Abstract
The envelope protein (Env) of murine leukemia viruses (MLVs) is composed of a surface subunit (SU) and a transmembrane subunit (TM), which mediates membrane fusion, resulting in infection. SU contains a discrete N-terminal receptor binding domain (RBD) that is connected to the remainder of Env by a short, proline-rich segment. Previous studies suggest that after receptor binding, the RBD interacts directly with the remainder of Env to trigger fusion (A. L. Barnett, R. A. Davey, and J. M. Cunningham, Proc. Natl. Acad. Sci. USA 98:4113–4118, 2001). To investigate the role of the RBD in activating fusion, we compared infection by several MLVs that are defective unless rescued in trans by the addition of soluble RBD to the culture medium. Infection by MLV lacking a critical histidine residue near the N terminus of the viral RBD is dependent on the expression of receptors for both the RBD in the viral Env and the soluble RBD supplied in trans. However, infection by MLVs in which the RBD has been deleted or replaced by the ligand erythropoietin are dependent only on expression of the receptor for the soluble RBD. We were able to expand the host range of xenotropic MLV to nonpermissive murine fibroblasts only if the RBD was deleted from the xenotropic viral envelope and the soluble RBD from ecotropic Friend MLV was supplied to the culture medium. These findings indicate that receptor binding transforms the RBD from an inhibitor to an activator of the viral fusion mechanism and that viruses lacking the critical histidine residue at the N terminus of the RBD are impaired at the activation step.
It is well established that retrovirus infection occurs by fusion of the virus to the target cell membrane (44). The energy that drives fusion may be derived, at least in part, from release of the viral envelope protein (Env) from a metastable conformation, analogous to the entry process of influenza virus (9, 10, 46). This conformation is achieved by posttranslational modification of the folded envelope polyprotein, including cleavage into surface (SU) and transmembrane (TM) subunits (14, 30, 47) and removal of the carboxy-terminal 16 amino acids of TM before budding (37, 48). There is strong evidence that fusion is coupled to exposure of the fusion peptide (19) and to refolding of TM into a highly stable helical hairpin (11, 16, 45). It has been proposed that formation of this hairpin may bring the viral and cellular membranes into proximity and also provide the free energy for lipid mixing (9, 10, 44, 46). In this model, infection is favored by events at the target cell membrane that reduce the kinetic barrier protecting the metastable conformation.
At present, a detailed understanding of how the transition from the metastable to the final conformation of TM is triggered has not been achieved. A key step is binding of the SU subunit to a specific receptor on the target cell membrane (21, 40). In the case of avian retroviruses, envelope binding to the receptor is necessary, but not sufficient, for infection, which also requires the low-pH environment in endosomes (29). Mammalian C-type retroviruses, including murine leukemia viruses (MLVs), share the requirement for a specific receptor but not for acidification (27, 29). However, the function of the MLV receptors is not simply to bring the viral and cell membranes into proximity, since infection is not observed if binding is redirected to other receptors by inserting ligands into the envelope (8, 50). This indicates that the role of the receptor in MLV infection is not solely to allow attachment of the virus to the membrane.
Studies of the organization of mammalian C-type retroviral Env proteins demonstrate that SU is composed of two domains that are linked by a short, proline-rich, “hinge” region (20, 23). Interference studies suggest that the amino-terminal half of SU forms a domain that binds directly to the receptor (5, 6, 33). This conclusion has been verified for Friend MLV (Fr-MLV), in which this domain, termed the receptor binding domain (RBD), has been shown to bind directly to its receptor, murine CAT1 (mCAT1) (1), with 1:1 stoichiometry (12). Structure-function studies informed by the atomic structure of the RBD have identified a discrete pocket at the top of the RBD that is required for receptor binding and for infection (13, 15). Recently, we observed that MLVs in which the RBD was deleted from Env were infectious if the deleted RBD was supplied as a soluble protein at the time of infection (4). This indicates that the RBD is not required for the assembly of the envelope into a fusion-competent conformation. It also indicates that after receptor binding, the RBD establishes contact with the remainder of Env (4, 22). We observed that the Fr-MLV RBD (Fr-RBD) was able to rescue infection of amphotropic and xenotropic MLV from which the RBD was deleted, indicating that the contact between the RBD and the remainder of Env is not subgroup specific (4). To explain these findings, we proposed that after receptor binding, the RBD is in a conformation that activates the remainder of Env to trigger fusion.
Previously, we proposed that the RBD and C-terminal portions of SU form two ends of a dumbbell-like structure that are connected by the proline-rich region (4). In one scenario, the two domains assemble independently and interact only following receptor binding to the RBD. Alternatively, the two domains may be in contact initially, but their relationship is either disrupted or changed by receptor binding. To address this issue, we compared infection by several MLVs rendered defective by changes in the RBD in the presence of defined concentrations of Fr-RBD in the culture medium.
MATERIALS AND METHODS
Cell lines.
All cell lines used in this study were propagated in Dulbecco's modified Eagle's medium (Gibco BRL, Gaithersburg, Md.) supplemented with 10% fetal calf serum, 4 mM l-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml at 37°C in 5% CO2. The preparation of human 293-derived cell lines expressing mCAT1 and mCAT1-EpoR (13) and the mouse NIH 3T3-derived cell line CL-13 (12) has been described previously.
Recombinant virus production and infection.
All of the viruses mentioned in this report were prepared by transfection of human 293 cells with the plasmids pMD.old.gagpol (20 μg) and pBABE-lacZ (20 μg) and the expression construct encoding the desired envelope protein (20 μg), as described in detail previously (41). In our recent report, we documented reproducible incorporation of gag into virions by using this protocol, and the same preparations of pMD.old.gagpol and pBabe-lacz plasmids were used in this study (4). Virus infection was determined by assaying for acquired β-galactosidase activity in indicator cells 2 days after overnight exposure to virus. The virus titer was determined by end point dilution.
In some experiments, an aliquot of the virus-containing supernatant was used to measure incorporation of envelope protein into virions. Lysates were prepared from virions purified by centrifugation over a sucrose cushion and, after sodium dodecyl sulfate-polyacrylamide gel electrophoresis, analyzed by immunoblotting with goat anti-MLV SU as previously described (4, 13).
Plasmid construction.
The expression plasmid encoding the Fr-MLV envelope protein with the deletion of the codon CAC for histidine at position 8 (Fr-MLV env ΔH8) was constructed by PCR-based mutagenesis using the plasmid pCMV-Frgp85, encoding the Fr-MLV57 envelope protein, as a template. The expression plasmid encoding the amphotropic MLV envelope protein with the deletion of the codon CAT for histidine at position 5 (A-MLV env ΔH5) was constructed by PCR-based mutagenesis using the plasmid pSLA-MLV, encoding the amphotropic 4070 envelope protein (26), as a template. Construction of an expression plasmid encoding the xenotropic MLV envelope protein with the deletion of codon CAC for histidine at position 7 (X-MLV env ΔH7) was achieved using the plasmid pCMV-Xenogp85 as a template for PCR-directed mutagenesis. pCMV-Xenogp85 contains the 5′ untranslated region from pCMV-Frgp85. Each of the plasmid constructs was validated by DNA sequencing.
The construction of the plasmids encoding Fr-MLV (env ΔRBD), Fr-MLV (Epo-env), and X-MLV (env ΔRBD) (4) and encoding Fr-MLV Env containing the substitution D86A, W102G, or S84I (13) have been described previously.
Purification of RBD proteins.
The preparation of the purified RBDs of Fr-MLV and amphotropic MLV from insect cells has been described previously (12). The yield of purified RBD was typically 1 mg/liter of original insect cell culture medium. RBD proteins with binding pocket mutations (W102G, D86A, and S84I) (13) were purified using the same protocol following the isolation of a high-titer recombinant baculovirus stock using the Bac-to-Bac system (Gibco BRL). Fr-RBD (D86A) eluted from the Mono-S column at a later point in the salt gradient than wild-type Fr-RBD. The purified protein samples were quantified using the bicinchoninic acid protein assay (Pierce, Rockford, Ill.), using bovine serum albumin as a standard.
Xenopus oocyte binding assay.
Capped mRNAs encoding mCAT1 and the RBD were transcribed using the mMESSAGE mMACHINE kit (Ambion, Austin, Tex.) following the manufacturer's instructions and were injected into Xenopus laevis oocytes. The protocol for preparation and binding of 125I-Fr-RBD to these oocytes has been previously reported (13).
RESULTS
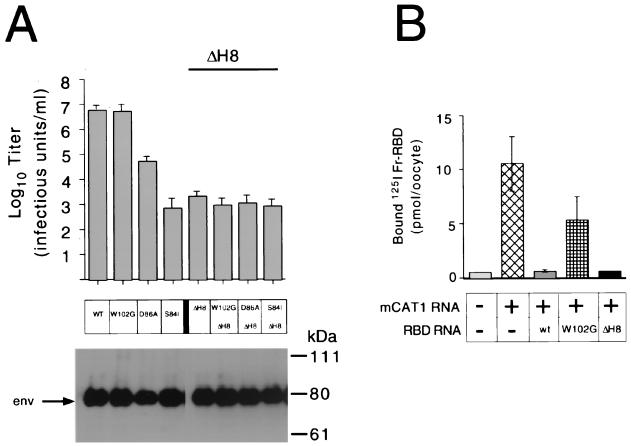
We measured the titers of infectious virions expressing Fr-MLV envelope protein and carrying lacZ on permissive mouse NIH 3T3 fibroblasts and on a cell line derived from human 293 cells that expresses the Fr-MLV receptor (1), mCAT1 (293 mCAT1). Deletion of His8 from the envelope protein reduced the titer of Fr-MLV (env ΔH8) from 107 to 103 infectious units (IU)/ml on NIH 3T3 cells (Fig. 1A) and from 107 to <10 IU/ml on 293 mCAT1 cells, similar to the findings of Bae et al. (3) and Lavillette et al. (24) in their studies of Moloney MLV infection. We compared the titer of Fr-MLV (env ΔH8) with those of three other viruses in which a single residue located in the receptor binding pocket had been altered. These substitutions, described previously (13), caused a negligible (W102G; 107-IU/ml), moderate (D86A; 5 × 104-IU/ml), or severe (S84I; 103-IU/ml) reduction in titer compared to wild-type Fr-MLV (107 IU/ml) on NIH 3T3 cells (Fig. 1A).
FIG. 1.
The effect of deletion of HIS8 (ΔH8) in Fr-MLV SU on receptor binding and infection in comparison with the effect of substitutions for residues in the receptor binding pocket that reduce binding affinity and titer. (A) Infection. End point dilution of virus on NIH 3T3 fibroblasts was used to measure the titers of Fr-MLVs with ΔH8 and/or substitutions for one of three residues (W102G, D86A, or S84I) in the receptor binding pocket of SU that reduce receptor binding affinity (13). Each titer was determined in triplicate by end point dilution from independent stocks of virus, and standard errors are indicated. Incorporation of Env into virions was monitored by immunoblotting with anti-gp70 antibody (below). (B) Binding. Binding of exogenous 125I-Fr-RBD to Xenopus oocytes was measured 2 days after injection with capped mRNA encoding mCAT1 alone or in combination with a capped mRNA encoding either Fr-RBD, Fr-RBD (W102G), or Fr-RBD (ΔH8). Each measurement is the mean of the counts per minute from five oocytes ± 1 standard error. +, present; −, absent; WT or wt, wild type.
An oocyte-based expression assay was used to determine if deletion of His8 alters binding of Fr-RBD (ΔH8) to the receptor (Fig. 1B). Binding of 125I-Fr-RBD was more than 10-fold greater (11 pmol/oocyte) to oocytes injected with mRNA encoding mCAT1 than to noninjected oocytes (0.8 pmol/oocyte). Coinjection of mRNA encoding the Fr-RBD with mCAT1 mRNA reduced binding of 125I-Fr-RBD to 1.3 pmol/oocyte, consistent with RBD-dependent down-regulation of mCAT1. Under these conditions, only a partial block of 125I-Fr-RBD binding (5 pmol/oocyte) was caused by expression of Fr-RBD (W102G), consistent with previous experiments demonstrating that W102 participates in receptor binding (13). However, coinjection of mRNA encoding Fr-RBD (ΔH8) blocked 125I-Fr-RBD binding to the same extent (0.9 pmol/oocyte) as coinjection of Fr-RBD mRNA. These experiments indicate that the reduction in infection caused by ΔH8 is not due to impaired binding of Fr-RBD to mCAT1.
We examined the consequences for infection of combining ΔH8 with each of the substitutions for the residues that compose the binding pocket. We observed that the presence of W102G, D86A, or S84I had no detectable effect on the titer of Fr-MLV (env ΔH8) infection, which remained 103 IU/ml on NIH 3T3 cells (Fig. 1A) and 10 IU/ml on 293 mCAT1 cells. Also, no infection by these viruses was observed on 293 cells that do not express mCAT1. These findings suggest that the His8 residue is critical for a step in infection that occurs after binding to mCAT1 and that, when His8 is deleted, this postbinding step is limiting for infection.
Biphasic relationship between soluble RBD concentration and infection by MLV (env ΔH).
RBDs from several MLVs were purified after expression in insect cells. These proteins were efficiently processed and secreted from insect cells as monomers. We demonstrated that addition of wild-type Fr-RBD, but not Fr-RBD (ΔH8), to the culture media markedly enhanced Fr-MLV (env ΔH8) infection of NIH 3T3 fibroblasts and human 293 mCAT1 cells (data not shown), confirming the findings of Lavillette et al. (24).
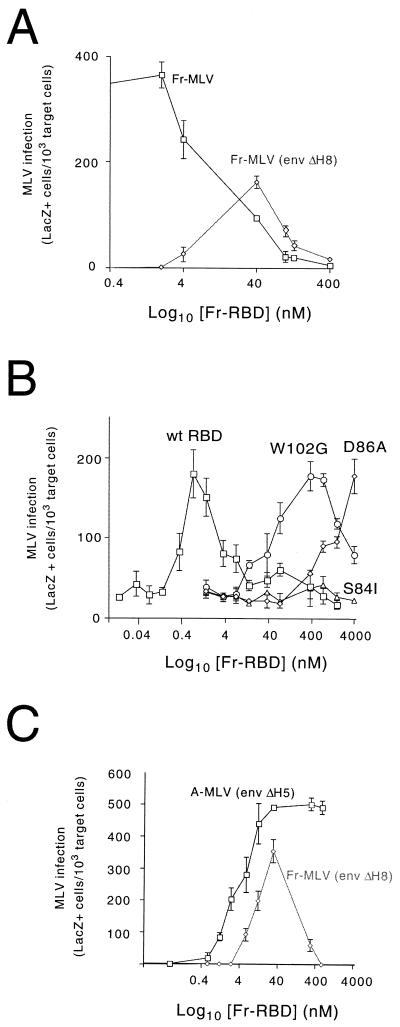
When Fr-RBD was added to human 293 mCAT1 cells, we observed an Fr-RBD concentration-dependent inhibition of wild-type Fr-MLV infection (Fig. 2A). When the concentration of Fr-RBD reached 400 nM, the titer of Fr-MLV was reduced from 107 to <10 IU/ml, indicating that this concentration of Fr-RBD is sufficient to occupy all of the virus receptors on the cell surface. The 50% inhibitory concentration under these conditions was ≈40 nM, which is comparable to the affinity binding constant of Fr-RBD for the receptor (Ka= 55 nM) determined previously by studies of Fr-RBD binding using oocytes that expressed mCAT1 (12).
FIG. 2.
(A) Relationship between the concentration of soluble Fr-RBD in the medium and infection by Fr-MLV or Fr-MLV (env ΔH8). Fr-MLV or Fr-MLV (env ΔH8) infection of human 293 mCAT1 cells as a function of the concentration of purified Fr-RBD in the medium (0 to 400 nM) was determined. Infection was assessed 2 days postexposure to a 1:10 dilution of virus by recording the proportion of target cells that were stained blue by 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside. The results for each RBD concentration were determined in triplicate wells of a six-well plate. Standard errors are indicated. (B) Effect of substitutions for residues in the binding pocket on the capacity of soluble Fr-RBD to rescue Fr-MLV (env ΔH8) infection. Fr-MLV (env ΔH8) infection of NIH 3T3 cells as a function of the soluble RBD concentration (0 to 4,000 nM) was determined using either purified wild-type (wt) Fr-RBD (squares), Fr-RBD (W102G) (circles), Fr-RBD (D86A) (diamonds), or Fr-RBD (S84I) (triangles). LacZ-positive cells were counted 2 days postexposure to a 1:10 dilution of virus. The effect of each RBD concentration was assessed in triplicate on a six-well plate. Standard errors are indicated. (C) Relationship between Fr-RBD concentration and infection by Fr-MLV (env ΔH8) and A-MLV (env ΔH5). Human 293 mCAT1 cells were exposed to 1:10 dilutions of Fr-MLV (env ΔH8) or A-MLV (env ΔH5) in the presence of the indicated concentration of purified Fr-RBD protein. The infection level was determined as described above.
We then studied infection by Fr-MLV (env ΔH8) as a function of the RBD concentration between 0 and 400 nM. We observed that the titer of Fr-MLV (env ΔH8) was <100 IU/ml when the Fr-RBD concentration was <4 or >100 nM. However, infection was markedly increased at RBD concentrations between 4 and 100 nM. The titer of Fr-MLV (env ΔH8) was greatest (5 × 105 IU/ml) in the presence of 40 nM Fr-RBD. Small changes in the concentration of soluble RBD between 4 and 100 nM resulted in large changes in the virus titer. This likely occurs because, in this concentration range, viral RBD and soluble RBD are competing for receptor binding. This indicates that Fr-MLV (env ΔH8) infection is strongly dependent on conditions under which receptor occupancy is near saturation and the ratio of viral to soluble RBD bound to the receptor is optimal. This is consistent with the likelihood that receptor-bound virus must be in close proximity to several receptor-bound RBDs for activation of fusion and infection. Indeed, when the receptor concentration on the cell surface is not limiting because the concentration of Fr-RBD is well below the affinity binding constant (<10 nM), the probability of infection was markedly reduced. For this reason, the measurement of the Fr-MLV (env ΔH8) titer under conditions where receptor binding is near saturation is not equivalent to the measurement of titer for viruses in which infection is the result of a single binding event under conditions where receptor availability is not limiting. To investigate the properties of the biphasic relationship, we chose to measure Fr-MLV (env ΔH8) infection as a function of RBD concentration in the presence of a fixed amount of virus supernatant (1:10 dilution). The results (Fig. 2A) are reported as the number of lacZ-positive cells per 103 target cells. At this virus dilution, the multiplicity of infection in the presence of 40 nM RBD is likely >1, and therefore, the number of lacZ-positive cells is an underestimate of the frequency of infection. However, when the virus supernatant was diluted further (1:100), infection was only observed when the Fr-RBD concentration was 40 nM. Consequently, the biphasic relationship between the RBD concentration and infection could not be seen.
Using this protocol, we observed a similar biphasic relationship between the RBD concentration and infection on NIH 3T3 cells. However, infection was greatest at an Fr-RBD concentration of 0.8 nM, 50-fold lower than the optimal concentration on 293 mCAT1 cells. We exploited the enhanced sensitivity of NIH 3T3 cells to measure Fr-MLV (env ΔH8) infection as a function of the concentration of three Fr-RBDs that each contain one of the substitutions (W102G, D86A, or S84I) which reduce affinity for receptor binding (13) (Fig. 2B). To compare the activities of these RBDs, we repeated the protocol in which the level of Fr-MLV (env ΔH8) infection was assessed using a fixed virus dilution (1:10). The Fr-RBD isoforms containing W102G and D86A restored Fr-MLV (env ΔH8) infection to the same extent as wild-type soluble Fr-RBD. However, the concentrations of these proteins required to achieve maximal infection are 500-fold greater for the W102G isoform and 2,000-fold greater for the D86A isoform than for wild-type RBD. No infection was detected in the presence of RBD (S84I) (0 to 4,000 nM). These findings are consistent with the previous conclusion that Fr-MLV (env ΔH8) infection is strongly dependent on the density of Fr-RBD bound to receptors on the membrane.
Viral RBD inhibits activation of infection by soluble RBD in trans.
To examine the relationship between the binding of RBD to the receptor and activation of infection in more detail, we measured the capacity of Fr-RBD to restore infection by defective MLVs that bind to receptors other than mCAT1. The deletion of the critical histidine residue (His5) in amphotropic MLV [A-MLV (env ΔH5)] decreased infection of 293 mCAT1 cells that express the A-MLV receptor, hPit2 (28, 43), from 106 to <10 IU/ml. When these cells were exposed to a 1:10 dilution of viral supernatant, A-MLV (env ΔH5) infection increased as a function of the Fr-RBD concentration in the medium, reaching a maximum at 40 nM (Fig. 2C). In the presence of 40 nM Fr-RBD, the titer of A-MLV (env ΔH5) determined by end point dilution was 8 × 105 IU/ml. In contrast to the behavior of Fr-MLV (env ΔH8), A-MLV (env ΔH5) infection was not inhibited by raising the Fr-RBD concentration to 1,000 nM (Fig. 2C). In a parallel experiment, the biphasic relationship between the RBD concentration and infection was observed when A-MLV RBD was used in place of Fr-RBD (data not shown). In this experiment, peak infection of A-MLV (env ΔH5) was achieved at 4 nM and infection was completely inhibited by 40 nM A-RBD.
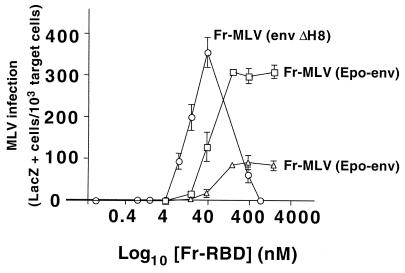
We then studied the relationship between the Fr-RBD concentration and infection by Fr-MLV in which RBD was replaced by the ligand erythropoietin [Fr-MLV (Epo-env)] (Fig. 3). Previously, we determined that binding of Fr-MLV (Epo-env) to membranes is mediated by the erythropoietin receptor (EpoR) and that soluble Fr-RBD is required in trans for infection (4). In addition, we determined that the titer of this virus in the presence of 40 nM Fr-RBD was 5 × 105 IU/ml on 293 cells that express mCAT1 and EpoR and 104 IU/ml on 293 cells that express mCAT1 alone (4). Upon exposure to a 1:10 dilution of virus supernatant, we observed that, like A-MLV (env ΔH5) infection, Fr-MLV (Epo-env) infection was saturated when the concentration of Fr-RBD reached 100 nM (Fig. 3). These experiments support the conclusion that the biphasic relationship between the soluble RBD concentration and infection is only observed when viral and soluble RBDs bind to the same receptor.
FIG. 3.
Relationship between Fr-RBD concentration and infection by Fr-MLV (Epo-env). Infection on 293 cells that express mCAT1 alone (triangles) or in combination with EpoR (squares) of a 1:10 dilution of Fr-MLV (Epo-env) supernatant was measured as a function of the concentration of Fr-RBD (0 to 2,000 nM). The cells were assayed for acquired β-galactosidase expression 48 h postinfection. The effect of each concentration of Fr-RBD on infection was assessed in triplicate wells of a six-well plate; standard errors are indicated. As a reference, the effect of Fr-RBD on Fr-MLV (env ΔH8) infection was replotted from Fig. 2C (circles). In a separate experiment, we observed no detectable difference in the relationship between Fr-RBD concentration and Fr-MLV (env ΔH8) infection on human 293 mCAT1 and human 293 mCAT1-EpoR cells (data not shown).
A low level of Fr-MLV (Epo-env) infection was also promoted by soluble Fr-RBD on 293 mCAT1 cells that lack EpoR. When the concentration of soluble Fr-RBD applied to these cells was greater than 1 μM, Fr-MLV (Epo-env) infection exceeded Fr-MLV (env ΔH8) infection. These findings strongly suggest that binding of viral RBD to the receptor is a prerequisite for activation of infection in trans by soluble RBD. Moreover, it shows that the requirement for receptor binding by viral RBD is not simply for virus attachment to the membrane, which is not needed for activation of infection by soluble RBD in trans. Rather, receptor binding is required to relieve an inhibitory effect of viral RBD (ΔH), thereby allowing the activation of infection by the action of soluble RBD in trans. Therefore, the activation of MLV infection in trans by soluble RBD bound to its receptor is blocked by the presence of viral RBD (ΔH) in the prebound state, and this inhibition is relieved by receptor binding to viral RBD.
Transfer of xenotropic MLV host range to nonpermissive mouse fibroblasts using soluble Fr-RBD.
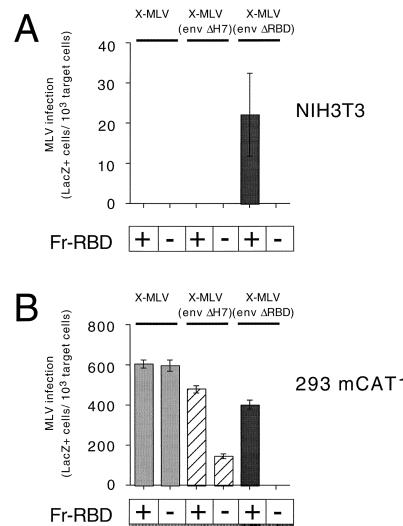
To determine if the inhibitory effect of viral RBD on activation is dependent on deletion of the critical histidine, we examined the capacity of soluble Fr-RBD to extend the host range of wild-type xenotropic MLV to nonpermissive mouse NIH 3T3 fibroblasts. Using a 1:10 dilution of virus supernatant, we observed that soluble Fr-RBD (100 nM) was unable to support X-MLV or X-MLV (env ΔH7) infection of these cells (Fig. 4A). However, Fr-RBD-dependent infection by X-MLV in which RBD was deleted from Env [X-MLV (env ΔRBD)] was observed. In the presence of 100 nM Fr-RBD, the titer of X-MLV (env ΔRBD) on NIH 3T3 cells was 5 × 103 IU/ml. We were unable to obtain stable clones of NIH 3T3 cells that expressed the X-MLV receptor, human SYG1 (hSYG1) (7, 42, 49), to determine if expression of this receptor was sufficient to reestablish Fr-RBD-dependent infection by X-MLV (env ΔH7). However, the inhibitory effect of viral RBD on X-MLV and X-MLV (env ΔH7) infection was not observed on human 293 mCAT1 cells that express native hSYG1 (Fig. 4B). Moreover, X-MLV (env ΔRBD) infection was not inhibited when the concentration of soluble Fr-RBD exceeded 100 nM (data not shown). These experiments demonstrate that, in the absence of a functional receptor, both X-MLV RBD and RBD (ΔH7) block activation of X-MLV infection by soluble Fr-RBD.
FIG. 4.
Infection of NIH 3T3 cells (A) and 293 mCAT1 cells (B) by X-MLV, X-MLV (env ΔH7), and X-MLV (env ΔRBD) was measured in the presence (100 nM) or absence of soluble Fr-RBD. The NIH 3T3 cells (CL-13) used in this experiment express fourfold more mCAT1 than normal NIH 3T3 cells (12). It should be noted that the deletion of the conserved histidine residue (His7) in X-MLV reduces the titer of X-MLV by 10-fold to 104 IU/ml compared to a 104-fold reduction in titer caused by the deletion of the equivalent His residue in A-MLV or Fr-MLV. +, present; −, absent.
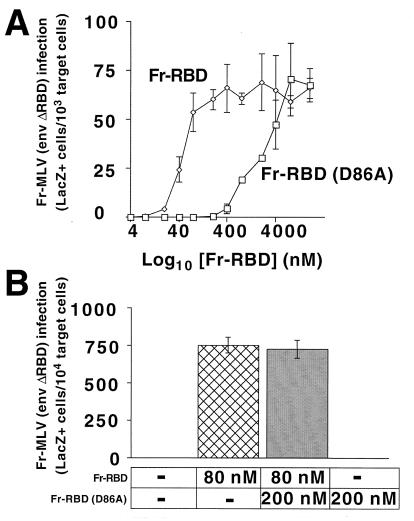
We tested the capacity of excess RBD in the prebound conformation to inhibit activation in trans. We identified the lowest concentration of wild-type Fr-RBD (80 nM) that fully restored Fr-MLV (env ΔRBD) infection and the highest concentration of Fr-RBD containing the D86A substitution (200 nM) that is unable to restore infection of this virus (Fig. 5A). At these concentrations, soluble RBD (D86A) did not inhibit the activation of Fr-MLV (env ΔRBD) infection by wild-type Fr-RBD (Fig. 5B). This indicates that, unlike the fusion-activating property of RBD bound to the receptor, an inhibitory effect of unbound RBD was not observed when it was supplied in trans.
FIG. 5.
(A) RBD-dependent infection by Fr-MLV (env ΔRBD) as a function of the concentration of Fr-RBD or Fr-RBD (D86A). Human 293 mCAT1 cells were exposed to Fr-MLV (env ΔRBD) carrying lacZ in the presence of Fr-RBD (diamonds) or Fr-RBD (D86A) (squares) over a concentration range of 0 to 8,000 nM. Two days after exposure to virus, the cells were stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside, and blue cells were counted. The experiments were performed in triplicate wells of a six-well plate. (B) Effect of an excess of Fr-RBD (D86A) on Fr-RBD-dependent infection by Fr-MLV (env ΔRBD). Human 293 mCAT1 cells were exposed to Fr-MLV (env ΔRBD) encoding lacZ alone or in the presence of either Fr-RBD (80 nM) or Fr-RBD (D86A) (200 nM) or both. Infection was assessed as described above. −, absent. The error bars represent ±1 standard error.
DISCUSSION
Activation of MLV fusion and infection is initiated by receptor binding and likely depends on the reduction of a disulfide bond between SU and TM (36). However, at present, a complete understanding of the mechanism of fusion and infection has not been achieved. It is well documented that binding of Fr-MLV to the receptor is required for infection and is mediated by a single domain composed of the amino-terminal portion of the SU subunit of Env (5, 6, 12, 33). The evidence to date suggests that receptor contact with Fr-MLV Env is limited to this domain (12) and, if true, indicates that this interaction is sufficient to trigger fusion.
To examine the triggering mechanism in more detail, we measured infection by defective MLVs on cells that were exposed to increasing concentrations of purified Fr-RBD. This approach was based on the experiments performed by Lavillette et al. (24), who observed that addition of soluble RBD to the culture medium markedly enhanced infection by MLV lacking the histidine residue in the conserved SPHQ motif near the amino terminus of viral RBD. We observed that the relationship between the Fr-RBD concentration and Fr-MLV (env ΔH8) infection was biphasic: as the concentration of Fr-RBD increased, infection increased to a maximum and then decreased to near zero. Several lines of evidence indicate that this relationship is a consequence of competitive inhibition by soluble RBD of viral RBD binding to the receptor. First, we established that Fr-RBD (ΔH8) binds to the receptor as well as wild-type Fr-RBD. Second, we observed that the concentration of soluble Fr-RBD required for maximum infection (inflection point) by Fr-MLV (env ΔH8) is directly correlated with the affinity of soluble Fr-RBD for the receptor. Third, we confirmed the findings of Lavillette et al. (24) that infection of nonpermissive hamster cells by amphotropic MLV (env ΔH5) is only restored by Fr-RBD on cell lines in which receptors for both viral (hPit2) and soluble (mCAT1) RBDs have been introduced (data not shown). Fourth, under conditions in which soluble Fr-RBD rescued A-MLV (env ΔH5) infection, competitive inhibition of infection by high concentrations of soluble Fr-RBD was not observed. These experiments suggest that Fr-MLV (env ΔH8) infection is highest when receptors are completely occupied, with a ratio of viral and soluble RBDs that allows the highest probability of the functional encounter required for infection. Taken together, these findings indicate that binding of both viral and soluble RBDs to receptors is required for MLV (env ΔH) infection and that a decrease in infection at high concentrations of soluble RBD is due to competitive inhibition of virus binding to receptors.
Previously, we observed that addition of purified Fr-RBD to the culture medium restored infection by xenotropic and amphotropic MLVs and Fr-MLV (env ΔRBD) on target cells that expressed the receptor for Fr-RBD (4). In these experiments, a role for the receptor for viral RBD was not evident, since it was deleted. These experiments strongly suggest that after receptor binding, Fr-RBD interacts directly with the C-terminal segment of SU to trigger fusion, perhaps by causing disruption of the disulfide bond between SU and TM. The same conclusion was reached in studies of RBD-dependent infection by chimeric MLVs (22). It remains possible that in the absence of viral RBD, the remainder of Env binds to an unidentified coreceptor. In addition, it remains possible that the receptor–Fr-RBD complex also recruits additional, unidentified host factors that are required for fusion and infection.
NIH 3T3 fibroblasts are 100-fold more efficient for RBD-independent Fr-MLV (env ΔH8) infection and are 50-fold more sensitive to Fr-RBD-dependent infection than 293 mCAT1 cells. It is possible that this difference is a function of the activity of an unidentified cofactor(s) discussed above or cell-type-specific differences in receptor mobility or trafficking. Whatever the cause, this difference may be related to the 50- to 100-fold-greater surface expression of mCAT1 on 293 mCAT1 cells than on NIH 3T3 cells measured by flow cytometry using fluorescein-labeled Fr-RBD (D. Wensel, unpublished data).
To examine why the presence of viral RBD (ΔH) confers a requirement for receptor binding, we studied infection by MLVs in which membrane binding was achieved by replacing RBD with the hormone erythropoietin. In this situation, the expression of EpoR on the target cells was not obligatory but enhanced infection activated by soluble Fr-RBD. This indicates that the requirement for expression of the receptor for viral RBD is not to allow attachment of the virus particle to the membrane. We propose that this requirement is indicative of an inhibitory activity of prebound viral RBD (ΔH) on MLV infection that is relieved upon receptor binding.
To directly test this hypothesis, we measured the capacity of Fr-RBD to establish infection by MLV on cells lacking the receptor for viral RBD. We observed that addition of soluble Fr-RBD to the medium is necessary and sufficient for infection by X-MLV (env ΔRBD) but not sufficient for infection by X-MLV or X-MLV (env ΔH7) on NIH 3T3 fibroblasts that do not express a functional X-MLV receptor. When these experiments were repeated using 293-derived cell lines that express functional receptors for X-MLV RBD and Fr-RBD, both X-MLV (env ΔRBD) and X-MLV (env ΔH7) infection was enhanced by addition of Fr-RBD to the culture medium. These experiments are consistent with the notion that, prior to receptor binding, viral RBD blocks the interaction of the C-terminal segment of SU with soluble-RBD–receptor complex. It is also possible that after deletion of the critical histidine or of RBD, the viral envelope is more sensitive to activation in trans. Additional experiments using viruses in which RBD is present but receptor binding is impaired may provide a test of this possibility.
Monomeric RBD encoded by defective proviruses has been observed in the sera of the mouse strain BALB/c-Fv-4Wr, derived from a cross between a wild mouse (Mus musculus molossinus) and inbred BALB/c mice (31). It was identified as the product of the genetic locus Fv-4, which confers resistance to virus-induced leukemia (18). Subsequent studies indicated that the reduction in leukemia in these mice is caused by down-regulation of receptor induced by binding to the Fv-4-encoded RBD (25). Recently, another monomeric RBD, termed FeLIX, has been identified in cats (2). FeLIX is encoded by a defective provirus related to subgroup B of feline leukemia virus (2). In contrast to Fv-4, the expression of FeLIX markedly enhanced infection by an immunosuppressive feline leukemia virus, 61C (2, 34). In this situation, the pathogenic 61C virus is defective in the absence of FeLIX, likely because 61C lacks the critical histidine in the SPHQ motif in SU. We speculate that susceptible cats express functional receptors for both FeLIX and the RBD of 61C. Alternatively, 61C may contain additional changes that prevent prebound viral RBD from blocking FeLIX-dependent activation of infection in trans. The behavior of the Fv-4 gene product and FeLIX supports the notion that the reservoir of proviruses in the genome of mammals provides a source of additional envelope proteins that, in the correct context, may provide key components to allow expansion of the viral host range. Conditions under which the host range is expanded may foster the generation of virus diversity by creating opportunities for recombination of viral RNA with RNAs transcribed from the cohort of endogenous viruses in the new host.
The physical basis for the inhibitory effect of viral RBD is unknown. One possible explanation is that the tethering of viral RBD by the flexible proline-rich hinge allows steric hindrance of the interaction between soluble RBD and the C-terminal segment of SU. If this is true, the observation that Epo is not inhibitory when inserted in place of RBD may reflect its slightly smaller size (147 residues compared to 206 residues for RBD). Alternatively, RBD may have a specific contact with the C-terminal segment of SU. Evidence from two laboratories indicates that viral RBD normally interacts with the C-terminal segment of SU, and this interaction enables optimal maturation and incorporation of Env into virions (17, 32, 35, 38). Both laboratories studied C-type retroviruses that were rendered defective by changes in either the RBD or the C-terminal segment that reduced the incorporation of mature Env into virions. After serial passage of these viruses, revertants were isolated in which replication was enhanced because correct processing of Env was restored. Analyses of several of these viruses revealed that the original changes in Env were still present and the improved processing was caused by compensatory changes located in the opposite segment of SU (17, 32, 35, 39). These findings suggest that RBD and the C-terminal segment are in contact during processing and that this contact is disrupted by changes in one segment and restored by complementary alterations in the opposite segment. If true, these observations suggest that in normal MLV infection, binding to the receptor changes RBD from an inhibitor to an activator of fusion by altering its preexisting relationship with the C-terminal segment. As a test of this model, we studied RBD-dependent infection by MLV (env ΔRBD) in the presence of an excess of RBD (D86A), which is deficient for receptor binding and therefore likely exists in the prebinding conformation. Under the conditions of the experiment, the presence of RBD (D86A) did not inhibit infection. This experiment indicates that either the association of Fr-RBD with the C-terminal segment in the pre-receptor binding conformation is weak or that the proline-rich segment is required for the prebinding interaction.
Our previous experiments demonstrated that the presence of RBD is not required for the processing of the remainder of Env into a fusion-competent state (4). This observation is potentially in conflict with the hypothesis that RBD interacts directly with the C-terminal segment in virions prior to receptor binding. This apparent conflict raises the possibility that the conformation of the C-terminal segment in MLV (env ΔRBD) is not the same as its prebound conformation in wild-type MLV. If this is true, the conformation of Env in MLV (env ΔRBD) may be an intermediate between the pre- and post-receptor-bound state. In this conformation, the C-terminal segment may be particularly susceptible to the “activated” form of RBD bound to the receptor. The relative stability of this conformation coupled with the flexibility provided by the proline-rich tether may favor intertrimeric interactions during normal infection that would likely facilitate formation of the fusion pore.
Studies of influenza infection indicate that before fusion, the hemagglutinin resides in a metastable conformation and that membrane fusion is tightly coupled to the assumption of the low-energy conformation (46). Although this concept has not been firmly established for retroviruses, including MLV, the similarities in the structures of the TM subunits and the requirement for cleavage of the envelope polyprotein suggest that the fusion mechanism of MLV is analogous to that of influenza virus (16). If the prefusion state of MLV is also metastable, it is unlikely that the C-terminal segment of SU and the TM subunit of MLV (env ΔRBD) and MLV (Epo-env) proteins are grossly misfolded, since they are incorporated into virions and remain fusion competent. Additional studies of MLV (env ΔRBD) are warranted to unequivocably establish this point.
We found no evidence for the participation of the conserved histidine in binding of Fr-RBD to the receptor. In addition, deletion of this residue did not relieve the receptor requirement for viral RBD, indicating that this residue is not critical for the prebinding interaction between RBD and the C-terminal segment. Moreover, we have confirmed the finding of Lavillette et al. (24) that soluble Fr-RBD (ΔH8) is unable to rescue infection by MLV (env ΔH). In the atomic structure of Fr-RBD, this histidine is located at the base of the domain, more than 25 Å from the pocket on the opposing surface that makes contact with the receptor (13, 15). This suggests that the portions of Fr-RBD which participate in receptor binding and in activation of infection are discrete and that His8 is a critical part of the postbinding contact with the C-terminal segment. Deletion of this residue from Env reduced the titer of Fr-MLV infection on NIH 3T3 cells by more than 5,000-fold, to 103 IU/ml. However, the introduction of additional mutations that reduced the affinity of RBD for the receptor did not cause additional reductions in the virus titer. We speculate that the reason for this observation is that after receptor binding, the rate of activation of fusion by RBD (ΔH) is low compared to the rate of dissociation of RBD from the receptor.
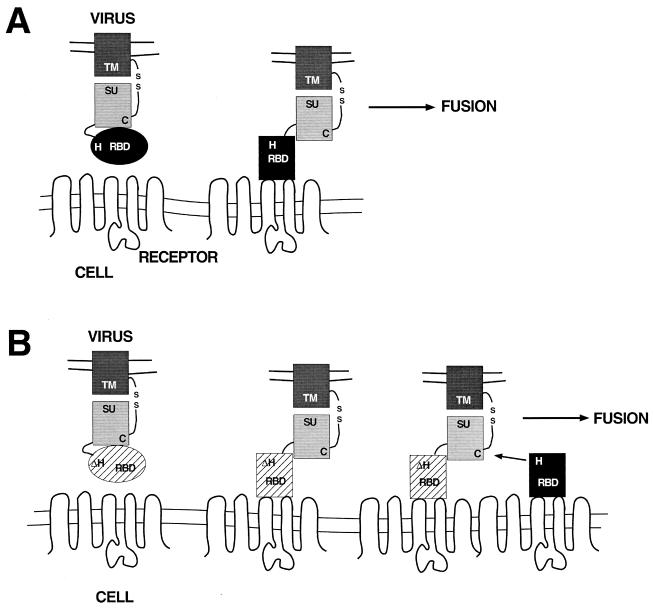
Taken together, the data presented in this report suggest that RBD engages in two distinct interactions with the C-terminal segment. Prior to receptor binding, this interaction inhibits fusion. After receptor binding, the interaction of RBD with the C-terminal segment is changed and, as a result, activates fusion. The stability of the C-terminal segment in the absence of RBD may allow activation in trans during normal infection. This step is strongly dependent on the conserved histidine in RBD, which may participate directly in the disruption of the disulfide bond between the C-terminal segment and TM. This proposed mechanistic scheme is summarized in the diagram in Fig. 6.
FIG. 6.
Schematic diagram to illustrate the proposed mechanism of soluble-RBD-dependent activation of fusion. The viral membrane containing the Env protein is at the top. Env is depicted with the RBD (oval) connected by the proline-rich region (curved line) to the C-terminal segment (light shaded rectangle). The transmembrane domain (dark shaded rectangle) is connected to the C-terminal segment by a disulfide bond. The cell membrane containing the virus receptor is at the bottom. (A) Proposed steps leading to the activation of fusion, assuming a mechanism in which the viral RBD and the C-terminal segment of SU interact in cis. The viral RBD is depicted as an oval that, on receptor contact, undergoes a conformational change (depicted as a rectangle). In this situation, fusion is triggered by a specific interaction between the bound conformation of the RBD and the C-terminal segment and is dependent upon the conserved histidine residue. (B) Proposed steps for RBD-dependent Fr-MLV (env ΔH8) infection in trans. Receptor binding to viral RBD (ΔH) results in exposure of the C-terminal segment of SU, enabling a productive interaction with soluble RBD bound to a distinct receptor. As in cis, the interaction between soluble RBD and the C-terminal segment in trans is strongly dependent on the conserved histidine residue.
ACKNOWLEDGMENTS
This work was supported by the Howard Hughes Medical Institute.
We thank Robert Davey for providing constructs to allow the production of RBD proteins with binding pocket mutations. We acknowledge Jason Smith and Walther Mothes for critical reading of the manuscript and helpful comments.
REFERENCES
- 1.Albritton L M, Tseng L, Scadden D, Cunningham J M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989;57:659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- 2.Anderson M M, Lauring A S, Burns C C, Overbaugh J. Identification of a cellular cofactor required for infection by feline leukemia virus. Science. 2000;287:1828–1830. doi: 10.1126/science.287.5459.1828. [DOI] [PubMed] [Google Scholar]
- 3.Bae Y, Kingsman S M, Kingsman A J. Functional dissection of the Moloney murine leukemia virus envelope protein gp70. J Virol. 1997;71:2092–2099. doi: 10.1128/jvi.71.3.2092-2099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnett A L, Davey R A, Cunningham J M. Modular organization of the Friend murine leukemia virus envelope protein underlies the mechanism of infection. Proc Natl Acad Sci USA. 2001;98:4113–4118. doi: 10.1073/pnas.071432398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battini J L, Danos O, Heard J M. Receptor choice determinants in the envelope glycoproteins of amphotropic, xenotropic, and polytropic murine leukemia viruses. J Virol. 1992;66:1468–1475. doi: 10.1128/jvi.66.3.1468-1475.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battini J L, Danos O, Heard J M. Receptor-binding domain of murine leukemia virus envelope glycoproteins. J Virol. 1995;69:713–719. doi: 10.1128/jvi.69.2.713-719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Battini J L, Rasko J F, Miller A D. A human cell-surface receptor for xenotropic and polytropic murine leukemia viruses: possible role in G protein-coupled signal transduction. Proc Natl Acad Sci USA. 1999;96:1385–1390. doi: 10.1073/pnas.96.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benedict C A, Tun R Y, Rubinstein D B, Guillaume T, Cannon P M, Anderson W F. Targeting retroviral vectors to CD34-expressing cells: binding to CD34 does not catalyze virus-cell fusion. Hum Gene Ther. 1999;10:545–557. doi: 10.1089/10430349950018625. [DOI] [PubMed] [Google Scholar]
- 9.Bullough P A, Hughes F M, Skehel J J, Wiley D C. Structure of influenza hemagglutinin at the pH of membrane fusion. Nature. 1994;371:37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- 10.Carr C M, Chaudry C, Kim P S. Influenza hemagglutinin is spring-loaded by a metastable native conformation. Proc Natl Acad Sci USA. 1997;94:14306–14313. doi: 10.1073/pnas.94.26.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan D C, Fass D, Berger J M, Kim P S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 12.Davey R A, Hamson C A, Healey J J, Cunningham J M. In vitro binding of purified murine ecotropic retrovirus envelope surface protein to its receptor, MCAT-1. J Virol. 1997;71:8096–8102. doi: 10.1128/jvi.71.11.8096-8102.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davey R A, Zuo Y, Cunningham J M. Identification of a receptor-binding pocket on the envelope protein of Friend murine leukemia virus. J Virol. 1999;73:3758–3763. doi: 10.1128/jvi.73.5.3758-3763.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.England J M, Bolognesi D P, Dietzschold B, Halpern M S. Evidence that a precursor glycoprotein is cleaved to yield the major glycoprotein of avian tumor virus. J Virol. 1977;21:810–814. doi: 10.1128/jvi.21.2.810-814.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fass D, Davey R A, Hamson C A, Kim P S, Cunningham J M, Berger J M. Structure of a murine leukemia virus receptor-binding glycoprotein at 2.0 angstrom resolution. Science. 1997;277:1662–1666. doi: 10.1126/science.277.5332.1662. [DOI] [PubMed] [Google Scholar]
- 16.Fass D, Harris S C, Kim P S. Retrovirus envelope domain at 1.7 angstroms resolution. Nat Struct Biol. 1996;3:465–468. doi: 10.1038/nsb0596-465. [DOI] [PubMed] [Google Scholar]
- 17.Gwynn S R, Hankenson F C, Lauring A S, Rohn J L, Overbaugh J. Feline leukemia virus envelope sequences that affect T-cell tropism and syncytium formation are not part of known receptor-binding domains. J Virol. 2000;74:5754–5761. doi: 10.1128/jvi.74.13.5754-5761.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeda H, Sugimura H. Fv-4 resistance gene: a truncated endogenous murine leukemia virus with ecotropic interference properties. J Virol. 1989;63:5405–5412. doi: 10.1128/jvi.63.12.5405-5412.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones J, Risser R. Cell fusion induced by the murine leukemia virus envelope glycoprotein. J Virol. 1993;67:67–74. doi: 10.1128/jvi.67.1.67-74.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kayman S C, Park H, Saxon M, Pinter A. The hypervariable domain of the murine leukemia virus surface protein tolerates large insertions and deletions, enabling development of a retroviral particle display system. J Virol. 1999;73:1802–1808. doi: 10.1128/jvi.73.3.1802-1808.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J W, Closs E I, Albritton L M, Cunningham J M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature. 1991;352:725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]
- 22.Lavillette D, Boson B, Russell S J, Cosset F L. Activation of membrane fusion by murine leukemia viruses is controlled in cis or in trans by interactions between the receptor-binding domain and a conserved disulfide loop of the carboxy terminus of the surface glycoprotein. J Virol. 2001;75:3685–3695. doi: 10.1128/JVI.75.8.3685-3695.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavillette D, Maurice M, Roche C, Russell S J, Sitbon M, Cosset F L. A proline-rich motif downstream of the receptor binding domain modulates conformation and fusogenicity of murine retroviral envelopes. J Virol. 1998;72:9955–9965. doi: 10.1128/jvi.72.12.9955-9965.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavillette D, Ruggieri A, Russell S J, Cosset F L. Activation of a cell entry pathway common to type C mammalian retroviruses by soluble envelope fragments. J Virol. 2000;74:295–304. doi: 10.1128/jvi.74.1.295-304.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Limjoco T I, Dickie P, Ikeda H, Silver J. Transgenic Fv-4 mice resistant to Friend virus. J Virol. 1993;67:4163–4168. doi: 10.1128/jvi.67.7.4163-4168.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markowitz D, Goff S, Bank A. Construction and use of a safe and efficient amphotropic packaging cell line. Virology. 1988;167:400–406. [PubMed] [Google Scholar]
- 27.McClure M O, Sommerfelt M A, Marsh M, Weiss R A. The pH independence of mammalian retrovirus infection. J Gen Virol. 1990;71:767–773. doi: 10.1099/0022-1317-71-4-767. [DOI] [PubMed] [Google Scholar]
- 28.Miller D G, Edwards R H, Miller A D. Cloning of the cellular receptor for amphotropic murine retroviruses reveals homology to that for gibbon ape leukemia virus. Proc Natl Acad Sci USA. 1994;91:78–82. doi: 10.1073/pnas.91.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mothes W, Boerger A L, Narayan S, Cunningham J M, Young J A. Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein. Cell. 2000;103:679–689. doi: 10.1016/s0092-8674(00)00170-7. [DOI] [PubMed] [Google Scholar]
- 30.Ng L V, Wood T G, Arlinghaus R B. Processing of the env gene products of Moloney murine leukemia virus. J Gen Virol. 1982;59:329–343. doi: 10.1099/0022-1317-59-2-329. [DOI] [PubMed] [Google Scholar]
- 31.Odaka T, Ikeda H, Yoshikura H, Moriwaki K, Suzuki S. Fv-4: gene controlling resistance to NB-tropic Friend murine leukemia virus. Distribution in wild mice, introduction into genetic background of BALB/c mice, and mapping of chromosomes. J Natl Cancer Inst. 1981;67:1123–1127. [PubMed] [Google Scholar]
- 32.O'Reilly L, Roth M J. Second-site changes affect viability of amphotropic/ecotropic chimeric enveloped murine leukemia viruses. J Virol. 2000;74:899–913. doi: 10.1128/jvi.74.2.899-913.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ott D, Rein A. Basis for receptor specificity of nonecotropic murine leukemia virus surface glycoprotein gp70SU. J Virol. 1992;66:4632–4638. doi: 10.1128/jvi.66.8.4632-4638.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Overbaugh J, Donahue P R, Quackenbush S L, Hoover E A, Mullins J I. Molecular cloning of a feline leukemia virus that induces fatal immunodeficiency disease in cats. Science. 1988;239:906–910. doi: 10.1126/science.2893454. [DOI] [PubMed] [Google Scholar]
- 35.Peredo C, O'Reilly L, Gray K, Roth M J. Characterization of chimeras between the ecotropic and amphotropic 4070A envelope proteins. J Virol. 1996;70:3142–3152. doi: 10.1128/jvi.70.5.3142-3152.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinter A, Kopelman R, Li Z, Kayman S C, Sanders D A. Localization of the labile disulfide bond between SU and TM of the murine leukemia virus envelope protein complex to a highly conserved CWLC motif in SU that resembles the active-site sequence of thiol-disulfide exchange enzymes. J Virol. 1997;71:8073–8077. doi: 10.1128/jvi.71.10.8073-8077.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rein A, Mirro J, Haynes J G, Ernst S M, Nagashima K. Function of the cytoplasmic domain of a retroviral transmembrane protein: p15E-p2E cleavage activates the membrane fusion capability of the murine leukemia virus Env protein. J Virol. 1994;68:1773–1781. doi: 10.1128/jvi.68.3.1773-1781.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rohn J L, Linenberger M L, Hoover E A, Overbaugh J. Evolution of feline leukemia virus variant genomes with insertions, deletions, and defective envelope genes in infected cats with tumors. J Virol. 1994;68:2458–2467. doi: 10.1128/jvi.68.4.2458-2467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rohn J L, Moser M S, Gwynn S R, Baldwin D N, Overbaugh J. In vivo evolution of a novel, syncytium-inducing and cytopathic feline leukemia virus variant. J Virol. 1998;72:2686–2696. doi: 10.1128/jvi.72.4.2686-2696.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sommerfelt M A. Retrovirus receptors. J Gen Virol. 1999;80:3049–3064. doi: 10.1099/0022-1317-80-12-3049. [DOI] [PubMed] [Google Scholar]
- 41.Soneoka Y, Cannon P M, Ramsdale E E, Griffiths J C, Romano G, Kingsman M S, Kingsman A J. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 1995;23:629–633. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tailor C S, Nouri A, Lee C G, Kozak C, Kabat D. Cloning and characterization of a cell surface receptor for xenotropic and polytropic murine leukemia viruses. Proc Natl Acad Sci. 1999;96:927–932. doi: 10.1073/pnas.96.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Zeijl M, Johann S V, Closs E, Cunningham J, Eddy R, Shows T B, O'Hara B. A human amphotropic retrovirus receptor is a second member of the gibbon ape leukemia virus receptor family. Proc Natl Acad Sci USA. 1994;91:1168–1172. doi: 10.1073/pnas.91.3.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weissenhorn W, Dessen A, Calder L J, Harrison S C, Skehel J J, Wiley D C. Structural basis for membrane fusion by enveloped viruses. Mol Membr Biol. 1999;16:3–9. doi: 10.1080/096876899294706. [DOI] [PubMed] [Google Scholar]
- 45.Weissenhorn W, Dessen A, Harrison S C, Skehel J J, Wiley D C. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:346–348. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 46.Wiley D, Skehel J. The structure of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- 47.Witte O N, Baltimore D. Relationship of retrovirus polyprotein cleavages to virion maturation studied with temperature-sensitive murine leukemia virus mutants. J Virol. 1978;26:750–761. doi: 10.1128/jvi.26.3.750-761.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Witte O N, Wirth D F. Structure of the murine leukemia virus envelope glycoprotein precursor. J Virol. 1979;29:735–743. doi: 10.1128/jvi.29.2.735-743.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Y L, Guo L, Xu S, Holland C A, Kitamura T, Hunter K, Cunningham J M. Receptors for polytropic and xenotropic mouse leukaemia viruses encoded by a single gene at Rmc1. Nat Genet. 1999;21:216–219. doi: 10.1038/6005. [DOI] [PubMed] [Google Scholar]
- 50.Zhao Y, Zhu L, Lee S, Li L, Chang E, Soong N, Douer D, Anderson W F. Identification of the block in targeted retroviral mediated gene transfer. Proc Natl Acad Sci USA. 1999;96:4005–4010. doi: 10.1073/pnas.96.7.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]