Abstract
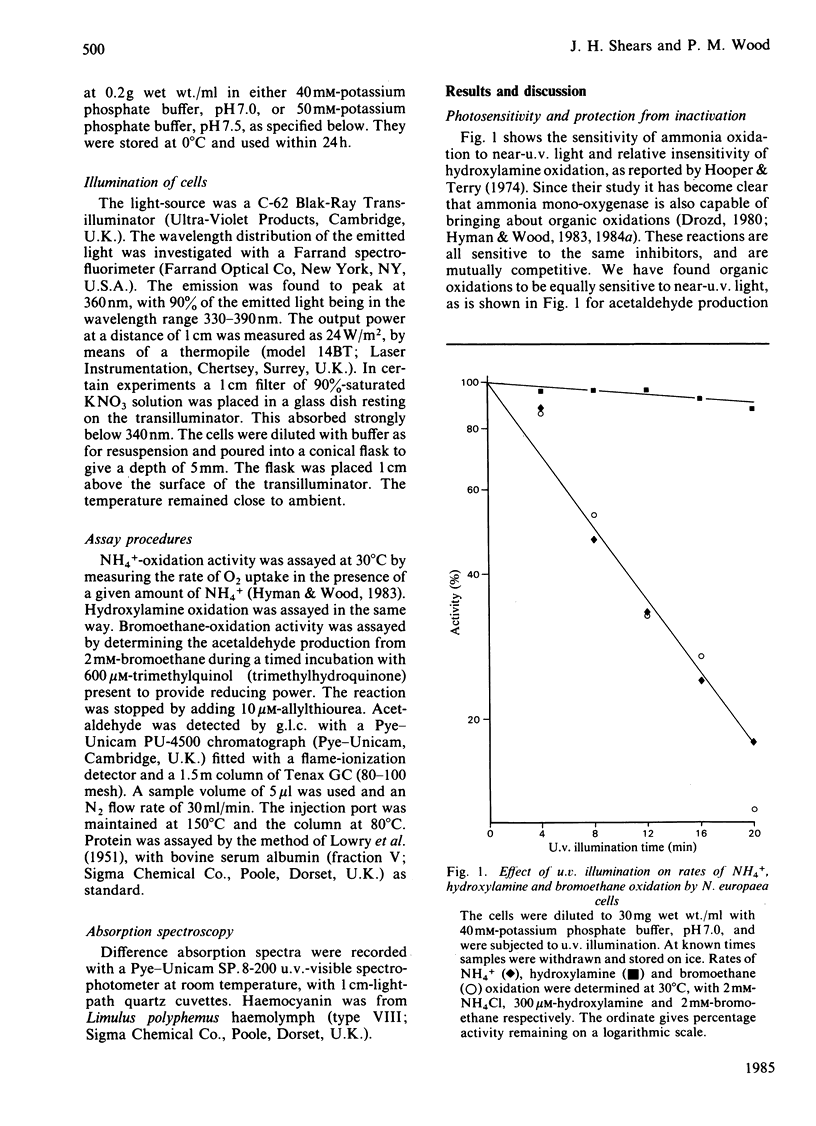
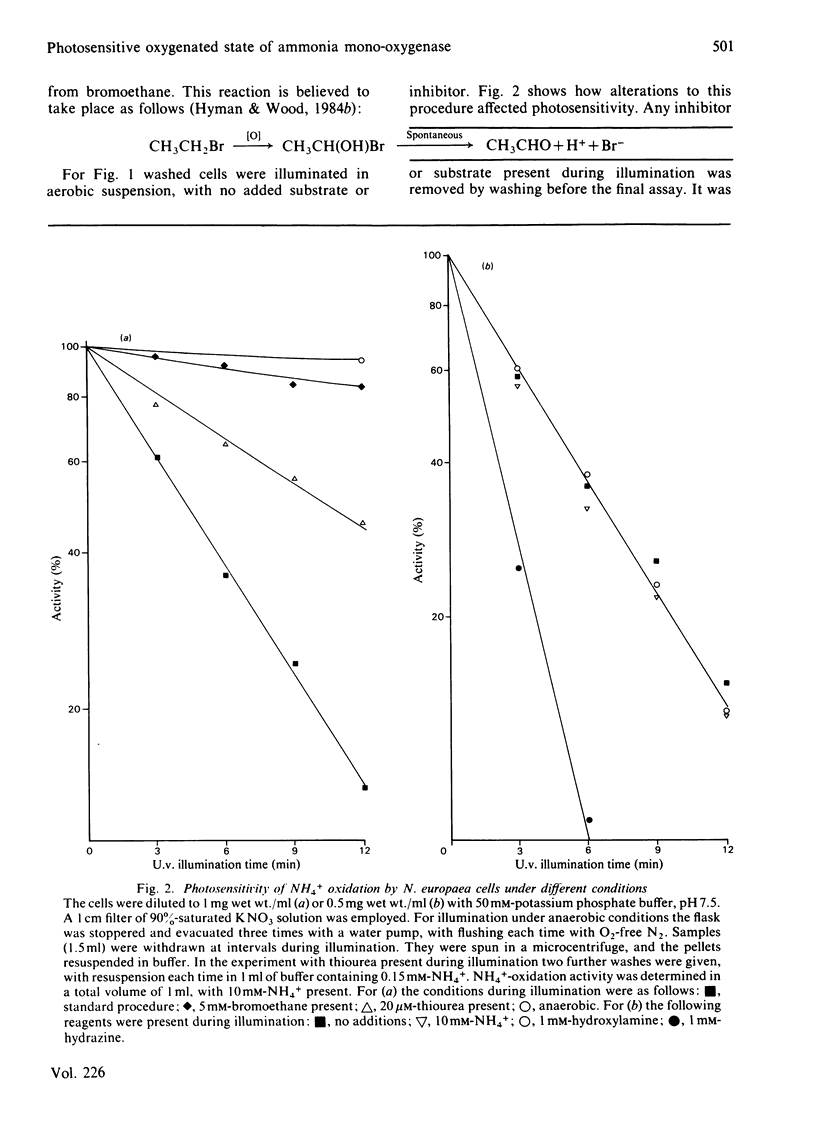
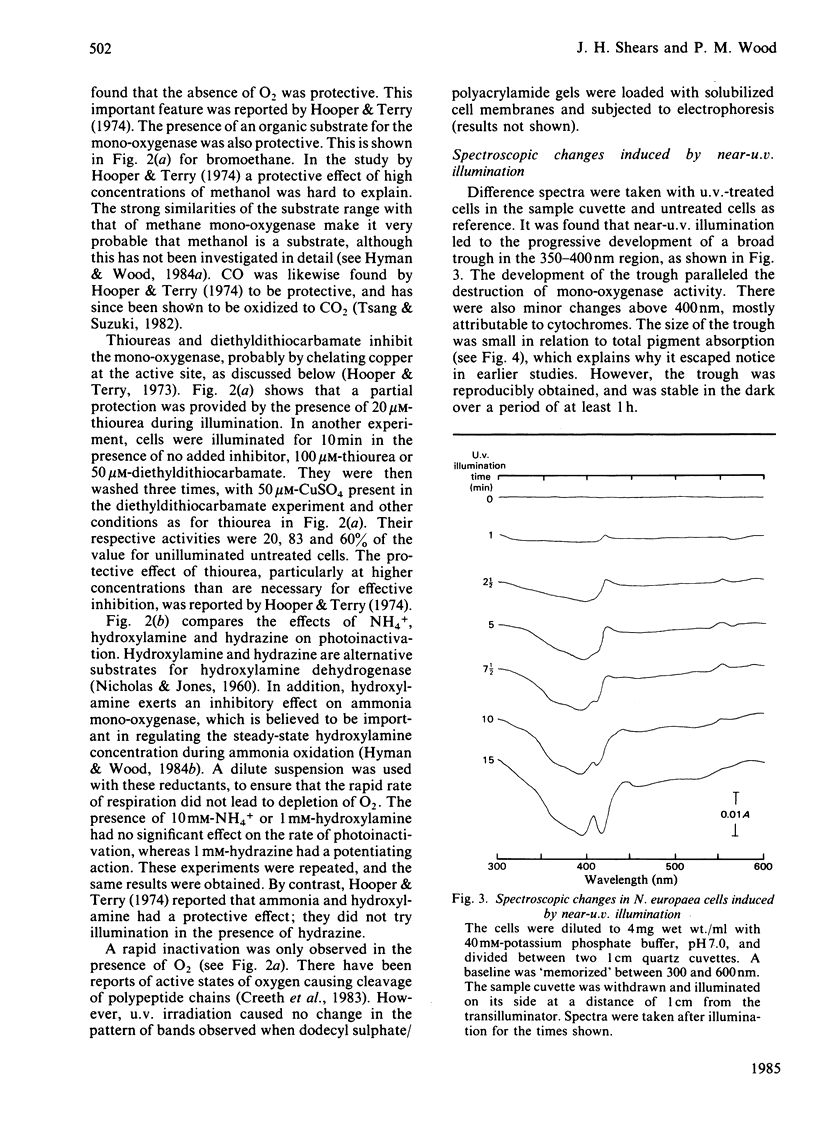
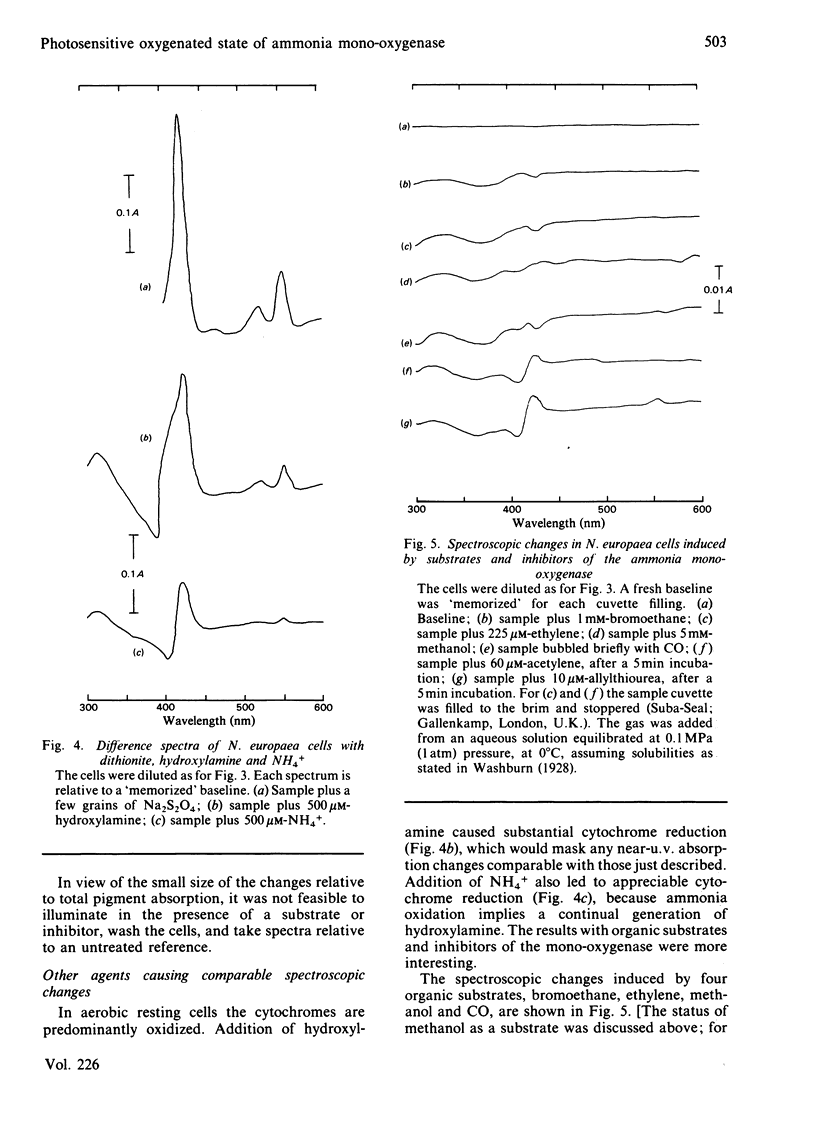
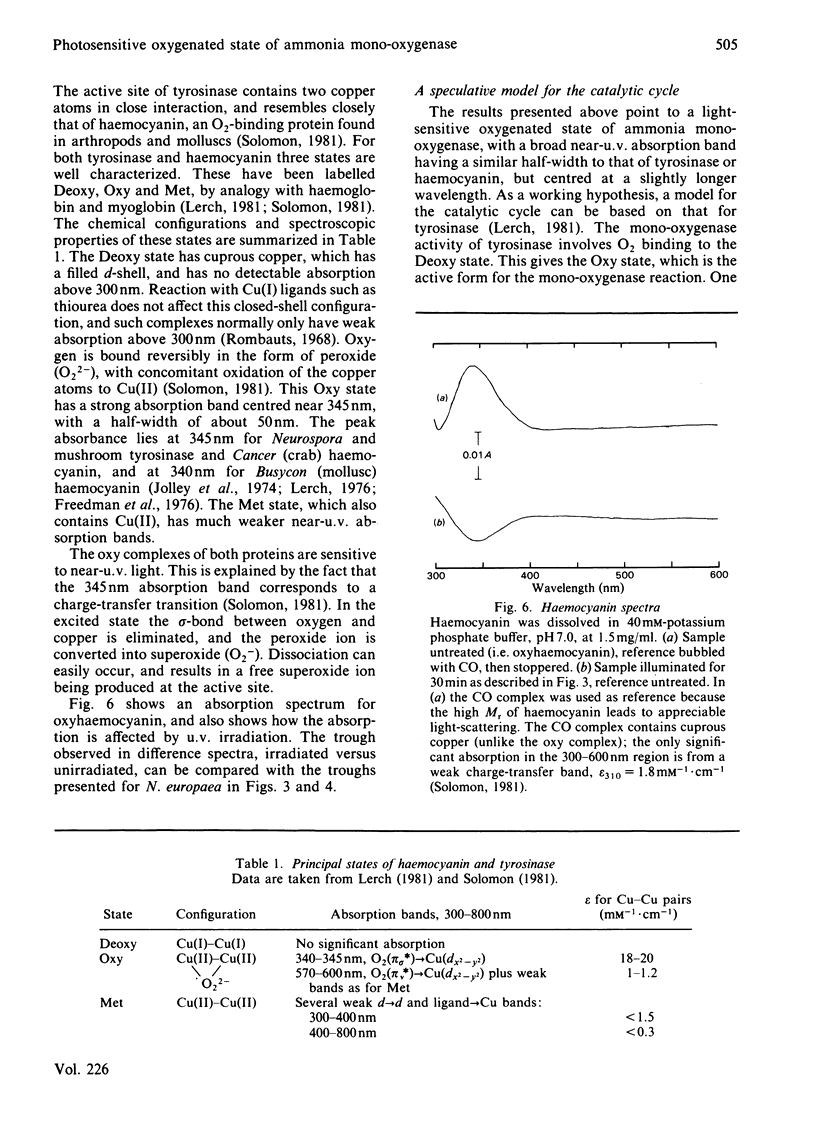
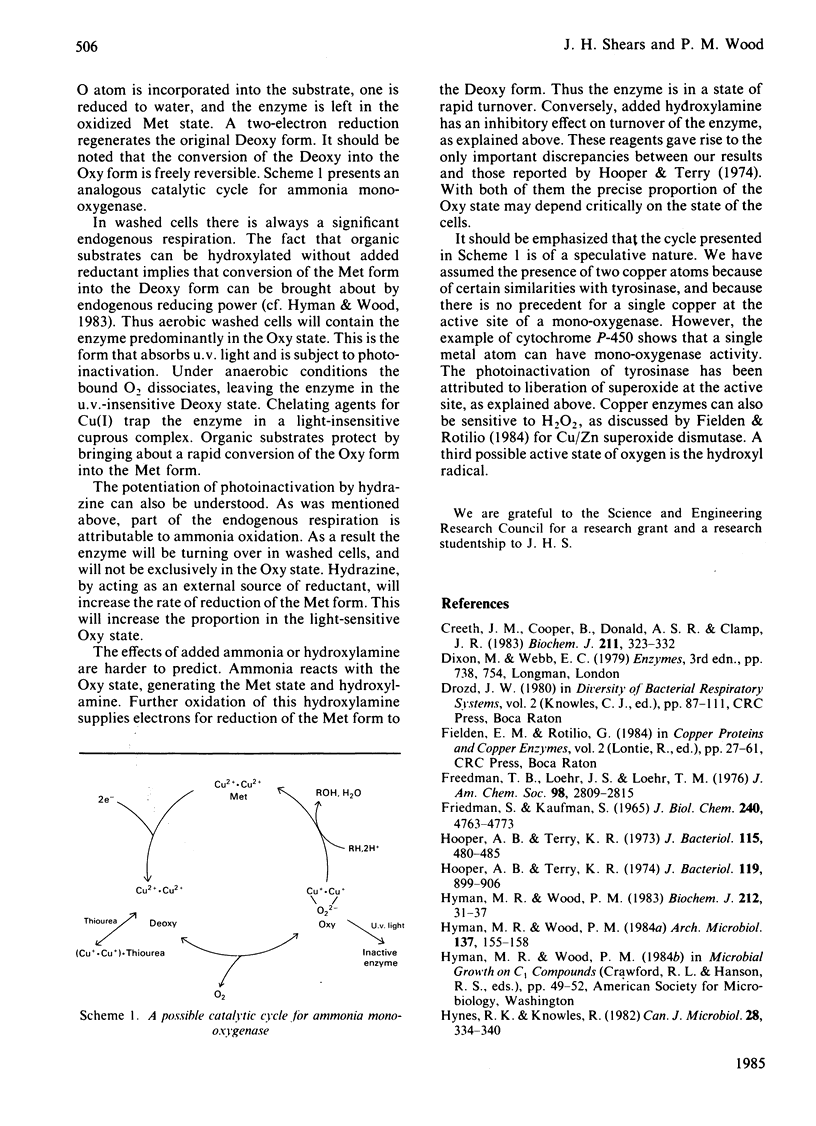
Photoinactivation of ammonia oxidation by Nitrosomonas europaea cells by near-u.v. light was confirmed and further shown to occur with the same rate constant as loss of bromoethane-oxidation activity. Hydroxylamine oxidation was much less photosensitive. Protection against inactivation was afforded by anaerobiosis, organic substrates of ammonia mono-oxygenase such as bromoethane, or metal-ion-chelating agents such as thiourea. The presence of 10 mM-NH4+ or 1 mM-hydroxylamine made little difference, whereas hydrazine had a potentiating effect. Illumination of cells also caused a bleaching in the absorption spectrum around 380 nm, along with changes in the cytochrome gamma-band region. Similar effects below 400 nm were obtained when organic substrates and inhibitors of the mono-oxygenase were added to cells in the dark. The copper proteins haemocyanin and tyrosinase have a photosensitive oxygenated state with a near-u.v. absorption band of similar half-width. They also have a sensitivity to chelating agents similar to that of ammonia mono-oxygenase. The experimental results are explained in terms of a three-stage catalytic cycle analogous to that for tyrosinase. In resting cells most of the enzyme is believed to be in an oxygenated (Oxy) form, which absorbs maximally at 378 nm and is photosensitive. In the presence of a substrate, one O atom is inserted into the substrate and the other is reduced to water, leaving the enzyme in an oxidized (Met) state. This is followed by a two-electron reduction of the proposed binuclear copper site to give a reduced (Deoxy) state, which can bind O2 to complete the cycle.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Creeth J. M., Cooper B., Donald A. S., Clamp J. R. Studies of the limited degradation of mucus glycoproteins. The effect of dilute hydrogen peroxide. Biochem J. 1983 May 1;211(2):323–332. doi: 10.1042/bj2110323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman T. B., Loehr J. S., Loehr T. M. A resonance Raman study of the copper protein, hemocyanin. New evidence for the structure of the oxygen-binding site. J Am Chem Soc. 1976 May 12;98(10):2809–2815. doi: 10.1021/ja00426a023. [DOI] [PubMed] [Google Scholar]
- Friedman S., Kaufman S. 3,4-dihydroxyphenylethylamine beta-hydroxylase. Physical properties, copper content, and role of copper in the catalytic acttivity. J Biol Chem. 1965 Dec;240(12):4763–4773. [PubMed] [Google Scholar]
- Hooper A. B., Terry K. R. Photoinactivation of ammonia oxidation in Nitrosomonas. J Bacteriol. 1974 Sep;119(3):899–906. doi: 10.1128/jb.119.3.899-906.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper A. B., Terry K. R. Specific inhibitors of ammonia oxidation in Nitrosomonas. J Bacteriol. 1973 Aug;115(2):480–485. doi: 10.1128/jb.115.2.480-485.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman M. R., Wood P. M. Methane oxidation by Nitrosomonas europaea. Biochem J. 1983 Apr 15;212(1):31–37. doi: 10.1042/bj2120031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. A., Boukma S. J., Kim E. G. Inhibition of dopamine beta-hydroxylase by aromatic and alkyl thioureas. J Pharmacol Exp Ther. 1969 Aug;168(2):229–234. [PubMed] [Google Scholar]
- Jolley R. L., Jr, Evans L. H., Makino N., Mason H. S. Oxytyrosinase. J Biol Chem. 1974 Jan 25;249(2):335–345. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lerch K. Neurospora tyrosinase: molecular weight, copper content and spectral properties. FEBS Lett. 1976 Oct 15;69(1):157–160. doi: 10.1016/0014-5793(76)80675-8. [DOI] [PubMed] [Google Scholar]
- Prabhakaran K., Kirchheimer W. F., Harris E. B. Oxidation of phenolic compounds by Mycobacterium leprae and inhibition of phenolase by substrate analogues and copper chelators. J Bacteriol. 1968 Jun;95(6):2051–2053. doi: 10.1128/jb.95.6.2051-2053.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. C., Lovenberg W. Dopamine-beta-hydroxylase. Essays Neurochem Neuropharmacol. 1980;4:163–209. [PubMed] [Google Scholar]
- SCHOEN G. H., ENGEL H. [The effect of light on Nitrosomonas europaea Win]. Arch Mikrobiol. 1962;42:415–428. [PubMed] [Google Scholar]
- Tsang D. C., Suzuki I. Cytochrome c554 as a possible electron donor in the hydroxylation of ammonia and carbon monoxide in Nitrosomonas europaea. Can J Biochem. 1982 Nov;60(11):1018–1024. doi: 10.1139/o82-131. [DOI] [PubMed] [Google Scholar]


