ABSTRACT
The number of pediatric respiratory tract infection cases in China has significantly increased this year, and Mycoplasma pneumoniae is one of the main pathogens. This study aimed to investigate the epidemiological characteristics of M. pneumoniae in children in the Anhui region and to provide evidence for the prevention and control strategies of M. pneumoniae in children in this region. A total of 66,488 pediatric patients with respiratory tract infection were enrolled from January 2015 to November 2023 in this study. The results of this study exhibited that M. pneumoniae infection in the Anhui region was characterized by a high positive rate during 2021–2023, especially this year is considered a year of pandemic for M. pneumoniae infection. Moreover, the positive rate of M. pneumoniae in female children is significantly higher than in male children, and the infection rate of M. pneumoniae in children increases significantly with age, particularly in school-aged children.
IMPORTANCE
The number of pediatric respiratory tract infection cases in China has significantly increased this year, and Mycoplasma pneumoniae is one of the main pathogens. This study aimed to investigate the epidemiological characteristics of M. pneumoniae in children in the Anhui region and provide evidence for the prevention and control strategies of M. pneumoniae in children in this region.
KEYWORDS: Mycoplasma pneumoniae, epidemic, children, COVID-19, respiratory tract infection
INTRODUCTION
Mycoplasma pneumoniae is one of the leading pathogens that cause respiratory tract infections in humans, especially in children and adolescents. M. pneumoniae has become a common pathogen of pediatric community-acquired mycoplasma pneumonia (CAMP), accounting for 10%–40% of CAMP cases (1–3). In recent years, the incidence of M. pneumoniae pneumonia in children has increased significantly in the post COVID-19 epidemic era (4, 5). M. pneumoniae infection can cause respiratory diseases, such as acute and chronic respiratory infections, asthma, and bronchitis. In addition, M. pneumoniae may cause extrapulmonary symptoms, such as diarrhea, inflammation of the central nervous system, otitis media, arthritis, or acute myocardial injury (6). These extrapulmonary symptoms may be the result of excessive immune response or inflammation triggered by M. pneumoniae pathogen, which can eventually develop into a life-threatening disease (7).
M. pneumoniae is the smallest bacterium without a cell wall (8). Infection caused by droplet transmission during close contact can facilitate the spread of the pathogen from person to person, leading to an epidemic outbreak. M. pneumoniae infection is widely distributed worldwide, and its epidemic occurs periodically (9). Usually, regional epidemics occur every 3–7 years, and each epidemic may last for 1–2 years (10–12). Previous studies have shown that pandemics of M. pneumoniae occurred in Beijing, China, and the United Kingdom in 2011–2013 and 2015–2016, respectively. Unfortunately, there has been an increase in M. pneumoniae cases pneumonia (MPP) in China recently. Data revealed that M. pneumoniae infection accounted for approximately 70% of all cases of CAP in children aged 5 years and older. The epidemiological characteristics of MPP may vary due to factors, such as seasonality, geographic region, and genotype, and may also be related to population immunity and the maturity of the immune system (1, 8, 13, 14). However, there have been few studies focusing on the epidemiology and dynamic changes of MPP in a large number of pediatric patients in recent years.
To better understand the epidemiological characteristics of M. pneumoniae in children in China, this study retrospectively analyzed the children with respiratory tract infection who were admitted to the Second Affiliated Hospital of Anhui Medical University from 2015 to 2023 and collected the basic information and serological results of M. pneumoniae in the children. The distribution of infections was analyzed based on factors, such as sex, age, month, and pre- and post-epidemic conditions, which can provide evidence for the clinical diagnosis, treatment, and prevention and control strategies of M. pneumoniae in children in the local region.
MATERIALS AND METHODS
Study patients
A total of 66,488 pediatric patients with respiratory tract infection, including 37,182 boys and 29,306 girls, ranging in age from 1 month to 14 years, were enrolled in the Second Affiliated Hospital of Anhui Medical University from January 2015 to November 2023 in this study. The inclusion criteria were as follows: (1) the cases presented with clinical manifestations of upper respiratory tract infection, pneumonia and bronchitis, such as cough, nasal congestion and sore throat; (2) the data of the pediatric patients were complete, and the antibody titer results of M. pneumoniae were performed in the hospital. The exclusion criteria were as follows: (1) patients over 14 years old; (2) patients with trachea or lung dysplasia or other congenital diseases; (3) patients with heart, liver, kidney, and other basic diseases; (4) Patients with immune deficiency or long-term use of immunosuppressants; (5) people with a history of tuberculosis. The study was a retrospective study, all patient data were reported anonymously, and the study was conducted in accordance with the Declaration of Helsinki (revised in 2013) and approved by the Ethics Committee of the Second Affiliated Hospital of Anhui Medical University.
Detection of M. pneumoniae antibody titers
Peripheral blood was extracted from the patient and centrifuged at 3,000 rpm(1,006×g) for 10 min. Then, the total antibody titer of M. pneumoniae in human serum was measured by passive agglutination method using the M. pneumoniae antibody detection kit (Fuji Ruibai Co., Ltd., Japan). All operations were strictly performed according to the manufacturer’s instructions. A serum M. pneumoniae antibody titer of ≥1:160 was considered as M. pneumoniae infection in the patient (10).
Statistical analysis
Statistical analysis was performed by using Graphpad 8.0 (Graphpad Software, USA). The continuous variables were exhibited as mean ± standard deviation (M ± SD), and the categorical variables were exhibited as numbers (%). Two groups of data that met the normal distribution and homogeneity of variance were analyzed using Student’s unpaired t-test, and the data that did not meet normal distribution and homogeneity of variance were analyzed using the Mann–Whitney U-test. Comparisons of multiple groups were performed using a one-way analysis of variance (ANOVA), followed by Tukey’s honest significant difference test. Comparisons between categorical variables were performed using χ or Fisher exact test. Bonferroni correction was applied to address the problem of multiple comparisons and was calculated as each P value multiplied by the number of comparisons. The differences were considered statistically significant when P < 0.05.
RESULTS
Positive rate of M. pneumoniae in different years
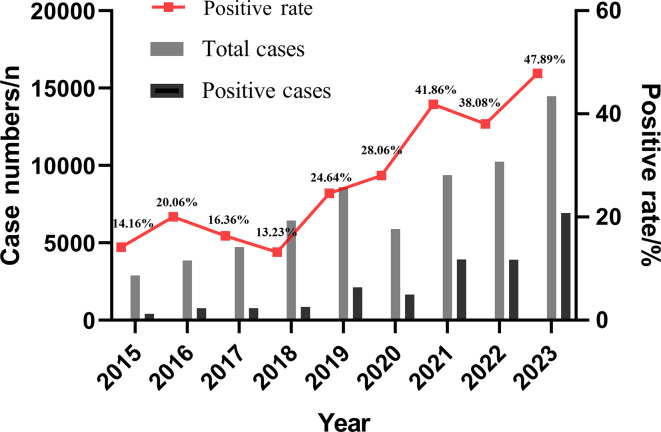
In this study, the total cases, positive cases, and positive rate of M. pneumoniae were calculated from 2015 to 2023. It was found that 66,488 children with respiratory tract infection were enrolled from 2015 to 2023, among which 21,331 cases were positive for M. pneumoniae, with a positive rate of 32.08%. The positive rate of M. pneumoniae showed significant differences among different years (χ2 = 4772.8, P < 0.0001). It can be observed from Fig. 1 that a peak epidemic of M. pneumoniae infection in children occurred in 2016 (20.06%), and a high prevalence of M. pneumoniae infection in children was observed in 2019–2023 (24.64%, 28.06%, 41.86%, 38.08%, and 47.89%) (see Fig. 1).
Fig 1.
Trend of positive rate of M. pneumoniae in different years.
Sex difference in positive rate of M. pneumoniae
Among the 21,331 positive cases of M. pneumoniae, 10,753 were male, and 10,578 were female, with a sex ratio of 1.017:1. The positive rate of M. pneumoniae in male pediatric patients was 28.92%, which was lower than that in female pediatric patients (36.09%), and the difference was statistically significant (χ2 = 387.2, P < 0.0001). In addition, the statistical analysis of the association between sex and the annual prevalence of M. pneumoniae was conducted using Pearson’s χ test with Bonferroni correction (see Table 1). The results revealed that in 2016, the positive rates of M. pneumoniae in males and females were 18.64% and 21.89%, respectively, and there was no statistically significant difference between the two groups after Bonferroni correction (Bonferroni-corrected P = 0.1116 > 0.05). However, in other years (2015, 2017–2023), there were significant differences in the positive rates of M. pneumoniae between male and female children (Bonferroni-corrected P < 0.05).
TABLE 1.
Sex difference in the prevalence of M. pneumoniae
| Year | Male | Female | Uncorrected P value | Corrected P valuea | ||
|---|---|---|---|---|---|---|
| Positive cases (%) | Negative cases (%) | Positive cases (%) | Negative cases (%) | |||
| 2015 | 201 (12.52) | 1,404 (87.48) | 209 (16.19) | 1,082 (83.81) | 0.0049 | 0.0441 |
| 2016 | 405 (18.64) | 1,768 (81.36) | 369 (21.89) | 1,317 (78.11) | 0.0124 | 0.1116 |
| 2017 | 391 (14.77) | 2,257 (85.23) | 383 (18.38) | 1,701 (81.62) | 0.0009 | 0.0081 |
| 2018 | 427 (11.70) | 3,222 (88.30) | 425 (15.23) | 2,366 (84.77) | <0.0001 | <0.0001 |
| 2019 | 1,095 (22.58) | 3,754 (77.42) | 1,022 (27.31) | 2,720 (72.69) | <0.0001 | <0.0001 |
| 2020 | 814 (23.64) | 2,632 (76.38) | 837 (34.33) | 1,601 (65.67) | <0.0001 | <0.0001 |
| 2021 | 1,931 (37.54) | 3,213 (62.46) | 1,991 (47.11) | 2,235 (52.89) | <0.0001 | <0.0001 |
| 2022 | 1,948 (34.64) | 3,675 (65.36) | 1,953 (42.25) | 2,669 (57.75) | <0.0001 | <0.0001 |
| 2023 | 3,541 (44.01) | 4,504 (55.99) | 3389(52.74) | 3,037 (47.26) | <0.0001 | <0.0001 |
Bonferroni-corrected P value was calculated as each P value multiplied by the number of tests (n = 9).
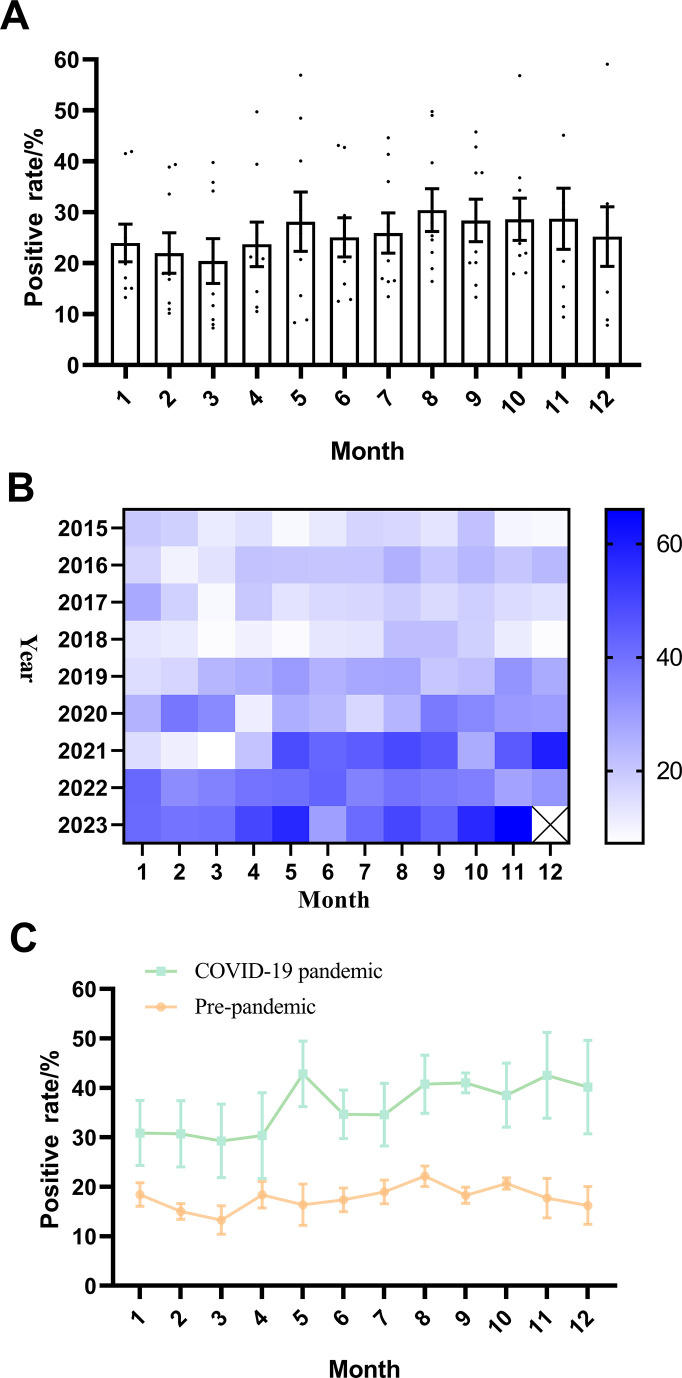
Changes in the positive rate of M. pneumoniae in different months
This study examined the differences in the positive rate of M. pneumoniae in different months and found no significant differences among different months (P = 0.8) (see Fig. 2A). In addition, we further analyzed the variation in the positive rate of M. pneumoniae in different months of different years using a heatmap and found that the overall trend was that the positive rate of M. pneumoniae was generally higher from August to October, and that the positive rate of M. pneumoniae was significantly higher in 2021–2023 than in 2015–2019 (χ2 = 22.38, P < 0.0001) (see Fig. 2B). Furthermore, with the outbreak of the COVID-19 pandemic in January 2020, this study considered January 2020 as the boundary point to analyze the trend in the variation of the positive rate of M. pneumoniae before and after the COVID-19 pandemic. The results revealed that the positive rate of M. pneumoniae was generally higher after the COVID-19 pandemic compared with before (see Fig. 2C). Changes in the number of M. pneumoniae positive cases in different months can be found in Fig. S1.
Fig 2.
Changes in the positive rate of M. pneumoniae in different months. (A) Changes in the positive rate of M. pneumoniae in different months. (B) The changes in the positive rate of M. pneumoniae in different months from 2015 to 2023 were demonstrated by heatmap. (C) Changes in the positive rate of M. pneumoniae in different months before and after the COVID-19 outbreak.
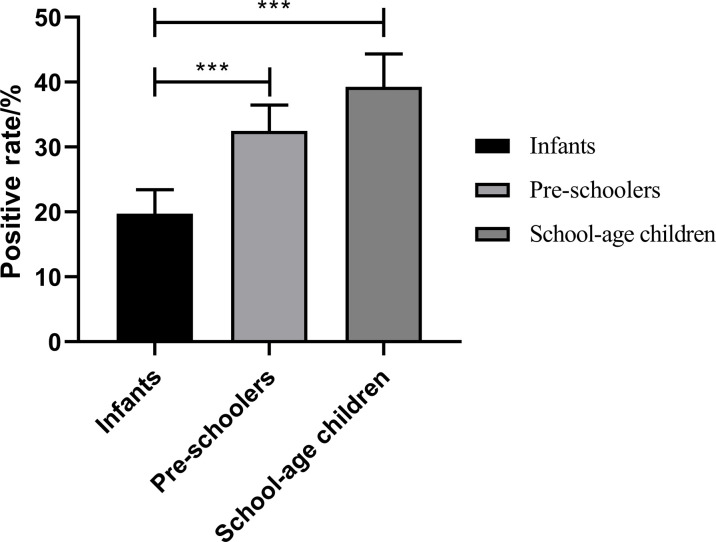
Difference of the positive rate of M. pneumoniae in different age groups
The positive rate of M. pneumoniae in children of different ages from 2015 to 2023 was calculated in this study. It was found that the positive rate of M. pneumoniae in infants and toddlers younger than 3 years old was 19.73% ± 11.09%, whereas the positive rate was 32.48% ± 12.02% in pre-schoolers aged between 3 and 6 years old. Moreover, the positive rate of M. pneumoniae was 39.27% ± 15.20% in the school-age group older than 6 years old. There was a significant difference in the positive rate of M. pneumoniae among these three groups (F = 480.0, P < 0.0001), as shown in Fig. 3. The difference of the number of positive cases of M. pneumoniae in different age groups can be found in Fig. S2.
Fig 3.
Difference of the positive rate of M. pneumoniae in different age groups, ***:P < 0.001
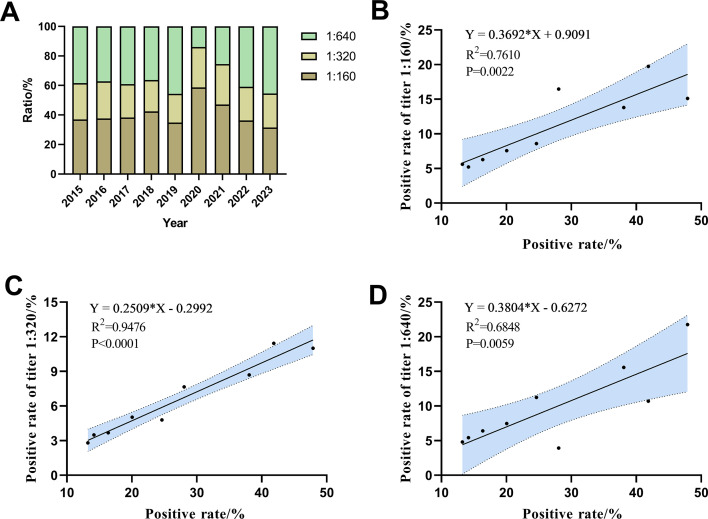
Comparison of M. pneumoniae antibody titers
The titer of serum M. pneumoniae antibody was detected by passive agglutination method in this study, and the proportion of different antibody titers in the positive cases was analyzed. It was found that the proportion of titers at 1:320 tended to stabilize, accounting for approximately 20%. During the period from 2015 to 2019, the proportion of antibody titers at 1:640 showed no remarkedly fluctuation. However, in the period from 2020 to 2023, with the increase in annual positive rate of M. pneumoniae, the proportion of titers 1:640 also increased. Conversely, the proportion of titers 1:160 showed the opposite trend (see Fig. 4A). Additionally, the correlation between the positive rate of antibody titers and the overall positive rate was analyzed, and the positive rates of the titer 1:160, 1:320, and 1:640 were all positively correlated with the overall positive rate, respectively (see Fig. 4B through D).
Fig 4.
Comparison of M. pneumoniae antibody titers. (A) Comparison of M. pneumoniae antibody titers between different years. (B) The correlation between the positive rate of titer 1:160 and the overall positive rate. (C) The correlation between the positive rate of titer 1:320 and the overall positive rate. (D) The correlation between the positive rate of titer 1:640 and the overall positive rate.
DISCUSSION
The number of pediatric respiratory tract infection cases in China has significantly increased this year, and M. pneumoniae is one of the main pathogens. M. pneumoniae infection occurs worldwide and is a common pathogen for acute upper respiratory tract infections in children of all ages (15–17). Studies have shown there are significant differences in the prevalence of M. pneumoniae infection among different countries and regions, different years and seasons, and different age groups (18). The prevalence of M. pneumoniae infection varies greatly in different countries worldwide, ranging from 8.7% to 37.5% (19). Gao et al. exhibited that the positive rate of M. pneumoniae in children with respiratory symptoms in northern China was 37.5% (7). In contrast, Jiang et al. revealed that the positive rate of M. pneumoniae in children with respiratory symptoms in southern China was only 12.2% (20). In this study, the number of M. pneumoniae detection cases and positive cases from 2015 to 2023 in a tertiary hospital in Anhui region was analyzed. This study found that the positive rate of M. pneumoniae from 2015 to 2023 was 32.08%, which is between the above two(12.2% and 37.5%). During 2023, 14,471 cases of respiratory tract infections were tested, among which 6,930 cases were positive for M. pneumoniae infection, accounting for 47.89%, suggesting that respiratory tract diseases caused by M. pneumoniae infection played a major role in pediatric cases in 2023. In addition, M. pneumoniae infection has periodic epidemic characteristics. Eun et al. found that the peak of M. pneumoniae prevalence in South Korea occurs every 3–4 years (21). Studies have shown that the infection rate of M. pneumoniae in children in Xi'an, China was relatively low in 2018 and 2019 (25.82% and 24.13%) (22). This study found that the lowest positive rate of M. pneumoniae occurred in 2018, only 13.23% (852/5588), which was consistent with previous studies (22).
In addition, there are sex differences in the prevalence of M. pneumoniae infection. Previous studies have revealed that the prevalence of M. pneumoniae infection is higher in females than in males, suggesting that females may be more susceptible to M. pneumoniae (7, 23). Similarly, in this study, the positive rate of M. pneumoniae in male children was 28.92%, lower than the 36.09% in female children, and the difference was statistically significant, which was consistent with previous studies (22, 24, 25). However, the number of positive cases of M. pneumoniae infection in females is not always higher than in males during this time period. According to the data from the seventh national census in China (https://www.stats.gov.cn/sj/pcsj/), the ratio of male to female children in Anhui province is 117:100, which may result in the higher overall detection cases and positive cases of M. pneumoniae in males than those in females. Previous literatures (7, 8) also reported that the number of positive cases of M. pneumoniae in male children was higher than that in female children in other regions of China, whereas the positive rate for M. pneumoniae in female children was significantly higher than that in male children, which is similar to our findings. This may be related to differences in lifestyle between girls and boys. Girls tend to have indoor activities during breaks, and indoor environments are relatively enclosed, which is more conducive to the transmission of M. pneumoniae (24, 25). There are also studies showing that the prevalence of M. pneumoniae in girls is significantly higher than that of boys, which may be related to the difference in hormone levels of girls and boys, making girls susceptible to M. pneumoniae, and the specific mechanism is still unknown, and this sex difference needs to be further studied. Children with M. pneumoniae infections occur throughout the year, but the seasonal trends can annually vary (9). A study in northern China showed the peak epidemics of M. pneumoniae infections in autumn, whereas a study in southern China found the peak epidemics in summer (23). Additionally, research in Korea indicated that the peak of M. pneumoniae outbreaks occurs in autumn or winter. Other studies have shown that there are no significant seasonal fluctuations in the prevalence of M. pneumoniae infections (24). Similarly, this study conducted in Anhui province found no significant differences in M. pneumoniae infections between different seasons or months. Nevertheless, an overall trend observed was that the positive rate of M. pneumoniae was generally higher from August to October, possibly related to the increased temperature during this period. In Anhui province, the temperature is higher, and people tend to gather in enclosed or semi-enclosed air-conditioned environments during August to October, which facilitates the transmission of M. pneumoniae (26, 27). Furthermore, M. pneumoniae outbreaks typically occur every 3–7 years and can last for 1–2 years (10–12). The most recent outbreaks were reported in 2013 and 2016 (25). The data from this study also showed that the peak epidemics for M. pneumoniae occurred in 2016 (20.06%). According to the epidemic pattern of M. pneumoniae outbreaks, the latest epidemic may last from autumn of 2019 to winter 2020 or spring 2021. Nevertheless, during the period from March 2020 to April 2021, the positive rate of M. pneumoniae did not increase significantly but significantly decreased. It was reported that the number of M. pneumoniae positive cases in Chengdu City decreased during the summer of 2020 (9, 10). Data in Finland and Japan also revealed a dramatic decrease in the incidence of M. pneumoniae in 2020 compared with 2012 and 2016 (28, 29). This may be due to the series of prevention and control measures issued by the National Health Commission of China after the outbreak of COVID-19 in 2020, including regular handwashing, restrictions on public gatherings, and wearing masks, effectively controlling the spread of the COVID-19 epidemic and markedly reducing the transmission of M. pneumoniae (4, 5, 9, 30). Notably, after April 2021, the positive rate of M. pneumoniae began to rebound, especially in children, with the highest positivity rate recorded for this year (47.89%). This may be because the COVID-19 pandemic delayed the timing of M. pneumoniae outbreaks.
M. pneumoniae infection can occur in any age group, especially in preschool and school-age children (31–33). The highest prevalence of M. pneumoniae is in the 7–10-year-age group, whereas in Australia, the highest prevalence of M. pneumoniae is in the 5–9-year-age group (34, 35). One study exhibited that the prevalence of M. pneumoniae in children older than 7 years old in South China is the highest. The results of this study showed that the positive rate of M. pneumoniae in infants and toddlers younger than 3 years old was 19.73% ± 11.09%, whereas in pre-schoolers aged between 3 and 6 years old, the positive rate was 32.48% ± 12.02%. Moreover, the positive rate of M. pneumoniae was 39.27±15.20% in the school-age group older than 6 years old, which is consistent with the results of most studies (9, 10). This indicates that the prevalence of M. pneumoniae infection in children increases significantly with age. School-age children between 6 and 14 years are more susceptible to infection, which may be due to their immature immune system and their frequent exposure to crowded places, such as kindergartens and schools, increasing the likelihood of respiratory pathogen infections and cross-transmission (23, 36). On the other hand, the prevalence of M. pneumoniae in infants aged 0–3 years is low, which may be related to the simple living environment and predominantly breastfeeding in this age group (22).
There are several limitations to this study. On the one hand, this study is a single-center retrospective analysis to explore the prevalence of M. pneumoniae infection in Anhui province based on clinical data from a large comprehensive tertiary hospital. To comprehensively analyze the epidemic patterns of M. pneumoniae in the region, multi-center analysis of the prevalence of M. pneumoniae infection can be considered in the future. On the other hand, the diagnosis of M. pneumoniae infection in this study was only based on the results of serum M. pneumoniae antibodies (passive agglutination method), and the antibody titer ≥1:160 was considered as recent M. pneumoniae infection.
In conclusion, the results of this study exhibited that M. pneumoniae infection in the Anhui region was characterized by a high positive rate during 2021–2023, especially this year is considered a year of pandemic for M. pneumoniae infection. Moreover, the positive rate of M. pneumoniae in female children is significantly higher than in male children, and the infection rate of M. pneumoniae in children increases significantly with age, particularly in school-aged (7–14 years old) children. Notably, during the period from April 2020 to March 2021, the positive rate of M. pneumoniae in children remained at a low level, indicating that the preventive and control measures for COVID-19 might have effectively controlled the transmission of M. pneumoniae infection.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Foundation of Anhui Educational Committee (No. 2023AH053165).
B.C., Y.-L.G., Q.-J.C., and Q.Z. participated in study design, data analysis, and manuscript writing. T.-D.Z., Y.T., N.H., and A.-H.W. participated in data collection, data sorting, and manuscript modification. All authors approved the final version of the manuscript submitted.
Contributor Information
Qiang Zhou, Email: zhouqiang1973@163.com.
Deena R. Altman, Icahn School of Medicine at Mount Sinai, New York, New York, USA
Robert Doug Hardy, Infectious Diseases Specialists, Plano, Texas, USA.
DATA AVAILABILITY
The authors confirm that the data supporting the findings of this study are available within the article and supplemental material.
ETHICS APPROVAL
This study was approved by the Institutional Ethics Committee of The Second Affiliated Hospital of Anhui Medical University (No. SZR2021038), and it was in compliance with national legislation and the Declaration of Helsinki guidelines.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.00651-24.
Fig. S1 and S2.
An accounting of the reviewer comments and feedback.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Wang Y, Yu X, Liu F, Tian X, Quan S, Jiao A, Yang X, Zeng X, Jiao W, Qi H, Xu F, Li Q, Liu S, Xu B, Sun L, Shen A. 2023. Respiratory microbiota imbalance in children with Mycoplasma pneumoniae pneumonia. Emerg Microbes Infect 12:2202272. doi: 10.1080/22221751.2023.2202272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rothstein TE, Cunningham SA, Rieke RA, Mainella JM, Mutchler MM, Patel R. 2022. Macrolide resistance in Mycoplasma pneumoniae, Midwestern United States, 2014 to 2021. Antimicrob Agents Chemother 66:e0243221. doi: 10.1128/aac.02432-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nantanda R, Bwanga F, Najjingo I, Ndeezi G, Tumwine JK. 2021. Prevalence, risk factors and outcome of Mycoplasma pneumoniae infection among children in Uganda: a prospective study. Paediatr Int Child Health 41:188–198. doi: 10.1080/20469047.2021.1980698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu T-H, Wu J-Y, Huang P-Y, Tsai Y-W, Lai C-C. 2023. The effect of nirmatrelvir plus ritonavir on the long-term risk of epilepsy and seizure following COVID-19: a retrospective cohort study including 91,528 patients. J Infect 86:256–308. doi: 10.1016/j.jinf.2023.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meyer Sauteur PM, Beeton ML, Uldum SA, Bossuyt N, Vermeulen M, Loens K, Pereyre S, Bébéar C, Keše D, Day J, Afshar B, Chalker VJ, Greub G, Nir-Paz R, Dumke R, ESGMAC–MyCOVID Study Team . 2022. Mycoplasma pneumoniae detections before and during the COVID-19 pandemic: results of a global survey, 2017 to 2021. Euro Surveill 27:19. doi: 10.2807/1560-7917.ES.2022.27.19.2100746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Akashi Y, Hayashi D, Suzuki H, Shiigai M, Kanemoto K, Notake S, Ishiodori T, Ishikawa H, Imai H. 2018. Clinical features and seasonal variations in the prevalence of macrolide-resistant Mycoplasma pneumoniae. J Gen Fam Med 19:191–197. doi: 10.1002/jgf2.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gao L-W, Yin J, Hu Y-H, Liu X-Y, Feng X-L, He J-X, Liu J, Guo Y, Xu B-P, Shen K-L. 2019. The epidemiology of paediatric Mycoplasma pneumoniae pneumonia in North China: 2006 to 2016. Epidemiol Infect 147:e192. doi: 10.1017/S0950268819000839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qu J, Yang C, Bao F, Chen S, Gu L, Cao B. 2018. Epidemiological characterization of respiratory tract infections caused by Mycoplasma pneumoniae during epidemic and post-epidemic periods in North China, from 2011 to 2016. BMC Infect Dis 18:335. doi: 10.1186/s12879-018-3250-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang L, Lai M, Ai T, Liao H, Huang Y, Zhang Y, Liu Y, Wang L, Hu J. 2021. Analysis of Mycoplasma pneumoniae infection among children with respiratory tract infections in hospital in Chengdu from 2014 to 2020. Transl Pediatr 10:990–997. doi: 10.21037/tp-21-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang Y, Huang Y, Ai T, Luo J, Liu H. 2021. Effect of COVID-19 on childhood Mycoplasma pneumoniae infection in Chengdu, China. BMC Pediatr 21. doi: 10.1186/s12887-021-02679-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee K-L, Lee C-M, Yang T-L, Yen T-Y, Chang L-Y, Chen J-M, Lee P-I, Huang L-M, Lu C-Y. 2021. Severe Mycoplasma pneumoniae pneumonia requiring intensive care in children, 2010-2019. J Formos Med Assoc 120:281–291. doi: 10.1016/j.jfma.2020.08.018 [DOI] [PubMed] [Google Scholar]
- 12. Kurkela S, Puolakkainen M, Hokynar K, Nieminen T, Saxen H, Mannonen L, Pietikäinen R. 2019. Mycoplasma pneumoniae outbreak, Southeastern Finland, 2017-2018: molecular epidemiology and laboratory diagnostic lessons. Eur J Clin Microbiol Infect Dis 38:1867–1871. doi: 10.1007/s10096-019-03619-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chalker V, Stocki T, Mentasti M, Fleming D, Harrison T. 2011. Increased incidence of Mycoplasma pneumoniae infection in England and Wales in 2010: multiocus variable number tandem repeat analysis typing and macrolide susceptibility. Euro Surveill 16:19. [PubMed] [Google Scholar]
- 14. Zhu Y, Luo Y, Li L, Jiang X, Du Y, Wang J, Li H, Gu H, Li D, Tang H, Qin H, Xu C, Liu Y, Zhao D, Guo Y, Liu F. 2023. Immune response plays a role in Mycoplasma pneumoniae pneumonia. Front Immunol 14:1189647. doi: 10.3389/fimmu.2023.1189647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Han Z, Zhang Y, Liao S, Zhou N. 2021. The clinical characteristics of Mycoplasma pneumoniae pneumonia and its relationship between hypokalemia in west China. Transl Pediatr 10:406–414. doi: 10.21037/tp-20-471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuo C-Y, Tsai W-C, Lee H-F, Ho T-S, Huang L-M, Shen C-F, Liu C-C. 2022. The epidemiology, clinical characteristics, and macrolide susceptibility of Mycoplasma pneumoniae pneumonia in children in Southern Taiwan, 2019–2020. J Microbiol Immunol Infect 55:611–619. doi: 10.1016/j.jmii.2021.09.010 [DOI] [PubMed] [Google Scholar]
- 17. Wang N, Zhang H, Yin Y, Xu X, Xiao L, Liu Y. 2022. Antimicrobial susceptibility profiles and genetic characteristics of Mycoplasma pneumoniae in Shanghai, China, from 2017 to 2019. Infect Drug Resist 15:4443–4452. doi: 10.2147/IDR.S370126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jiang F-C, Wang R-F, Chen P, Dong L-Y, Wang X, Song Q, Wan Y-Q, Song Q-Q, Song J, Wang Y-H, Xia Z-Q, Xia D, Han J. 2021. Genotype and mutation patterns of macrolide resistance genes of Mycoplasma pneumoniae from children with pneumonia in Qingdao, China, in 2019. J Glob Antimicrob Resist 27:273–278. doi: 10.1016/j.jgar.2021.10.003 [DOI] [PubMed] [Google Scholar]
- 19. Xiao L, Ratliff AE, Crabb DM, Mixon E, Qin X, Selvarangan R, Tang Y-W, Zheng X, Dien Bard J, Hong T, Prichard M, Brooks E, Dallas S, Duffy LB, Fowler KB, Atkinson TP, Waites KB. 2020. Molecular characterization of Mycoplasma pneumoniae isolates in the United States from 2012 to 2018. J Clin Microbiol 58:e00710-20. doi: 10.1128/JCM.00710-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiang Q, Yang F, Peng Y, Dong X, Ge Y. 2020. Epidemiology and molecular identification of mycoplasma pneumoniae associated with respiratory infections in Zhejiang province, China, 2008-2017. J Clin Lab Anal 34:e23460. doi: 10.1002/jcla.23460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eun BW, Kim NH, Choi EH, Lee HJ. 2008. Mycoplasma pneumoniae in Korean children: the epidemiology of pneumonia over an 18-year period. J Infect 56:326–331. doi: 10.1016/j.jinf.2008.02.018 [DOI] [PubMed] [Google Scholar]
- 22. Meng G-L, Kang R, Cheng X-Y, Wang Q, Xie Y. 2022. Laboratory analysis of positive rate of Mycoplasma pneumoniae antibody among 53,273 children with respiratory tract infections in Xi’an from 2017 to 2020. Transl Pediatr 11:625–630. doi: 10.21037/tp-22-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu Y-C, Zhu L-J, Xu D, Tao X-F, Li S-X, Tang L-F, Chen Z-M. 2011. Epidemiological characteristics and meteorological factors of childhood Mycoplasma pneumoniae pneumonia in Hangzhou. World J Pediatr 7:240–244. doi: 10.1007/s12519-011-0318-0 [DOI] [PubMed] [Google Scholar]
- 24. Lv Y-T, Sun X-J, Chen Y, Ruan T, Xu G-P, Huang J-A. 2022. Epidemic characteristics of Mycoplasma pneumoniae infection: a retrospective analysis of a single center in Suzhou from 2014 to 2020. Ann Transl Med 10:1123. doi: 10.21037/atm-22-4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cheng Y, Cheng Y, Dai S, Hou D, Ge M, Zhang Y, Fan L, Pei Y, Yu L, Xue G, Ma L, Sun H. 2022. The prevalence of Mycoplasma pneumoniae among children in Beijing before and during the COVID-19 pandemic. Front Cell Infect Microbiol 12:854505. doi: 10.3389/fcimb.2022.854505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang X, Han M-N, Dong J-H, Li X-X, Hu X-Y, Wang Z, Qin E-Q, Li J, Tan J-Y, Wang F-S, Huang L. 2020. Outbreak of Mycoplasma pneumoniae at a military academy. Mil Med Res 7:60. doi: 10.1186/s40779-020-00289-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Suzuki Y, Seto J, Shimotai Y, Itagaki T, Katsushima Y, Katsushima F, Ikeda T, Mizuta K, Hongo S, Matsuzaki Y. 2019. Polyclonal spread of multiple genotypes of Mycoplasma pneumoniae in semi-closed settings in Yamagata, Japan. J Med Microbiol 68:785–790. doi: 10.1099/jmm.0.000969 [DOI] [PubMed] [Google Scholar]
- 28. Haapanen M, Renko M, Artama M, Kuitunen I. 2021. The impact of the lockdown and the re-opening of schools and day cares on the epidemiology of SARS-CoV-2 and other respiratory infections in children - a nationwide register study in Finland. EClinMed 34:100807. doi: 10.1016/j.eclinm.2021.100807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fujita J. 2021. Mycoplasma pneumoniae pneumonia and respiratory syncytial virus infection in Japan during the severe acute respiratory syndrome coronavirus 2 pandemic. Respir Investig 59:5–7. doi: 10.1016/j.resinv.2020.11.002 [DOI] [PubMed] [Google Scholar]
- 30. Wang X, Li M, Luo M, Luo Q, Kang L, Xie H, Wang Y, Yu X, Li A, Dong M, Huang F, Gong C. 2022. Mycoplasma pneumoniae triggers pneumonia epidemic in autumn and winter in Beijing: a multicentre, population-based epidemiological study between 2015 and 2020. Emerg Microbes Infect 11:1508–1517. doi: 10.1080/22221751.2022.2078228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lu Y, Wang Y, Hao C, Ji W, Chen Z, Jiang W, Yan Y, Gu W. 2018. Clinical characteristics of pneumonia caused by Mycoplasma pneumoniae in children of different ages. Int J Clin Exp Pathol 11:855–861. [PMC free article] [PubMed] [Google Scholar]
- 32. Su M, Wang Q, Li D, Wang L-L, Wang C-Y, Wang J-L, Zhang Q, Du L-Y, Liu J-Y, Xie G-C. 2021. Prevalence and clinical characteristics of hospitalized children with community-acquired Mycoplasma pneumoniae pneumonia during 2017/2018, Chengde, China. Medicine (Abingdon) 100:e23786. doi: 10.1097/MD.0000000000023786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang B, Zhang W, Gu W, Zhang X, Wang M, Huang L, Zhu C, Yan Y, Ji W, Ni H, Chen Z. 2021. Differences of clinical features and prognosis between Mycoplasma pneumoniae necrotizing pneumonia and non-Mycoplasma pneumoniae necrotizing pneumonia in children. BMC Infect Dis 21:797. doi: 10.1186/s12879-021-06469-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ito I, Ishida T, Osawa M, Arita M, Hashimoto T, Hongo T, Mishima M. 2001. Culturally verified Mycoplasma pneumoniae pneumonia in Japan: a long-term observation from 1979-99. Epidemiol Infect 127:365–367. doi: 10.1017/s0950268801005982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Othman N, Isaacs D, Kesson A. 2005. Mycoplasma pneumoniae infections in Australian children. J Paediatr Child Health 41:671–676. doi: 10.1111/j.1440-1754.2005.00757.x [DOI] [PubMed] [Google Scholar]
- 36. Cai F, Shou X, Ye Q. 2022. Epidemiological study on Mycoplasma pneumoniae and Chlamydia pneumoniae infection of hospitalized children in a single center during the COVID-19 pandemic. Front Cell Infect Microbiol 12:843463. doi: 10.3389/fcimb.2022.843463 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 and S2.
An accounting of the reviewer comments and feedback.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and supplemental material.