Abstract
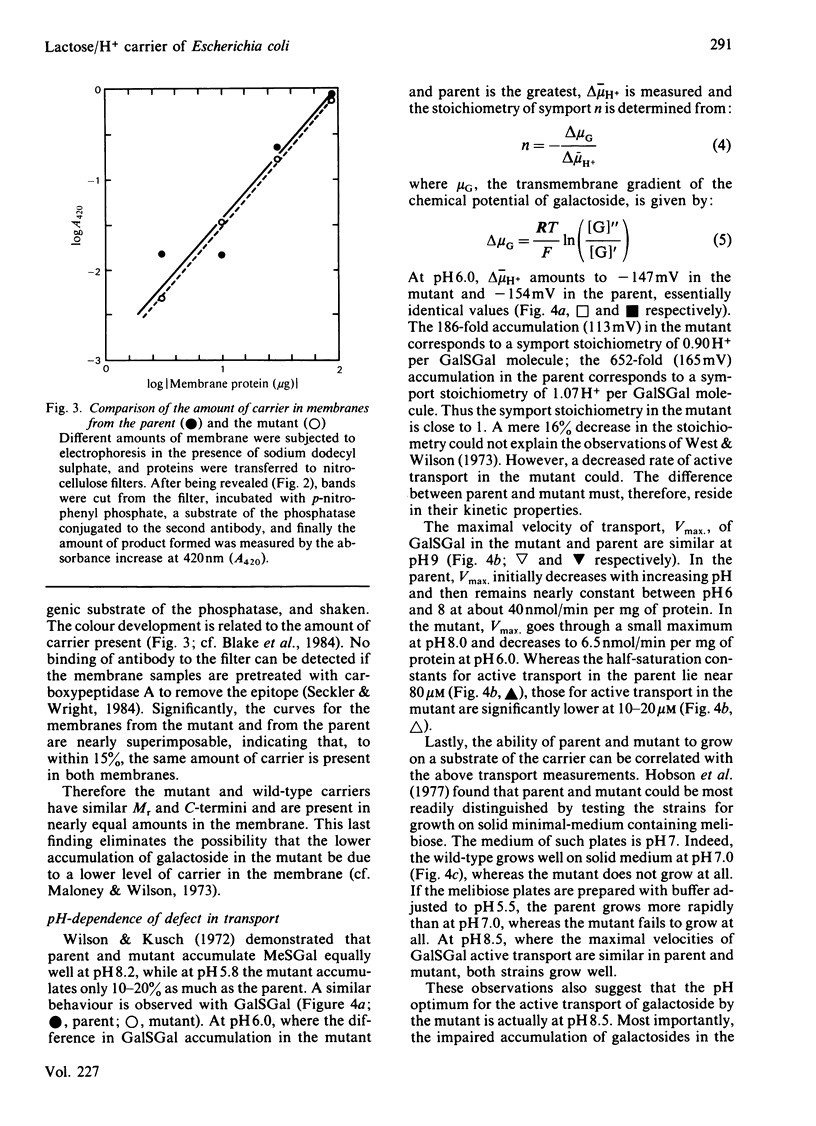
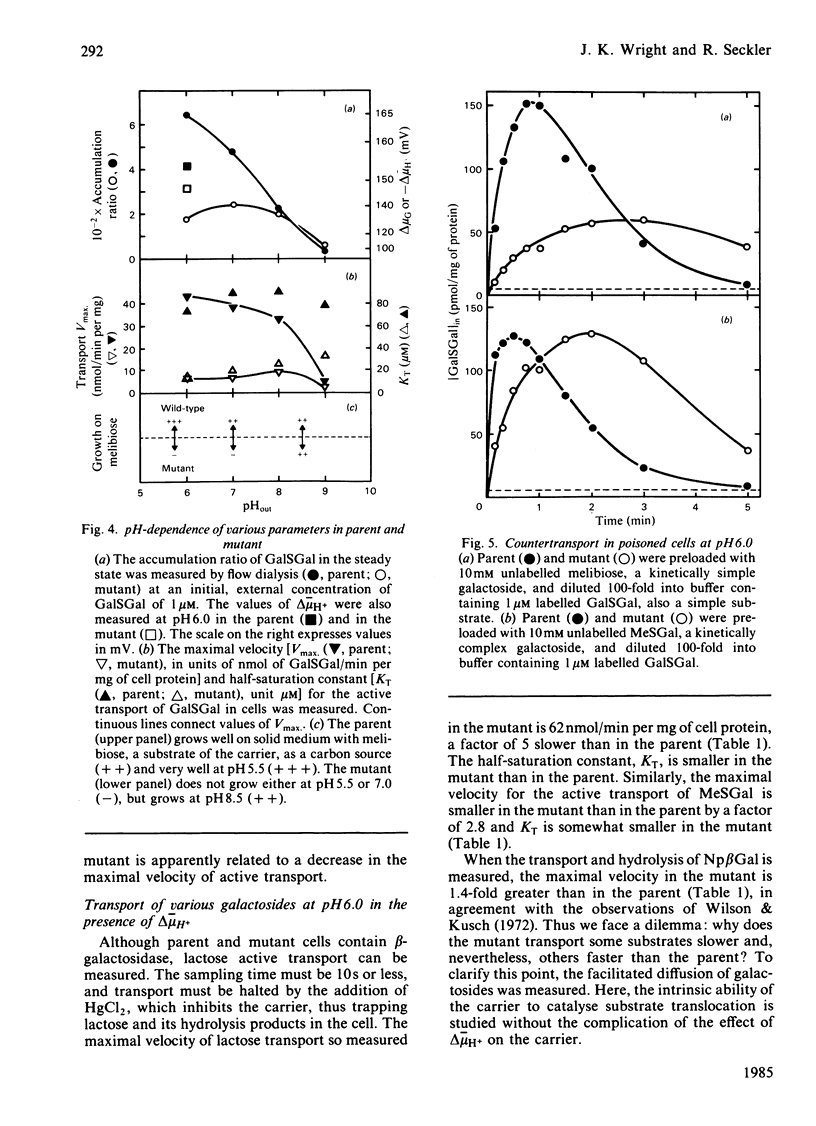
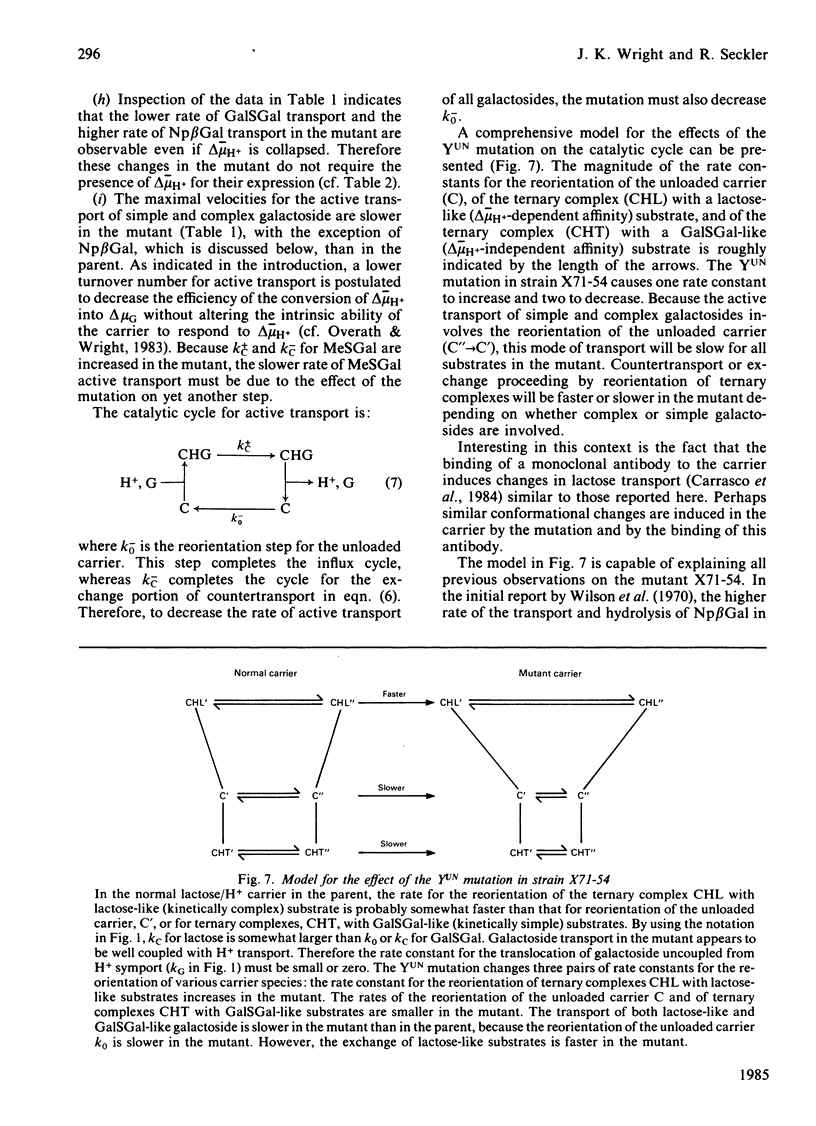
The Escherichia coli K12 strain X71-54 carries the lac YUN allele, coding for a lactose/H+ carrier defective in the accumulation of a number of galactosides [Wilson, Kusch & Kashket (1970) Biochem. Biophys. Res. Commun. 40, 1409-1414]. Previous studies proposed that the lower accumulation in the mutant be due to a faulty coupling of H+ and galactoside fluxes via the carrier. Immunochemical characterization of the carriers in membranes from mutant and parent strains with an antibody directed against the C-terminal decapeptide of the wild-type carrier leads to the conclusion that the mutant carrier is similar to the wild-type in terms of apparent Mr, C-terminal sequence, and level of incorporation into the membrane. The pH-dependence of galactoside transport was compared in the mutant and the parent. At pH 8.0-9.0, mutant and parent behave similarly with respect to the accumulation of beta-D-galactosyl 1-thio-beta-D-galactoside and to the ability to grow on the carrier substrate melibiose. At pH 6.0, both the maximal velocity for active transport and the level of accumulation of beta-D-galactosyl-1-thio-beta-D-galactoside are lower in the mutant. The mutant also is unable to grow on melibiose at pH 5.5. However, at pH 6.0 and low galactoside concentrations, the symport stoichiometry is 0.90 H+ per galactoside in the mutant as compared with 1.07 in the parent. These observations suggest that symport is normal in the mutant and that the lower rate of transport in the mutant is responsible for the phenotype. At higher galactoside concentrations, accumulation is determined not only thermodynamically but also kinetically, contrary to a simple interpretation of the chemiosmotic theory. Therefore lower rates of active transport can mimic the effect of uncoupling H+ and galactoside symport. Examination of countertransport in poisoned cells at pH 6.0 reveals that the rate constants for the reorientation of the loaded and unloaded carrier are altered in the mutant. The reorientation of the unloaded carrier is slower in the mutant. However, the reorientation of the galactoside-H+-carrier complex is slower for substrates like melibiose, but faster for substrates like lactose. These findings suggest that lactose-like and melibiose-like substrates interact with the carrier in slightly different ways.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTTIN G., COHEN G. N., MONOD J., RICKENBERG H. V. La galactoside-perméase d'Escherichia coli. Ann Inst Pasteur (Paris) 1956 Dec;91(6):829–857. [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Carrasco N., Herzlinger D., Mitchell R., DeChiara S., Danho W., Gabriel T. F., Kaback H. R. Intramolecular dislocation of the COOH terminus of the lac carrier protein in reconstituted proteoliposomes. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4672–4676. doi: 10.1073/pnas.81.15.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane R. K. The gradient hypothesis and other models of carrier-mediated active transport. Rev Physiol Biochem Pharmacol. 1977;78:99–159. doi: 10.1007/BFb0027722. [DOI] [PubMed] [Google Scholar]
- Hobson A. C., Gho D., Müller-Hill B. Isolation, genetic analysis, and characterization of Escherichia coli mutants with defects in the lacY gene. J Bacteriol. 1977 Sep;131(3):830–838. doi: 10.1128/jb.131.3.830-838.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Maloney P. C., Wilson T. H. Quantitative aspects of active transport by the lactose transport system of Escherichia coli. Biochim Biophys Acta. 1973 Dec 13;330(2):196–205. doi: 10.1016/0005-2736(73)90225-3. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Performance and conservation of osmotic work by proton-coupled solute porter systems. J Bioenerg. 1973 Jan;4(1):63–91. doi: 10.1007/BF01516051. [DOI] [PubMed] [Google Scholar]
- Overath P., Teather R. M., Simoni R. D., Aichele G., Wilhelm U. Lactose carrier protein of Escherichia coli. Transport and binding of 2'-(N-dansyl)aminoethyl beta-D-thiogalactopyranoside and p-nitrophenyl alpha-d-galactopyranoside. Biochemistry. 1979 Jan 9;18(1):1–11. doi: 10.1021/bi00568a001. [DOI] [PubMed] [Google Scholar]
- Quiocho F. A., Vyas N. K. Novel stereospecificity of the L-arabinose-binding protein. Nature. 1984 Aug 2;310(5976):381–386. doi: 10.1038/310381a0. [DOI] [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The electrochemical gradient of protons and its relationship to active transport in Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1892–1896. doi: 10.1073/pnas.73.6.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandermann H., Jr beta-D-Galactoside transport in Escherichia coli: substrate recognition. Eur J Biochem. 1977 Nov 1;80(2):507–515. doi: 10.1111/j.1432-1033.1977.tb11906.x. [DOI] [PubMed] [Google Scholar]
- Seckler R., Wright J. K., Overath P. Peptide-specific antibody locates the COOH terminus of the lactose carrier of Escherichia coli on the cytoplasmic side of the plasma membrane. J Biol Chem. 1983 Sep 25;258(18):10817–10820. [PubMed] [Google Scholar]
- Seckler R., Wright J. K. Sidedness of native membrane vesicles of Escherichia coli and orientation of the reconstituted lactose: H+ carrier. Eur J Biochem. 1984 Jul 16;142(2):269–279. doi: 10.1111/j.1432-1033.1984.tb08281.x. [DOI] [PubMed] [Google Scholar]
- Seto-Young D., Bedu S., Wilson T. H. Transport by reconstituted lactose carrier from parental and mutant strains of Escherichia coli. J Membr Biol. 1984;79(2):185–193. doi: 10.1007/BF01872122. [DOI] [PubMed] [Google Scholar]
- Smith-Gill S. J., Rupley J. A., Pincus M. R., Carty R. P., Scheraga H. A. Experimental identification of a theoretically predicted "left-sided" binding mode for (GlcNAc)6 in the active site of lysozyme. Biochemistry. 1984 Feb 28;23(5):993–997. doi: 10.1021/bi00300a030. [DOI] [PubMed] [Google Scholar]
- Teather R. M., Bramhall J., Riede I., Wright J. K., Fürst M., Aichele G., Wilhelm U., Overath P. Lactose carrier protein of Escherichia coli. Structure and expression of plasmids carrying the Y gene of the lac operon. Eur J Biochem. 1980;108(1):223–231. doi: 10.1111/j.1432-1033.1980.tb04715.x. [DOI] [PubMed] [Google Scholar]
- Teather R. M., Müller-Hill B., Abrutsch U., Aichele G., Overath P. Amplification of the lactose carrier protein in Escherichia coli using a plasmid vector. Mol Gen Genet. 1978 Feb 27;159(3):239–248. doi: 10.1007/BF00268260. [DOI] [PubMed] [Google Scholar]
- West I. C., Wilson T. H. Galactoside transport dissociated from proton movement in mutants of Escherichia coli. Biochem Biophys Res Commun. 1973 Jan 23;50(2):551–558. doi: 10.1016/0006-291x(73)90875-9. [DOI] [PubMed] [Google Scholar]
- Wilson T. H., Kusch M. A mutant of Escherichia coli K 12 energy-uncoupled for lactose transport. Biochim Biophys Acta. 1972 Mar 17;255(3):786–797. doi: 10.1016/0005-2736(72)90391-4. [DOI] [PubMed] [Google Scholar]
- Wilson T. H., Kusch M., Kashket E. R. A mutant in Escherichia coli energy-uncoupled for lactose transporta defect in the lactose-operon. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1409–1414. doi: 10.1016/0006-291x(70)90024-0. [DOI] [PubMed] [Google Scholar]
- Wong P. T., Kashket E. R., Wilson T. H. Energy coupling in the lactose transport system of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Jan;65(1):63–69. doi: 10.1073/pnas.65.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. K., Overath P. Purification of the lactose:H+ carrier of Escherichia coli and characterization of galactoside binding and transport. Eur J Biochem. 1984 Feb 1;138(3):497–508. doi: 10.1111/j.1432-1033.1984.tb07944.x. [DOI] [PubMed] [Google Scholar]
- Wright J. K., Riede I., Overath P. Lactose carrier protein of Escherichia coli: interaction with galactosides and protons. Biochemistry. 1981 Oct 27;20(22):6404–6415. doi: 10.1021/bi00525a019. [DOI] [PubMed] [Google Scholar]



