophagy | Melanoma | 81 |
| (+)-Bornyl p-coumarate | Upregulation of the expression of Beclin 1, ATG3, ATG5, p62, LC3-I, and LC3-II proteins | Melanoma | 82 |
| 1,25(OH)2D3 | Increased DDIT4 expression and inhibition of mTORC1 activity to promote autophagy | Squamous cell carcinoma | 83 |
| Lycopene | Increased levels of the autophagy protein p62, causing Keap1 to be degraded through an autophagy-lysosomal pathway | Cutaneous tumor | 84 |
| Vanillic acid | Induction of STAT3-mediated autophagy | Melanoma | 85 |
| Honokiol | Activation of LC3B | Melanoma | 86 |
| Nitidine chloride | Inhibition of the mTOR pathway | Melanoma | 87 |
| Isokotomolide A | Formation of autophagic vacuoles | Melanoma | 88 |
| Secokotomolide A | Formation of autophagic vacuoles | Melanoma | 88 |
| Gracillin | Suppression of phosphorylation/activation of PI3K, Akt, and mTOR | Melanoma | 89 |
| Hibiscus leaf polyphenolic (HLP) extract | Increased expression of ATG5, Beclin 1, and LC3-II | Melanoma | 90 |
| Diethyl ether (Et2O) fraction of Jasione montana extract | Induction of autophagy | Melanoma | 91 |
| Natto | Formation of acidic autophagic vacuoles and autophagolysosomes | Melanoma | 92 |
| East Indian sandalwood oil | Activation of LC3B | Skin cancer | 93 |
| Chinese propolis | Decreased ratio of LC3-I/LC3-II and amplification of ATG5/ATG12 complex and p62 protein | Melanoma | 94 |
| Ibrutinib | Induction of autophagy and inhibition of cell proliferation by concentration and time-dependent manner | Skin cancer | 95 |
| Novel binuclear palladium metallacycle complex with 1,2-bis(dipenylphosphino)ethane | Increased levels of LC3-II and Beclin 1 | Melanoma | 96 |
| Terfenadine | Promotion of autophagy in both ROS-independent and ROS-dependent manner | Melanoma | 97 |
| Bortezomib | Breakdown of LC3 and autophagic formation | Melanoma | 98 |
| Triclosan | Through the AMPK/p62/LC3 pathway | Melanoma | 99 |
| Neratinib and entinostat combination | ROS-dependent activation of ATM via AMPK-ULK1-ATG13-Beclin 1/ATG5 | Melanoma | 100 |
| Monobenzone | Induction of tyrosinase ubiquitination and the autophagocytic degradation of melanosomes | Melanoma | 101 |
| BAY 87-2243 | Promotion of autophagosome formation and mitophagy | Melanoma | 102 |
| HA15 | Increased ER stress, causing the induction of autophagy | Melanoma | 103 |
| Flavonoid GL-V9 | Blockage of the Akt/mTOR pathway | Squamous cell carcinoma | 104 |
| Lapatinib | Inhibition of the PI3K/Akt/mTOR signaling pathway | Squamous cell carcinoma | 105 |
| Temozolomide | Induction of autophagy | Melanoma | 1 |
| Dacarbazine | Induction of autophagy | Melanoma | 1 |
| Dacarbazine and pitavastatin combination | Increased LC3-II expression | Melanoma | 106 |
| Ag/ZnO nanoparticles | Induction of autophagy in response to oxidative stress caused by nanoparticles | Melanoma | 107 |
| Nitrogen-doped titanium dioxide nanoparticles | Induction of autophagy in the dark and inhibiting autophagy in the light | Melanoma | 108 |
| Nitrogen-phosphorous-doped carbon dot | Upregulation of the protein expression levels of LC3-II and ATG-5 | Melanoma | 109 |
| Pheophorbide a | Increased expression of the ATGs Beclin 1, LC3B, and ATG5 | Melanoma | 110 |
| Berberine | Increased level of LC3-related autophagy | Melanoma | 111 |
| 2-Deoxy-d-glucose | Mitochondria hyperpolarization | Melanoma | 112 |
| Pyrimethamine | Increased autophagosome and LC3-II expression | Melanoma | 113 |
| Metformin | Regulation of LC3 and Beclin 1 proteins | Melanoma | 114 |
| Ivermectin | ROS generation | Melanoma | 115 |
| AC-1001-H3 | ROS generation and increased LC3/LC3-II and Beclin 1 expression | Melanoma | 116 |
| Pyr-1 | Enhanced Akt-dependent autophagy | Melanoma | 117 |
| Protoapigenone 1′-O-butyl ether | ROS generation | Melanoma | 65 |
| Rapamycin | Inhibition of the mTOR pathway and increased Bcl-2, Bax, and LC3-II expressions | Melanoma | 118 |
| Hydroxychloroquine | Inhibition of autophagy by preventing the fusion of autophagosomes with lysosomes | Melanoma | 119 |
| Hydroxychloroquine and temsirolimus | Concomitant inhibition of mTOR and autophagy | Melanoma | 106 |
| Climacostol | Inhibition of autophagy by increasing p53 protein levels and activating AMPKα | Melanoma | 120 |
| Decursinol angelate | Inhibition of autophagy by decreasing ATG5, ATG7, Beclin 1, and LC3-II expressions | Melanoma | 121 |
| Pinocembrin (5,7-dihydroxy flavanone) | Inhibition of autophagy via activating the PI3K/Akt/mTOR pathway | Melanoma | 122 |
| Chloroquine | Modification of mTOR-mediated autophagy, causing the inhibition of autophagy | Melanoma | 123 |
| Bafilomycin A1 and ammonium chloride combination | Activation of the p38 MAPK and autophagy inhibition | Melanoma | 124 |
| 3-Methyladenine and 5-fluorouracil | Inhibition of the LC3 gene in cancer cells | Squamous cell carcinoma | 125 |
| Analog of photoactive NADPH NS1 | Selective modification of autophagosome formation and autophagy inhibition at the late stage | Melanoma | 126 |
| Esomeprazole | Increased accumulation of LC3-II and autophagy inhibition | Melanoma | 127 |
| Mibefradil and pimozide | Increased LC3-II/I ratio, accumulation of p62/SQSTM1, and autophagy inhibition | Melanoma | 128 |
| Sorafenib | Inhibition of autophagy | Melanoma | 1 |
| Sorafenib + α-mangostin | α-Mangostin enhances the activity of sorafenib | NRAS-mutant melanoma | 129 |
Akt: Protein kinase B; AMPK: Adenosine monophosphate-activated protein kinase; ATG: Autophagy-related gene; ATM: Ataxia telangiectasia mutated; DDIT4: Deoxyribonucleic acid damage-inducible transcript 4; ECG: Epicatechin gallate; ER: Endoplasmic reticulum; Et2O: Diethyl ether; HLP: Hibiscus leaf polyphenolic; IGF-1R: Insulin-like growth factor 1 receptor; JAK: Janus kinase; LC3: Light chain 3; MAPK: Mitogen-activated protein kinase; MITF: Microphthalmia-associated transcription factor; mTOR: Mammalian target of rapamycin; mTORC1: Mammalian target of rapamycin complex 1; NADPH: Nicotinamide adenine dinucleotide phosphate; NF-κB: Nuclear factor kappa B; NRAS: Neuroblastoma RAS viral oncogene homolog; p70S6K: p70 ribosomal S6 kinase; PI3K: Phosphoinositide 3-kinase; ROS: Reactive oxygen species; STAT3: Signal transducer and activator of transcription 3; ULK1: Unc-51-like autophagy-activating kinase 1.
Natural compounds and extracts inducing autophagy in skin cancer
Panduratin A, apigenin, harmine, sinomenine, curcumin, resveratrol, erufosine, WYE-354, and α-mangostin are some of the natural compounds involved in inducing autophagy in skin cancer by targeting the PI3K/Akt/mTOR pathway. Curcumin (diferuloylmethane) is an active polyphenolic compound found in the rhizome of Curcuma longa that induces autophagy in human melanoma cells by downregulating the Akt/mTOR signaling pathway and ROS generation and upregulating Beclin 1.50 Panduratin A is a natural chalcone obtained from the rhizome of Boesenbergia rotunda that induces autophagy by downregulating the mTOR pathway and activating the AMPK pathway in MSC.51 This mechanism has also been observed for apigenin, which induces autophagy in keratinocytes, including basal and squamous cells.52 Harmine is an alkaloid found in Peganum harmala seeds that suppresses the Akt/mTOR pathway to activate autophagy in B16 cells and is a possible therapeutic agent for MSC.60 Sinomenine, which is a plant alkaloid obtained from the root of Sinomenium acutum, enhances autophagy in B16 melanoma cells by inhibiting the PI3K/Akt/mTOR pathway and increasing the expression of Beclin 1 and LC3-II/LC3-I.53 Inhibition of the PI3K/Akt/mTOR pathway to enhance autophagy and overexpression of Beclin 1, LC3, and LC3-II are also observed in α-mangostin and polyphyllin I in melanoma cells.54,55 Physalin A, a steroidal compound of physalis plants, induces autophagy in melanoma A375-S2 cells by upregulating the p38-NF-κB pathway and activating the expression of Beclin 1 and regulating LC3-II/LC3-I proportions.56 Resveratrol (trans-3,5,4′-trihydroxystilbene), which is a stilbenoid phenolic compound found in berries, grapes, and peanuts, induces autophagy by modulating Rictor (a component of mammalian target of rapamycin complex [mTORC]2) in squamous carcinoma cells57 and activates autophagy in B16 melanoma cells by accumulating ceramide and suppressing the Akt/mTOR pathway.58 Erucylphospho-N,N,N-trimethylpropylammonium (Erufosine) belongs to the alkylphosphocholine class and enhances autophagy in SCC by inhibiting the Akt/mTOR pathway.59 Sasanquasaponin III is a triterpenoid saponin obtained from Schima crenata Korth that induces autophagy in melanoma A375 cells by Akt/mTOR/p70 ribosomal S6 kinase (p70S6K) pathway inhibition, ROS accumulation, and LC3-II expression upregulation.61 Ellagic acid, a polyphenol obtained from the hydrolysis of ellagitannins, induces autophagy in melanocytes by downregulating the phosphorylation of PI3K/Akt and the mTOR pathway.62 An active form of vitamin D, 1,25(OH)2D3 has an important protective role against SCC through autophagy. Treatment with 1,25(OH)2D3 increases DNA damage-inducible transcript 4 (DDIT4) expression, inhibits mTORC1 activity to promote autophagy and induces an antiproliferative reaction in SCC cells. Additionally, according to a previous study, after receiving 1,25(OH)2D3, there was a notable reduction in the size and expansion of the tumor, and the tumor-suppressive effects were dependent on vitamin D-DDIT4 interaction.83 Dihydroartemisinin, which is an active metabolite of artemisinin isolated from Artemisia annua, and thymoquinone obtained from Nigella sativa show increased accumulation of autophagosomes and induction of autophagy in human tongue SSC (with increased Beclin 1 and LC3B-II levels) and head and neck SCC (with increased LC3-II protein levels), respectively.63,64
Some compounds, such as capsaicin, protoapigenone, shikonin, graveoline, flavokawain B, epigallocatechin gallate (EGCG), epicatechin gallate (ECG), usnic acid, icariside II, and kalantuboside B, induce autophagy in skin cancer by regulating and accumulating ROS or by increasing the expression of autophagy-related proteins. Capsaicin is a bioactive compound found in chili peppers and is derived from plants of the genus Capsicum, the most widely used chili worldwide. Capsaicin directly inhibits cellular tumor-associated nicotinamide adenine dinucleotide (NADH) oxidase (tNOX), leading to a concurrent decrease in silent mating type information regulation 2 homolog 1 (SIRT1) expression. In melanoma cells, this results in increased ULK1 acetylation, which promotes ROS-dependent autophagy. Furthermore, in an in vivo xenograft study employing tNOX-depleted melanoma cells, capsaicin therapy effectively suppressed tumor expansion by reducing tNOX and SIRT1 expression.81 Graveoline (obtained from Ruta graveolens) stimulates ROS generation, is cytotoxic in melanoma A375 cells, and induces autophagy by upregulating p53 and Beclin 1 expression.66 Additionally, autophagy in melanoma A375 cells is induced by shikonin, a naphthoquinone isolated from Lithospermum erythrorhizon, and is involved in ROS-mediated ER stress, activation of p38 pathways, and upregulation of p-p38, LC3B-II, and Beclin 1 expression.67 Flavokawain B, a chalcone, induces ROS-mediated autophagy in melanoma A375 cells by inhibiting mTOR, increasing LC3-II levels, and dysregulating Beclin 1/Bcl-2 levels.68 Moreover, ECG and other polyphenols found at high concentrations in hibiscus leaf polyphenolic (HLP) extract cause human melanoma cell death and autophagy. In A375 cells, HLP and ECG cause the cleavage of caspases, regulation of Bcl-2 family proteins, and activation of Fas/FasL. HLP may increase the expression of ATG5, Beclin 1, LC3-II, and autophagy-related proteins and cause autophagic cell death in A375 cells.90 (+)-Bornyl p-coumarate is a bioactive substance abundantly found in Piper betle stem. It induces autophagy in melanoma cells, as evidenced by the upregulated expression of Beclin 1, ATG3, ATG5, p62, LC3-I, and LC3-II proteins and the inhibition of these proteins by autophagy, preventing 3-methyladenine (3-MA).82 Usnic acid, a supercritical CO2 extract of Usnea barbata, exhibits cytotoxic activity, upregulates ROS production in melanoma B16 cells, and induces autophagy.70 Icariside II is a flavonoid extracted from Herba Epimedii that induces autophagy in melanocytes by activating ROS production and inhibiting the microphthalmia-associated transcription factor (MITF), Janus kinase (JAK)-signal transducer and activator of transcription 3 (STAT3), and MAPK pathways.71,72 Kalantuboside B is a bufadienolide obtained from Kalanchoe tubiflora that induces autophagy in melanoma A2058 cells through ROS generation, p53 downregulation, extracellular signal-regulated kinase (ERK) pathway upregulation, and LC3-II accumulation.73 β-β-Dimethyl acryl shikonin, obtained from the roots of Onosma paniculata, induces autophagy in melanoma cell lines by ROS generation and increases LC3B-II expression.74 3′-Hydroxydaidzein is an ortho-dihydroxyisoflavone that upregulates ATG5 expression to enhance autophagy in melanoma. Luteolin is a flavone that induces autophagy by initiating intracellular acidic lysosomal vacuolization and buildup of LC3-II in SCC.75 Silibinin, a flavonolignan, enhances autophagy in carcinoma A431 cells by inhibiting the insulin-like growth factor 1 receptor (IGF-1R) pathway and upregulating Beclin 1 and ATG5 expression.76 Pterostilbene, an analog of resveratrol, induces autophagy through the activation of ULK1 and ATG13 via the AMPK pathway.77 Silvestrol, obtained from the fruits and twigs of Aglaia foveolata, activates early autophagy by autophagosome formation and the buildup of LC3-II in melanoma cells.78 Dorsomorphin, an AMPK inhibitor, induces autophagy in BSC via ROS generation and conversion of LC3-I to LC3-II.79 Polygonatum cyrtonema lectin (mannose/sialic acid-binding lectin), found in the rhizomes of P. cyrtonema Hua, induces autophagy in melanoma A375 cells by regulating the ROS-p38-p53 pathway.80 Lycopene is a key bioactive compound found in tomatoes. The topical application of lycopene blocks tripropylamine (TPA)-induced intracellular redox disturbances and mouse cutaneous tumors in the growth phase by speeding up nuclear factor erythroid 2-related factor 2 (Nrf2) nuclear localization. This effect may have been caused by an elevation in the levels of the autophagy protein p62, which makes it easier for Keap1 to be degraded via the autophagy-lysosomal pathway.84 Vanillic acid (VA), a benzoic acid derivative, ameliorates obesity and melanoma through the STAT3 pathway in mice. In a melanoma cancer-obesity comorbidity model, oral VA treatment reduced body weight, white adipose tissue weight, and tumor growth. The levels of the corresponding biomarkers confirmed the enhanced levels of autophagy.85 By focusing on Notch signaling, a biphenolic naturally occurring honokiol (HNK) suppresses melanoma stem cells. HNK dramatically reduces the viability, clonogenicity, proliferation, and increased autophagy of melanoma cells. Additionally, HNK substantially and dose-dependently reduces melanosphere development.86 Two naturally occurring compounds, isokotomolide A (Iso A) and secokotomolide A (Sec A), obtained from Cinnamomum kotoense, may be effective against human melanoma, particularly against B16F10, A2058, MeWo, and A375 cells. Autophagy induces and causes DNA damage and cell cycle arrest.88 Gracillin, another natural compound, also induces autophagy in melanoma cells. It suppresses the phosphorylation and activation of PI3K, AKT, and mTOR in melanoma cells.89
Similar to the HLP extract, some natural extracts regulate autophagy in skin cancer. A study found that treatment of spontaneously transformed human keratinocyte cell culture (HaCaT) with East Indian sandalwood oil (EISO), where the main component is α-santol, blocked the advancement of the cell cycle and inhibited the function of AP-1, a protein known to cause skin cancer, in a concentration-dependent manner. Additionally, EISO-treated cells exhibited extensively damaged plasma membranes, which might have caused LC3 cleavage and autophagy induction.93 Natto, a soy product fermented by the bacteria Bacillus subtilis, is used to prepare the natto freeze-drying extract (NFDE) and natto water extract (NWE). Natto is believed to play a significant role in cell death by regulating ROS and autophagy. NFDE and NWE have strong dose-dependent anti-melanoma effects but have little effect on healthy skin cells.92 Another medicinal plant from Belarus, Jasione montana L. (Campanulaceae), and its main active compound luteolin are effective against melanoma. Diethyl ether (Et2O) fraction of the plant extract (JM4), in which luteolin is present in high amounts, can induce autophagy in a dose-dependent manner. JM4 and its main constituents can cause cell cycle arrest and a significant reduction in the mitochondrial membrane potential.91 Chinese propolis (CP) has a strong antitumor effect against different malignancies. According to a previous study, the antiproliferative and anti-inflammatory properties of CP worked together to slow the growth of the human melanoma cell line A375. CP-induced autophagy was also observed in A375 cells. Interestingly, the anticancer activity of CP-treated cells decreased when autophagy was inhibited.94
Natural compounds inhibiting autophagy in skin cancer
Some compounds inhibit autophagy in skin cancer, indicating a dual role of autophagy in skin cancer. Climacostol, from the protozoan Climacostomum virens, inhibits autophagy in melanoma B16-F10 cells by increasing p53 protein levels and activating AMPKα.120 Decursinol angelate is a coumarin compound that inhibits autophagy in melanoma B16-F10 cells by inhibiting ATG5, ATG7, and Beclin 1 expression and LC3-I to LC3-II conversion.121 Pinocembrin (5,7-dihydroxy flavanone) is a naturally occurring flavanone found in CP with diverse medicinal properties. It reduces the growth of melanoma cells (A375 and B16F10) in vitro in a dose-dependent manner, induces ER stress via the inositol-requiring enzyme type 1 (IRE1)/X-box binding protein 1 (Xbp1) pathway, and prompts caspase-12/caspase-4-mediated death in both cell lines. Pinocembrin also inhibits autophagy by activating the PI3K/Akt/mTOR pathway, which acts as a dual mechanism to enhance the pro-apoptotic effects of this compound.122
Synthetic and semisynthetic compounds inducing autophagy in skin cancer
Ibrutinib, a Food and Drug Association (FDA)-approved synthetic medicine (also known as PCI-32765), irreversibly inhibits Bruton's tyrosine kinase.130 A previous study demonstrated that ibrutinib caused skin cancer cells to undergo both autophagy and apoptosis. According to western blot analysis, the ibrutinib concentration and treatment duration were associated with the development of autophagy in skin cancer cells.95 A novel EGCG analog 4-(S)-(2,4,6-trimethylthiobenzyl)-EGCG is a polyphenol isolated from Camellia sinensis L. that selectively induces ROS accumulation and autophagy in melanoma B16-F10 cells.69 Protoapigenone 1′-O-butyl ether, a semisynthetic p-quinol, activates autophagy in melanoma A375 cells by upregulating ROS generation and p53 levels.65 Dacarbazine (DTIC) is also an FDA-approved drug used in the treatment of melanoma; it induces autophagy. However, another study found that pitavastatin and DTIC were more successful than DTIC alone in treating the human melanoma cancer cell lines WM115 and A375. Pitavastatin and DTIC therapy cause synergistic cell death and G1 cell cycle arrest, and pitavastatin and DTIC therapy together activate autophagy as a component of cell death. Another drug temozolomide, which is currently in phase II clinical trial, possesses the same effect as the aforementioned two drugs do. Another combination of hydroxychloroquine and temsirolimus is responsible for inducing autophagy through the mTOR pathway and cancer cell death in human melanoma.1,106 A novel binuclear palladacycle complex (AJ-5) effectively induces autophagy and cell death in melanoma cancer cell lines. In a study on ME1402 and WM1158 melanoma cells, it effectively inhibited the proliferation of both cell types. AJ-5 therapy also stimulates the development of autophagosomes and increases the levels of several autophagy indicators, such as LC3-II and Beclin 1.96 2-Deoxy-d-glucose, a synthetic glucose analog, induces autophagy in melanoma cells.112 Pyrimethamine is a synthetic therapeutic agent that induces autophagy in metastatic melanoma cells by increasing the autophagosome and LC3-II expression levels.113 Metformin, an antidiabetic drug, induces autophagy in melanoma cells by regulating LC3 and Beclin 1 protein levels.114 Ivermectin, a semisynthetic antiparasitic compound, enhances TFE3-dependent autophagy in melanoma cells via the ROS pathway.115 Terfenadine, an H1 histamine receptor antagonist, is a strong inducer of death in melanoma cells by manipulating Ca2+ homeostasis and promoting autophagy in skin melanoma cells in ROS-independent and ROS-dependent manner, according to two separate studies.97 Accordingly, the ROS-mitochondrial dysregulation-associated pathways are involved, at least in part, in the induction of autophagy and death by the proteasome inhibitor bortezomib in the A375 and BLM cell lines.98 Treatment of the antibiotic triclosan with the cell type A375 also causes mitochondrial malfunction. Triclosan induces cytotoxicity and cell death by regulating ROS signaling and promoting autophagy in melanoma A375 cells.99 Monobenzone treatment causes M0508A melanocytes and melanoma cells to undergo autophagocytic destruction of melanosomes and produce ROS. In addition to suppressing cellular pigment synthesis and activating dendritic cells, the vitiligo-causing substance monobenzone stimulates cytotoxic melanoma-reactive T lymphocytes, which subsequently eliminate melanoma in vivo.101 AC-1001-H3, a synthetic peptide, induces autophagy through ROS generation and elevates the levels of LC3/LC3-II and Beclin 1 in melanoma cells.116 Another novel substance, HA15, directly inhibits HSPA5 and increases the unfolded protein response (UPR), causing cancer cells to simultaneously die in vitro and in vivo by inducing autophagy. This substance offers strong evidence in favor of the hypothesis that ER stress inducers can be useful innovative therapeutic approaches for addressing melanoma.103 Lead compound Pyr-1 induces AKT-dependent autophagy in A375 cells.117 Under UV irradiation, Ag/ZnO nanoparticles enhance ROS generation in A375 cells, which in turn cause cytotoxicity, increased autophagic turnover, and cell death through apoptosis.107 Nitrogen-doped titanium dioxide nanoparticles (N-TiO2 NPs) activated by light increase ROS generation, which causes necroptosis and blocks autophagy due to a change in the lysosomal pH. Instead, as a pro-survival strategy in the dark, N-TiO2 NPs increase autophagic flow. Similar to N-P-doped carbon dots, which increase cytotoxicity and induce apoptosis, oxidative stress, and autophagy in B16-F10 cells, these particles exhibit anticancer effects.108,109 Notably, after therapy with BAY 87-2243, a strong inhibitor of mitochondrial complex I, tumor growth is inhibited in vivo. In G361 and SK-Mel 28 cells, it also promotes cell death, increases cellular ROS levels, enhances lipid peroxidation, and decreases glutathione levels, while promoting autophagosome formation and mitophagy.102 Rapamycin promotes autophagy in melanoma cells by blocking the mTOR pathway and increasing the protein levels of Bcl-2, Bax, and LC3-II.118 Lapatinib also inhibits the PI3K/Akt/mTOR signaling pathway to induce autophagy in A431 cells in vitro.105 Additionally, the synthetic flavonoid GL-V9 is developed from the naturally occurring bioactive component wogonin. In the human cutaneous SCC cell line A431 cells, GL-V9 promotes autophagy and stops the progression of chemically produced primary skin cancer in mice by blocking the Akt/mTOR pathway and triggering autophagy.104 Another study was conducted to determine the effect of nitidine chloride (NC) on A375 and WM35 melanoma cancer cells. It induces autophagy in melanoma cells and activates the AMPK-mTOR pathway, which is crucial for autophagy initiation. Additionally, the induction of autophagy by NC may exert a cytoprotective effect on melanoma cells because suppressing autophagy using a 3-MA or AMPK pathway inhibitor significantly enhanced apoptosis and cell death caused by NC.87 In this study, neratinib, an irreversible inhibitor of erythroblastic leukemia viral oncogene homolog (ErbB) 1/2/4, was investigated in uveal melanoma cells along with the histone deacetylase inhibitor entinostat. Although various mechanisms are involved, neratinib and entinostat kill cancer cells by enhancing autophagy.100 Delivery of an analog of photoactive nicotinamide adenine dinucleotide phosphate (NADPH) NS1 in A375, SK-Mel 28, and primary melanoma cells promotes cancer cell death by inhibiting NADPH oxidase (NOX). Although autophagy is triggered by this compound, it is only partially complete.126 Pheophorbide a (Pa), a derivative of chlorophyll, is a photosensitizer that exerts substantial anticancer effects in several cancer cell types. Photodynamic therapy (PDT) effectively reduces the proliferation of the two human skin cancer cell lines, A431 and G361, in a concentration-dependent manner. PDT therapy results in increased expression of the ATGs Beclin 1, LC3B, and ATG5 in A431 cells, but not in G361 cells. According to an in vivo investigation, Pa-PDT significantly increased autophagy and/or apoptosis in tumors that had been transplanted with A431 or G361 cells, respectively.110 Moreover, berberine-mediated PDT (BBR-PDT) is effective against human melanoma cells. BBR-PDT increases LC3-related autophagy. Additionally, it induces ER stress, resulting in a sharp increase in ROS. Interestingly, BBR-PDT-induced apoptosis, autophagy, and ER stress levels are decreased by C/EBP homologous protein (CHOP) production, indicating that CHOP is possibly associated with apoptosis, autophagy, and ER stress in MMCs treated with BBR-PDT.111 According to another study, T-type Ca2+ channel blockers, such as mibefradil and pimozide, could treat melanoma cells in which basal autophagy was observed. Thus, they inhibit autophagy and promote cell death in malignant melanoma cells.128
Synthetic and semisynthetic compounds inhibiting autophagy in skin cancer
Sorafenib, an FDA-approved drug and multi-kinase inhibitor, inhibits autophagy during melanoma treatment.1 Natural compound α-mangostin enhances the activity of sorafenib and synergistic effects in the suppression of autophagy by inhibiting Akt, and ERK is observed in neuroblastoma RAS viral oncogene homolog-mutant melanoma cells.129 Hydroxychloroquine is a therapeutic compound that inhibits autophagy by blocking autophagosome–lysosome fusion in melanoma cells.131 Chloroquine also acts as a lysosomal autophagy inhibitor and kills melanoma cells in an autophagy-independent manner. In addition to chloroquine, the lysosomal autophagy inhibitors bafilomycin A1 and ammonium chloride possess the same effect in B16-F0 cells exposed to high levels of oxidative stress and autophagy-independent mitochondrial depolarization, which together cause caspase-mediated death.124 2-Phenylethynesulfonamide in combination with NVP-AUY922 also inhibits autophagy by increasing glutathione levels in melanoma cells.43 Additionally, esomeprazole induces ROS-dependent cell death in Me30966, Mel501, and WM793 cell lines by inhibiting autophagy, one of the adverse modes of action of the drug. Through a caspase-dependent mechanism, it kills melanoma cells while decreasing autophagic flow.127 3-MA is a known autophagy inhibitor that has a synergistic effect against SSC in combination with 5-fluorouracil. This combination inhibits the proliferation and metastasis of cancer cells. LC3 expression is negatively associated with Bcl-2 expression and/or survival, confirming autophagy in SCC.125
Limitations and future prospects
In this review, we systematically examined the involvement of autophagy in skin cancer and evaluated the efficacy of autophagy modulators as potential treatments. However, this study has some limitations. First, the precise role of autophagy in cancer remains debatable, necessitating further experiments to comprehensively elucidate its functions and signaling pathways in the context of skin cancer. Second, considering that autophagy is a ubiquitous metabolic process in human physiology, the safety profile of autophagy modulators for the treatment of skin cancer warrants careful investigation. Furthermore, although this study explored promising autophagy modulators identified in vitro and in vivo studies, their effectiveness must be rigorously validated in human models for translation.
Conclusion
Skin cancer remains one of the major health problems to be solved, and alternative targeted therapies have been explored owing to their optimum safety, effectiveness, and potential. Autophagy is considered one of these factors as it plays a dual role in skin cancer. Although the exact role of autophagy in skin cancer remains controversial, it plays an important role throughout cancer development, making it a crucial target for understanding and effectively treating skin cancer. In the initial stages, autophagy suppresses tumor progression and prevents the progression of cancer to advanced stages. However, when cancer reaches advanced stages, autophagy helps cancer cells survive and grow exponentially. Thus, autophagy inducers and inhibitors, collectively referred to as autophagy modulators, can be used to inhibit skin cancer progression and effectively reduce cancer cell growth. Based on our current understanding, this review provides the first comprehensive examination of a clinical approach for targeting autophagy and the role of autophagy modulators in the treatment of skin cancer. Based on our findings, both the promotion and suppression of autophagy are beneficial in addressing skin cancer. Particularly, our study delineated the involvement of autophagy-related molecular pathways, including the AMPK and mTOR pathways, in the regulation of skin cancer using natural, semisynthetic, and synthetic molecules within the human body. Further investigations are necessary to fully understand the role of autophagy at different stages of skin cancer and the effectiveness of autophagy modulators in treating skin cancer.
Funding
None.
Authors contribution
Md. Liakot Ali: conceptualization, writing – original draft, data extraction, and data analysis; Amdad Hossain Roky: writing – original draft and data extraction; S.M. Asadul Karim Azad: writing – original draft; Abdul Halim Shaikat: writing – original draft; Jannatul Naima Meem: writing – original draft; Emtiajul Hoque: writing – original draft; Abu Mohammed Fuad Ahasan: writing – original draft; Mohammed Murshedul Islam: writing – original draft; Md. Saifur Rahaman Arif: writing – original draft; Md. Saqline Mostaq: writing – original draft; Md. Zihad Mahmud: writing – original draft; Mohammad Nurul Amin: project administration; Md. Ashiq Mahmud: conceptualization, supervision, and manuscript revision. All authors reviewed and approved the submission of the final version of the manuscript.
Ethics statement
None.
Data availability statement
The datasets used in this study can be obtained from the corresponding author upon reasonable request.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
None.
Managing Editor: Peng Lyu
Contributor Information
Mohammad Nurul Amin, Email: amin.pharma07@gmail.com.
Md. Ashiq Mahmud, Email: mahmudashiq0@gmail.com.
References
- 1.Rahmati M., Ebrahim S., Hashemi S., Motamedi M., Moosavi M.A. New insights on the role of autophagy in the pathogenesis and treatment of melanoma. Mol Biol Rep. 2020;47:9021–9032. doi: 10.1007/s11033-020-05886-6. [DOI] [PubMed] [Google Scholar]
- 2.Wu S., Han J., Laden F., Qureshi A.A. Long-term ultraviolet flux, other potential risk factors, and skin cancer risk: a cohort study. Cancer Epidemiol Biomarkers Prev. 2014;23:1080–1089. doi: 10.1158/1055-9965.EPI-13-0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cullen J.K., Simmons J.L., Parsons P.G., Boyle G.M. Topical treatments for skin cancer. Adv Drug Deliv Rev. 2020;153:54–64. doi: 10.1016/j.addr.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Simões M.C.F., Sousa J.J.S., Pais A.A.C.C. Skin cancer and new treatment perspectives: a review. Cancer Lett. 2015;357:8–42. doi: 10.1016/j.canlet.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Guo Y., Zhang X., Wu T., Hu X., Su J., Chen X. Autophagy in skin diseases. Dermatology. 2019;235:380–389. doi: 10.1159/000500470. [DOI] [PubMed] [Google Scholar]
- 6.Liu H., He Z., Simon H.U. Targeting autophagy as a potential therapeutic approach for melanoma therapy. Semin Cancer Biol. 2013;23:352–360. doi: 10.1016/j.semcancer.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Russell R.C., Guan K.L. The multifaceted role of autophagy in cancer. EMBO J. 2022;41 doi: 10.15252/embj.2021110031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao W., Li J., Yang K., Cao D. An overview of autophagy: mechanism, regulation and research progress. Bull Cancer. 2021;108:304–322. doi: 10.1016/j.bulcan.2020.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Yao R.Q., Ren C., Xia Z.F., Yao Y.M. Organelle-specific autophagy in inflammatory diseases: a potential therapeutic target underlying the quality control of multiple organelles. Autophagy. 2021;17:385–401. doi: 10.1080/15548627.2020.1725377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarzynska J.M. The importance of autophagy regulation in breast cancer development and treatment. Biomed Res Int. 2014;2014 doi: 10.1155/2014/710345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakthivel K.M., Rasmia R.R. Role and regulation of autophagy in cancer. BBA Mol Basis Dis. 2022;1868 doi: 10.1016/j.bbadis.2022.166400. [DOI] [PubMed] [Google Scholar]
- 12.Ávalos Y., Canales J., Bravo-Sagua R., Criollo A., Lavandero S., Quest A.F.G. Tumor suppression and promotion by autophagy. Biomed Res Int. 2014;2014 doi: 10.1155/2014/603980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brech A., Ahlquist T., Lothe R.A., Stenmark H. Autophagy in tumour suppression and promotion. Mol Oncol. 2009;3:366–375. doi: 10.1016/j.molonc.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kardideh B., Samimi Z., Norooznezhad F., Kiani S., Mansouri K. Autophagy, cancer and angiogenesis: where is the link? Cell Biosci. 2019;9:65. doi: 10.1186/s13578-019-0327-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dikic I., Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19:349–364. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- 16.Yun C.W., Lee S.H. The roles of autophagy in cancer. Int J Mol Sci. 2018;19:3466. doi: 10.3390/ijms19113466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaaf M.B., Houbaert D., Meçe O., Agostinis P. Autophagy in endothelial cells and tumor angiogenesis. Cell Death Differ. 2019;26:665–679. doi: 10.1038/s41418-019-0287-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craythorne E., Al-Niami F. Skin cancer. Medicine. 2017;45:431–434. doi: 10.1016/j.mpmed.2017.04.003. [DOI] [Google Scholar]
- 19.Lopes J., Rodrigues C.M.P., Gaspar M.M., Reis C.P. Melanoma management: from epidemiology to treatment and latest advances. Cancers. 2022;14:4652. doi: 10.3390/cancers14194652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linares M.A., Zakaria A., Nizran P. Skin cancer. Prim Care. 2015;42:645–659. doi: 10.1016/j.pop.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Apalla Z., Lallas A., Sotiriou E., Lazaridou E., Ioannides D. Tendencias epidemiológicas en cáncer de piel. Dermatol Pract Concept. 2017;7:1–6. doi: 10.5826/dpc.0702a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sánchez-Danés A., Blanpain C. Deciphering the cells of origin of squamous cell carcinomas. Nat Rev Cancer. 2018;18:549–561. doi: 10.1038/s41568-018-0024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dika E., Scarfì F., Ferracin M., et al. Basal cell carcinoma: a comprehensive review. Int J Mol Sci. 2020;21:5572. doi: 10.3390/ijms21155572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X., Yu D.D., Yan F., et al. The role of autophagy induced by tumor microenvironment in different cells and stages of cancer. Cell Biosci. 2015;5:14. doi: 10.1186/s13578-015-0005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanida I. Autophagosome formation and molecular mechanism of autophagy. Antioxid Redox Signal. 2011;14:2201–2214. doi: 10.1089/ars.2010.3482. [DOI] [PubMed] [Google Scholar]
- 26.Sun K., Deng W., Zhang S., et al. Paradoxical roles of autophagy in different stages of tumorigenesis: protector for normal or cancer cells. Cell Biosci. 2013;3:35. doi: 10.1186/2045-3701-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen N., Debnath J. Autophagy and tumorigenesis. FEBS Lett. 2010;584:1427–1435. doi: 10.1016/j.febslet.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo J.Y., Xia B., White E. Autophagy-mediated tumor promotion. Cell. 2013;155:1216–1219. doi: 10.1016/j.cell.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer. 2012;12:401–410. doi: 10.1038/nrc3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi K.S. Autophagy and cancer. Exp Mol Med. 2012;44:109–120. doi: 10.3858/emm.2012.44.2.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathew R., Karp C.M., Beaudoin B., et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yue Z., Jin S., Yang C., Levine A.J., Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee S.J., Kim H.P., Jin Y., Choi A.M.K., Ryter S.W. Beclin 1 deficiency is associated with increased hypoxia-induced angiogenesis. Autophagy. 2011;7:829–839. doi: 10.4161/auto.7.8.15598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qu X., Yu J., Bhagat G., et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang X.H., Jackson S., Seaman M., et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 36.Degenhardt K., Mathew R., Beaudoin B., et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White E., DiPaola R.S. The double-edged sword of autophagy modulation in cancer. Clin Cancer Res. 2009;15:5308–5316. doi: 10.1158/1078-0432.CCR-07-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu H., He Z., von Rütte T., Yousefi S., Hunger R.E., Simon H.U. Down-regulation of autophagy-related protein 5 (ATG5) contributes to the pathogenesis of early-stage cutaneous melanoma. Sci Transl Med. 2013;5:202ra123. doi: 10.1126/scitranslmed.3005864. [DOI] [PubMed] [Google Scholar]
- 39.Miracco C., Cevenini G., Franchi A., et al. Beclin 1 and LC3 autophagic gene expression in cutaneous melanocytic lesions. Hum Pathol. 2010;41:503–512. doi: 10.1016/j.humpath.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Hara Y., Nakamura M. Overexpression of autophagy-related beclin-1 in advanced malignant melanoma and its low expression in melanoma-in-situ. Eur J Dermatol. 2012;22:128–129. doi: 10.1684/ejd.2011.1562. [DOI] [PubMed] [Google Scholar]
- 41.Maes H., Martin S., Verfaillie T., Agostinis P. Dynamic interplay between autophagic flux and Akt during melanoma progression in vitro. Exp Dermatol. 2014;23:101–106. doi: 10.1111/exd.12298. [DOI] [PubMed] [Google Scholar]
- 42.Klapan K., Simon D., Karaulov A., et al. Autophagy and skin diseases. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.844756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sivridis E., Giatromanolaki A., Karpathiou G., Karpouzis A., Kouskoukis C., Koukourakis M.I. LC3A-positive “stone-like” structures in cutaneous squamous cell carcinomas. Am J Dermatopathol. 2011;33:285–290. doi: 10.1097/DAD.0b013e3181f10de0. [DOI] [PubMed] [Google Scholar]
- 44.Qiang L., Wu C., Ming M., Viollet B., He Y.Y. Autophagy controls p38 activation to promote cell survival under genotoxic stress. J Biol Chem. 2013;288:1603–1611. doi: 10.1074/jbc.M112.415224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lazova R., Camp R.L., Klump V., Siddiqui S.F., Amaravadi R.K., Pawelek J.M. Punctate LC3B expression is a common feature of solid tumors and associated with proliferation, metastasis, and poor outcome. Clin Cancer Res. 2012;18:370–379. doi: 10.1158/1078-0432.CCR-11-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma X.H., Piao S., Wang D., et al. Measurements of tumor cell autophagy predict invasiveness, resistance to chemotherapy, and survival in melanoma. Clin Cancer Res. 2011;17:3478–3489. doi: 10.1158/1078-0432.CCR-10-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marino M.L., Pellegrini P., Di Lernia G., et al. Autophagy is a protective mechanism for human melanoma cells under acidic stress. J Biol Chem. 2012;287:30664–30676. doi: 10.1074/jbc.M112.339127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verschooten L., Barrette K., Van Kelst S., et al. Autophagy inhibitor chloroquine enhanced the cell death inducing effect of the flavonoid luteolin in metastatic squamous cell carcinoma cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0048264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romano S., D'angelillo A., Pacelli R., et al. Role of FK506-binding protein 51 in the control of apoptosis of irradiated melanoma cells. Cell Death Differ. 2010;17:145–157. doi: 10.1038/cdd.2009.115. [DOI] [PubMed] [Google Scholar]
- 50.Zhao G., Han X., Zheng S., et al. Curcumin induces autophagy, inhibits proliferation and invasion by downregulating AKT/mTOR signaling pathway in human melanoma cells. Oncol Rep. 2016;35:1065–1074. doi: 10.3892/or.2015.4413. [DOI] [PubMed] [Google Scholar]
- 51.Lai S.L., Mustafa M.R., Wong P.F. Panduratin A induces protective autophagy in melanoma via the AMPK and mTOR pathway. Phytomedicine. 2018;42:144–151. doi: 10.1016/j.phymed.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 52.Tong X., Smith K.A., Pelling J.C. Apigenin, a chemopreventive bioflavonoid, induces AMP-activated protein kinase activation in human keratinocytes. Mol Carcinog. 2012;51:268–279. doi: 10.1002/mc.20793. [DOI] [PubMed] [Google Scholar]
- 53.Sun Z., Zheng L., Liu X., Xing W., Liu X. Sinomenine inhibits the growth of melanoma by enhancement of autophagy via PI3K/AKT/mTOR inhibition. Drug Des Devel Ther. 2018;12:2413–2421. doi: 10.2147/DDDT.S155798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang F., Ma H., Liu Z., Huang W., Xu X., Zhang X. α-Mangostin inhibits DMBA/TPA-induced skin cancer through inhibiting inflammation and promoting autophagy and apoptosis by regulating PI3K/Akt/mTOR signaling pathway in mice. Biomed Pharmacother. 2017;92:672–680. doi: 10.1016/j.biopha.2017.05.129. [DOI] [PubMed] [Google Scholar]
- 55.Long J., Pi X. Polyphyllin I promoted melanoma cells autophagy and apoptosis via PI3K/Akt/mTOR signaling pathway. Biomed Res Int. 2020;2020 doi: 10.1155/2020/5149417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He H., Zang L.H., Feng Y.S., et al. Physalin A induces apoptosis via p53-Noxa-mediated ROS generation, and autophagy plays a protective role against apoptosis through p38-NF-κB survival pathway in A375-S2 cells. J Ethnopharmacol. 2013;148:544–555. doi: 10.1016/j.jep.2013.04.051. [DOI] [PubMed] [Google Scholar]
- 57.Back J.H., Zhu Y., Calabro A., et al. Resveratrol-mediated downregulation of Rictor attenuates autophagic process and suppresses UV-induced skin carcinogenesis. Photochem Photobiol. 2012;88:1165–1172. doi: 10.1111/j.1751-1097.2012.01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang M., Yu T., Zhu C., et al. Resveratrol triggers protective autophagy through the ceramide/Akt/mTOR pathway in melanoma B16 cells. Nutr Cancer. 2014;66:435–440. doi: 10.1080/01635581.2013.878738. [DOI] [PubMed] [Google Scholar]
- 59.Kapoor V., Zaharieva M.M., Das S.N., Berger M.R. Erufosine simultaneously induces apoptosis and autophagy by modulating the Akt–mTOR signaling pathway in oral squamous cell carcinoma. Cancer Lett. 2012;319:39–48. doi: 10.1016/j.canlet.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 60.Zou N., Wei Y., Li F., Yang Y., Cheng X., Wang C. The inhibitory effects of compound Muniziqi granule against B16 cells and harmine induced autophagy and apoptosis by inhibiting Akt/mTOR pathway. BMC Complement Altern Med. 2017;17:517. doi: 10.1186/s12906-017-2017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liang Q.P., Xu T.Q., Liu B.L., et al. Sasanquasaponin ΙΙΙ from Schima crenata Korth induces autophagy through Akt/mTOR/p70S6K pathway and promotes apoptosis in human melanoma A375 cells. Phytomedicine. 2019;58 doi: 10.1016/j.phymed.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 62.Yang H.L., Lin C.P., Vudhya Gowrisankar Y.V., et al. The anti-melanogenic effects of ellagic acid through induction of autophagy in melanocytes and suppression of UVA-activated α-MSH pathways via Nrf2 activation in keratinocytes. Biochem Pharmacol. 2021;185 doi: 10.1016/j.bcp.2021.114454. [DOI] [PubMed] [Google Scholar]
- 63.Deng S., Shanmugam M.K., Kumar A.P., Yap C.T., Sethi G., Bishayee A. Targeting autophagy using natural compounds for cancer prevention and therapy. Cancer. 2019;125:1228–1246. doi: 10.1002/cncr.31978. [DOI] [PubMed] [Google Scholar]
- 64.Chu S.C., Hsieh Y.S., Yu C.C., Lai Y.Y., Chen P.N. Thymoquinone induces cell death in human squamous carcinoma cells via caspase activation-dependent apoptosis and LC3-II activation-dependent autophagy. PLoS One. 2014;9 doi: 10.1371/journal.pone.0101579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Csekes E., Vágvölgyi M., Hunyadi A., Račková L. Protoflavones in melanoma therapy: prooxidant and pro-senescence effect of protoapigenone and its synthetic alkyl derivative in A375 cells. Life Sci. 2020;260 doi: 10.1016/j.lfs.2020.118419. [DOI] [PubMed] [Google Scholar]
- 66.Ghosh S., Bishayee K., Khuda-Bukhsh A.R. Graveoline isolated from ethanolic extract of Ruta graveolens triggers apoptosis and autophagy in skin melanoma cells: a novel apoptosis-independent autophagic signaling pathway. Phytother Res. 2014;28:1153–1162. doi: 10.1002/ptr.5107. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y., Kang X., Niu G., et al. Shikonin induces apoptosis and prosurvival autophagy in human melanoma A375 cells via ROS-mediated ER stress and p38 pathways. Artif Cells Nanomed Biotechnol. 2019;47:626–635. doi: 10.1080/21691401.2019.1575229. [DOI] [PubMed] [Google Scholar]
- 68.WalyEldeen A.A., Sabet S., El-Shorbagy H.M., Abdelhamid I.A., Ibrahim S.A. Chalcones: promising therapeutic agents targeting key players and signaling pathways regulating the hallmarks of cancer. Chem Biol Interact. 2023;369 doi: 10.1016/j.cbi.2022.110297. [DOI] [PubMed] [Google Scholar]
- 69.Xie J., Yun J.P., Yang Y.N., et al. A novel ECG analog 4-(S)-(2,4,6-trimethylthiobenzyl)-epigallocatechin gallate selectively induces apoptosis of B16-F10 melanoma via activation of autophagy and ROS. Sci Rep. 2017;7 doi: 10.1038/srep42194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zugic A., Jeremic I., Isakovic A., Arsic I., Savic S., Tadic V. Evaluation of anticancer and antioxidant activity of a commercially available CO2 supercritical extract of old man's beard (Usnea barbata) PLoS One. 2016;11 doi: 10.1371/journal.pone.0146342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu J., Xu J., Eksioglu E.A., et al. Icariside II induces apoptosis of melanoma cells through the downregulation of survival pathways. Nutr Cancer. 2013;65:110–117. doi: 10.1080/01635581.2013.741745. [DOI] [PubMed] [Google Scholar]
- 72.Liu X., Li Z., Li M., et al. Icariside II overcomes BRAF inhibitor resistance in melanoma by inducing ROS production and inhibiting MITF. Oncol Rep. 2020;44:360–370. doi: 10.3892/or.2020.7582. [DOI] [PubMed] [Google Scholar]
- 73.Hseu Y.C., Cho H.J., Gowrisankar Y.V., et al. Kalantuboside B induced apoptosis and cytoprotective autophagy in human melanoma A2058 cells: an in vitro and in vivo study. Free Radic Biol Med. 2019;143:397–411. doi: 10.1016/j.freeradbiomed.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 74.Kretschmer N., Deutsch A., Durchschein C., et al. Comparative gene expression analysis in WM164 melanoma cells revealed that β-β-dimethylacrylshikonin leads to ROS generation, loss of mitochondrial membrane potential, and autophagy induction. Molecules. 2018;23:2823. doi: 10.3390/molecules23112823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moosavi M.A., Haghi A., Rahmati M., et al. Phytochemicals as potent modulators of autophagy for cancer therapy. Cancer Lett. 2018;424:46–69. doi: 10.1016/j.canlet.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 76.Liu W., Otkur W., Li L., et al. Autophagy induced by silibinin protects human epidermoid carcinoma A431 cells from UVB-induced apoptosis. J Photochem Photobiol B. 2013;123:23–31. doi: 10.1016/j.jphotobiol.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 77.Chen R.J., Lee Y.H., Yeh Y.L., et al. Autophagy-inducing effect of pterostilbene: a prospective therapeutic/preventive option for skin diseases. J Food Drug Anal. 2017;25:125–133. doi: 10.1016/j.jfda.2016.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen W.L., Pan L., Kinghorn A.D., Swanson S.M., Burdette J.E. Silvestrol induces early autophagy and apoptosis in human melanoma cells. BMC Cancer. 2016;16:17. doi: 10.1186/s12885-015-1988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang S.W., Wu C.Y., Wang Y.T., et al. p53 modulates the AMPK inhibitor compound C induced apoptosis in human skin cancer cells. Toxicol Appl Pharmacol. 2013;267:113–124. doi: 10.1016/j.taap.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 80.Liu B., Cheng Y., Bian H.J., Bao J.K. Molecular mechanisms of Polygonatum cyrtonema lectin-induced apoptosis and autophagy in cancer cells. Autophagy. 2009;5:253–255. doi: 10.4161/auto.5.2.7561. [DOI] [PubMed] [Google Scholar]
- 81.Islam A., Hsieh P.F., Liu P.F., et al. Capsaicin exerts therapeutic effects by targeting tNOX-SIRT1 axis and augmenting ROS-dependent autophagy in melanoma cancer cells. Am J Cancer Res. 2021;11:4199–4219. doi: 10.21203/rs.3.rs-117713/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu Y.J., Su T.R., Chang C.I., Chen C.R., Hung K.F., Liu C. (+)-Bornyl p-coumarate extracted from stem of Piper betle induced apoptosis and autophagy in melanoma cells. Int J Mol Sci. 2020;21:3737. doi: 10.3390/ijms21103737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vishlaghi N., Lisse T.S. Exploring vitamin D signalling within skin cancer. Clin Endocrinol. 2020;92:273–281. doi: 10.1111/cen.14150. [DOI] [PubMed] [Google Scholar]
- 84.Wang S., Wu Y.Y., Wang X., et al. Lycopene prevents carcinogen-induced cutaneous tumor by enhancing activation of the Nrf2 pathway through p62-triggered autophagic Keap1 degradation. Aging (Albany NY) 2020;12:8167–8190. doi: 10.18632/aging.103132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park J., Cho S.Y., Kang J., et al. Vanillic acid improves comorbidity of cancer and obesity through stat3 regulation in high-fat-diet-induced obese and b16bl6 melanoma-injected mice. Biomolecules. 2020;10:1098. doi: 10.3390/biom10081098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaushik G., Venugopal A., Ramamoorthy P., et al. Honokiol inhibits melanoma stem cells by targeting notch signaling. Mol Carcinog. 2015;54:1710–1721. doi: 10.1002/mc.22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang X., Hu M., Li K., Luo F., Zhu H. Nitidine chloride efficiently induces autophagy and apoptosis in melanoma cells via AMPK-mTOR signaling pathway. Pharmazie. 2020;75:440–442. doi: 10.1691/ph.2020.0478. [DOI] [PubMed] [Google Scholar]
- 88.Li J., Chen C.Y., Huang J.Y., et al. Isokotomolide a from Cinnamomum kotoense induce melanoma autophagy and apoptosis in vivo and in vitro. Oxid Med Cell Longev. 2020;2020 doi: 10.1155/2020/3425147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li J.K., Zhu P.L., Wang Y., et al. Gracillin exerts anti-melanoma effects in vitro and in vivo: role of DNA damage, apoptosis and autophagy. Phytomedicine. 2023;108 doi: 10.1016/j.phymed.2022.154526. [DOI] [PubMed] [Google Scholar]
- 90.Chiu C.T., Hsuan S.W., Lin H.H., Hsu C.C., Chou F.P., Chen J.H. Hibiscus sabdariffa leaf polyphenolic extract induces human melanoma cell death, apoptosis, and autophagy. J Food Sci. 2015;80:H649–H658. doi: 10.1111/1750-3841.12790. [DOI] [PubMed] [Google Scholar]
- 91.Juszczak A.M., Czarnomysy R., Strawa J.W., Zovko Končić M., Bielawski K., Tomczyk M. In vitro anticancer potential of Jasione montana and its main components against human amelanotic melanoma cells. Int J Mol Sci. 2021;22:3345. doi: 10.3390/ijms22073345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chou H.Y., Liu L.H., Chen C.Y., et al. Bifunctional mechanisms of autophagy and apoptosis regulations in melanoma from Bacillus subtilis natto fermentation extract. Food Chem Toxicol. 2021;150 doi: 10.1016/j.fct.2021.112020. [DOI] [PubMed] [Google Scholar]
- 93.Dickinson S.E., Olson E.R., Levenson C., et al. A novel chemopreventive mechanism for a traditional medicine: East Indian sandalwood oil induces autophagy and cell death in proliferating keratinocytes. Arch Biochem Biophys. 2014;558:143–152. doi: 10.1016/j.abb.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zheng Y., Wu Y., Chen X., Jiang X., Wang K., Hu F. Chinese propolis exerts anti-proliferation effects in human melanoma cells by targeting NLRP1 inflammatory pathway, inducing apoptosis, cell cycle arrest, and autophagy. Nutrients. 2018;10:1170. doi: 10.3390/nu10091170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sun F.D., Wang P.C., Shang J., Zou S.H., Du X. Ibrutinib presents antitumor activity in skin cancer and induces autophagy. Eur Rev Med Pharmacol Sci. 2018;22:561–566. doi: 10.26355/eurrev_201801_14210. [DOI] [PubMed] [Google Scholar]
- 96.Aliwaini S., Swarts A.J., Blanckenberg A., Mapolie S., Prince S. A novel binuclear palladacycle complex inhibits melanoma growth in vitro and in vivo through apoptosis and autophagy. Biochem Pharmacol. 2013;86:1650–1663. doi: 10.1016/j.bcp.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 97.Nicolau-Galmés F., Asumendi A., Alonso-Tejerina E., et al. Terfenadine induces apoptosis and autophagy in melanoma cells through ROS-dependent and -independent mechanisms. Apoptosis. 2011;16:1253–1267. doi: 10.1007/s10495-011-0640-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Selimovic D., Porzig B.B.O.W., El-Khattouti A., et al. Bortezomib/proteasome inhibitor triggers both apoptosis and autophagy-dependent pathways in melanoma cells. Cell Signal. 2013;25:308–318. doi: 10.1016/j.cellsig.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 99.Jin J., Chen N., Pan H., et al. Triclosan induces ROS-dependent cell death and autophagy in A375 melanoma cells. Oncol Lett. 2020;20:73. doi: 10.3892/ol.2020.11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Booth L., Roberts J.L., Sander C., et al. Neratinib and entinostat combine to rapidly reduce the expression of K-RAS, N-RAS, Gαq and Gα11 and kill uveal melanoma cells. Cancer Biol Ther. 2019;20:700–710. doi: 10.1080/15384047.2018.1551747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Van Den Boorn J.G., Picavet D.I., Van Swieten P.F., et al. Skin-depigmenting agent monobenzone induces potent T-cell autoimmunity toward pigmented cells by tyrosinase haptenation and melanosome autophagy. J Invest Dermatol. 2011;131:1240–1251. doi: 10.1038/jid.2011.16. [DOI] [PubMed] [Google Scholar]
- 102.Basit F., van Oppen L.M., Schöckel L., et al. Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis. 2017;8 doi: 10.1038/cddis.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cerezo M., Rocchi S. New anti-cancer molecules targeting HSPA5/BIP to induce endoplasmic reticulum stress, autophagy and apoptosis. Autophagy. 2017;13:216–217. doi: 10.1080/15548627.2016.1246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhu Y., Liu M., Yao J., Guo Q., Wei L. The synthetic flavonoid derivative GL-V9 induces apoptosis and autophagy in cutaneous squamous cell carcinoma via suppressing AKT-regulated HK2 and mTOR signals. Molecules. 2020;25:5033. doi: 10.3390/molecules25215033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yao M., Shang Y.Y., Zhou Z.W., et al. The research on lapatinib in autophagy, cell cycle arrest and epithelial to mesenchymal transition via Wnt/ErK/PI3K-AKT signaling pathway in human cutaneous squamous cell carcinoma. J Cancer. 2017;8:220–226. doi: 10.7150/jca.16850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Al-Qatati A., Aliwaini S. Combined pitavastatin and dacarbazine treatment activates apoptosis and autophagy resulting in synergistic cytotoxicity in melanoma cells. Oncol Lett. 2017;14:7993–7999. doi: 10.3892/ol.2017.7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ghaemi B., Moshiri A., Herrmann I.K., et al. Supramolecular insights into domino effects of Ag@ZnO-induced oxidative stress in melanoma cancer cells. ACS Appl Mater Interfaces. 2019;11:46408–46418. doi: 10.1021/acsami.9b13420. [DOI] [PubMed] [Google Scholar]
- 108.Mohammadalipour Z., Rahmati M., Khataee A., Moosavi M.A. Differential effects of N-TiO2 nanoparticle and its photo-activated form on autophagy and necroptosis in human melanoma A375 cells. J Cell Physiol. 2020;235:8246–8259. doi: 10.1002/jcp.29479. [DOI] [PubMed] [Google Scholar]
- 109.Bajpai V.K., Khan I., Shukla S., et al. Multifunctional N-P-doped carbon dots for regulation of apoptosis and autophagy in B16F10 melanoma cancer cells and in vitro imaging applications. Theranostics. 2020;10:7841–7856. doi: 10.7150/thno.42291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yoon H.E., Oh S.H., Kim S.A., Yoon J.H., Ahn S.G. Pheophorbide a-mediated photodynamic therapy induces autophagy and apoptosis via the activation of MAPKs in human skin cancer cells. Oncol Rep. 2014;31:137–144. doi: 10.3892/or.2013.2856. [DOI] [PubMed] [Google Scholar]
- 111.Fang J., Huang X., Yang Y., Wang X., Liang X., Liu J. Berberine-photodynamic induced apoptosis by activating endoplasmic reticulum stress-autophagy pathway involving CHOP in human malignant melanoma cells. Biochem Biophys Res Commun. 2021;552:183–190. doi: 10.1016/j.bbrc.2021.02.147. [DOI] [PubMed] [Google Scholar]
- 112.Giammarioli A.M., Gambardella L., Barbati C., et al. Differential effects of the glycolysis inhibitor 2-deoxy-D-glucose on the activity of pro-apoptotic agents in metastatic melanoma cells, and induction of a cytoprotective autophagic response. Int J Cancer. 2012;131:E337–E347. doi: 10.1002/ijc.26420. [DOI] [PubMed] [Google Scholar]
- 113.Tommasino C., Gambardella L., Buoncervello M., et al. New derivatives of the antimalarial drug pyrimethamine in the control of melanoma tumor growth: an in vitro and in vivo study. J Exp Clin Cancer Res. 2016;35:137. doi: 10.1186/s13046-016-0409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tomic T., Botton T., Cerezo M., et al. Metformin inhibits melanoma development through autophagy and apoptosis mechanisms. Cell Death Dis. 2011;2 doi: 10.1038/cddis.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Deng F., Xu Q., Long J., Xie H. Suppressing ROS-TFE3-dependent autophagy enhances ivermectin-induced apoptosis in human melanoma cells. J Cell Biochem. 2019;120:1702–1715. doi: 10.1002/jcb.27490. [DOI] [PubMed] [Google Scholar]
- 116.Rabaça A.N., Arruda D.C., Figueiredo C.R., et al. AC-1001 H3 CDR peptide induces apoptosis and signs of autophagy in vitro and exhibits antimetastatic activity in a syngeneic melanoma model. FEBS Open Bio. 2016;6:885–901. doi: 10.1002/2211-5463.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ghosh S.K., Ganta A., Spanjaard R.A. Discovery and cellular stress pathway analysis of 1,4-naphthoquinone derivatives with novel, highly potent broad-spectrum anticancer activity. J Biomed Sci. 2018;25:12. doi: 10.1186/s12929-018-0408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li X., Wu D.I., Shen J., Zhou M., Lu Y. Rapamycin induces autophagy in the melanoma cell line M14 via regulation of the expression levels of Bcl-2 and Bax. Oncol Lett. 2013;5:167–172. doi: 10.3892/ol.2012.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Corazzari M., Fimia G.M., Lovat P., Piacentini M. Why is autophagy important for melanoma? Molecular mechanisms and therapeutic implications. Semin Cancer Biol. 2013;23:337–343. doi: 10.1016/j.semcancer.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 120.Zecchini S., Proietti Serafini F., Catalani E., et al. Dysfunctional autophagy induced by the pro-apoptotic natural compound climacostol in tumour cells. Cell Death Dis. 2018;10:10. doi: 10.1038/s41419-018-1254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chang S.N., Khan I., Kim C.G., et al. Decursinol angelate arrest melanoma cell proliferation by initiating cell death and tumor shrinkage via induction of apoptosis. Int J Mol Sci. 2021;22:4096. doi: 10.3390/ijms22084096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zheng Y., Wang K., Wu Y., et al. Pinocembrin induces ER stress mediated apoptosis and suppresses autophagy in melanoma cells. Cancer Lett. 2018;431:31–42. doi: 10.1016/j.canlet.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 123.Georgakilas A.G. Oxidative stress, DNA damage and repair in carcinogenesis: have we established a connection? Cancer Lett. 2012;327:3–4. doi: 10.1016/j.canlet.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 124.Stamenkovic M., Janjetovic K., Paunovic V., Ciric D., Kravic-Stevovic T., Trajkovic V. Comparative analysis of cell death mechanisms induced by lysosomal autophagy inhibitors. Eur J Pharmacol. 2019;859 doi: 10.1016/j.ejphar.2019.172540. [DOI] [PubMed] [Google Scholar]
- 125.Zhang L., Zhang J., Chen L., Wang J. Autophagy in human skin squamous cell carcinoma: inhibition by 3-MA enhances the effect of 5-FU-induced chemotherapy sensitivity. Oncol Rep. 2015;34:3147–3155. doi: 10.3892/or.2015.4302. [DOI] [PubMed] [Google Scholar]
- 126.Rouaud F., Boucher J.L., Slama-Schwok A., Rocchi S. Mechanism of melanoma cells selective apoptosis induced by a photoactive NADPH analogue. Oncotarget. 2016;7:82804–82819. doi: 10.18632/oncotarget.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Marino M.L., Fais S., Djavaheri-Mergny M., et al. Proton pump inhibition induces autophagy as a survival mechanism following oxidative stress in human melanoma cells. Cell Death Dis. 2010;1:e87. doi: 10.1038/cddis.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Das A., Pushparaj C., Herreros J., et al. T-type calcium channel blockers inhibit autophagy and promote apoptosis of malignant melanoma cells. Pigment Cell Melanoma Res. 2013;26:874–885. doi: 10.1111/pcmr.12155. [DOI] [PubMed] [Google Scholar]
- 129.Xia Y., Li Y., Westover K.D., et al. Inhibition of cell proliferation in an NRAS mutant melanoma cell line by combining sorafenib and α-mangostin. PLoS One. 2016;11 doi: 10.1371/journal.pone.0155217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Charalambous A., Schwarzbich M.-A., Witzens-Harig M. Ibrutinib. Recent Results Cancer Res. 2018;212:133–168. doi: 10.1007/978-3-319-91439-8_7. [DOI] [PubMed] [Google Scholar]
- 131.Xu R., Ji Z., Xu C., Zhu J. The clinical value of using chloroquine or hydroxychloroquine as autophagy inhibitors in the treatment of cancers: a systematic review and meta-analysis. Medicine. 2018;97 doi: 10.1097/MD.0000000000012912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used in this study can be obtained from the corresponding author upon reasonable request.