Abstract
The biotechnological development of monoclonal antibodies and their immunotherapeutic use in oncology have grown exponentially in the last decade, becoming the first-line therapy for some types of cancer. Their mechanism of action is based on the ability to regulate the immune system or by interacting with targets that are either overexpressed in tumor cells, released into the extracellular milieu or involved in processes that favor tumor growth. In addition, the intrinsic characteristics of each subclass of antibodies provide specific effector functions against the tumor by activating antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity, and antibody-dependent cellular phagocytosis, among other mechanisms. The rational design and engineering of monoclonal antibodies have improved their pharmacokinetic and pharmacodynamic features, thus optimizing the therapeutic regimens administered to cancer patients and improving their clinical outcomes. The selection of the immunoglobulin G subclass, modifications to its crystallizable region (Fc), and conjugation of radioactive substances or antineoplastic drugs may all improve the antitumor effects of therapeutic antibodies. This review aims to provide insights into the immunological and pharmacological aspects of therapeutic antibodies used in oncology, with a rational approach at molecular modifications that can be introduced into these biological tools, improving their efficacy in the treatment of cancer.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-024-03814-2.
Keywords: Monoclonal antibodies, Cancer therapy, Immunotherapy, Immunobiotechnology, Bispecifics, Antibody-drug conjugates, Bispecific T-cell engagers (BiTEs)
Introduction
The pharmaceutical industry has grown along with the development of biotechnology to generate innovative drugs [1]. Among these, monoclonal antibodies (mAbs) to treat diseases, including cancer, are distinguished: of a total of 213 mAbs globally approved or under review up to May 15, 2024, 99 are indicated for cancer treatment, a highly prevalent and deadly pathology worldwide [2, 3]. Among these anti-cancer mAbs, 76 are currently approved by the World Health Organization (WHO)-Listed Authorities (WLA) in several country of the world [4].
Over time, the technologies used for the development of therapeutic mAbs have evolved from the first therapeutic molecules of murine origin to completely human antibodies, which included modifications in their amino acid sequences, further optimizing the exceptional characteristics of these drugs. The engineering of antibodies has enabled the development of mAbs with enhanced binding affinity to therapeutic targets and prolonged serum half-life, even far exceeding 20 days. Moreover, they have achieved increased capacity to activate mediators that contribute to either eliminate tumor cells, modulate antibody effector functions, or facilitate the entry of cytotoxic drugs into the cells of interest [5].
Here, we provide an overview of the mAbs approved for oncology treatment by the WLA, focusing on their therapeutic mechanisms of action; we also examine the rational design of modifications applied to antibodies in order to improve their therapeutic efficacy. Anti-cancer mAbs that received approval by the WLA, but have been withdrawn or marketing discontinued, will not be covered in this review.
Structure and function of antibodies
Antibodies (Abs) or immunoglobulins (Igs) are soluble glycoproteins responsible for the specific and selective recognition of antigens. Structurally, the basic unit of Abs is a monospecific monomer composed of four polypeptide chains: two identical heavy chains (HC) with a molecular mass of 50–75 kDa each and two identical light chains (LC) of approximately 25 kDa each (Fig. 1A). The modular domains that compose each HC consist of one variable heavy domain (VH) and three or four constant heavy domains (CH1, CH2, CH3, CH4), depending on the antibody isotype. The modular domains that form each LC consist of one variable light domain (VL) and one constant light domain (CL). The combination of a VH domain with a VL domain forms the antigen-binding region, which can also be referred to as the Fv region. Each variable domain contains three hypervariable regions called complementary-determining regions (CDRs). The amino acids of CDRs are different in sequence and structure between different antigen-specific antibodies. Upon protein folding, in each VH and VL chain, the CDR regions give rise to three loops that connect to β-strands; the six loops are in proximity to each other; together, they form the antigen-binding site, also known as the paratope. The strands of the two β-sheets and the non-hypervariable loops are called framework regions (FRs) [5]. Although the paratope aligns harmoniously with the antigen’s epitope, only a few residues on the paratope contribute to the binding energy. The antibody-antigen binding strength, measured in KD (dissociation constant in the order of nM to pM), as well as the antibody specificity, is useful properties to design therapeutic monoclonal Abs (TmAbs) for treating several diseases, including cancer. In addition, the selection of targets that discriminate between a tumor and a non-tumor cell contributes to the ability of the TmAb to neutralize or selectively block molecules that facilitate tumor growth or even induce cell death.
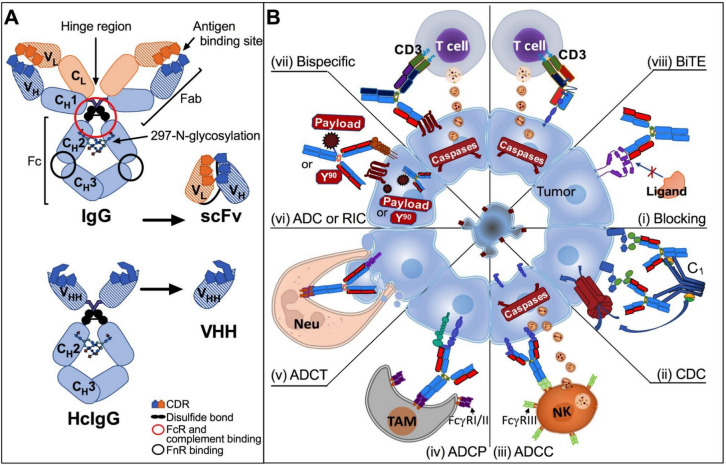
Fig. 1.
A Structure of therapeutic monoclonal antibodies (TmAbs). Human IgG (top) is formed by two heavy chains and two light chains linked to each other by disulfide bonds, which originate Fab and Fc fragments. Fab fragment contains the antigen-binding site formed by complementary-determining regions (CDRs). The Fc fragment is responsible for effector functions and binds to the neonatal receptor. The single-chain fragment variable (scFv) structure is composed of the variable domains of heavy and light chains. The structure of heavy chain-only antibodies (HcIgG) from camelids (bottom) includes their respective variable domains (VHH), which are being used in the design of TmAbs. B Mechanism of action of TmAbs. i Blockage of immunoregulators (CTLA-4, PD-1, or PDL-1), cytokines or growth factors. The antibody binds to its target expressed on the tumor cell membrane and prevents its interaction with its ligand; ii complement activation-dependent cytotoxicity (CDC). Complement molecule C1q directly interacts with target-bound IgG CH2 domain, resulting in the activation of the complement cascade and the formation of the membrane attack complex, which induces cell lysis. iii Antibody-dependent cellular cytotoxicity (ADCC). FcγRIIIa (CD16a), a low-affinity activation receptor present on Natural Killer (NK) cells, binds to target-bound IgG1 CH2 domain. Activation of CD16 triggers the release of perforin and granzymes, resulting in target cell death. iv Antibody-dependent cellular phagocytosis (ADCP). ADCP is mediated through the interaction of the Fc domain of target cell-bound IgG with FcγRs on phagocytic cells, such as tumor-associated macrophages (TAM). FcγRI (CD64) and FcγRII (CD32) recognize overlapping but not identical sites in the lower hinge region of IgGs, which promotes tumor cell phagocytosis. v Antibody-dependent cellular trogocytosis (ADCT). Neutrophils can remove tumor cell surface content in the presence of TmAbs targeting specific cell membrane proteins through Fc binding to FcγR on the effector immune cell, which may trigger tumor cell lysis. vi Antibody–drug conjugate (ADC) and radioimmunoconjugate (RIC). Antineoplastic drugs or radioactive elements can be conjugated to the antibody through a peptide linker to be directed to the target tissue, increasing its cytotoxic concentration in situ and decreasing its unwanted effects on other tissues. vii,viii Bispecific molecules. vii Bispecific antibodies. They have dual binding sites that bind two different antigens. viii Bispecific T-cell engager (BiTE). They are recombinant proteins composed of two scFvs linked by a short flexible linker. These bispecific molecules are designed to target tumor antigens and CD3, thereby creating a link between tumor cells and T cells. Created with Microsoft® PowerPoint for Mac. Version 15.32 and Prism 6 for Mac OS X
The two HCs are linked by disulfide bonds between their hinge regions; the VH and CH1 domains, together with the LCs, form the antigen-binding fragment (Fab), and the CH2, CH3, and CH4 domains of both HCs form the crystallizable fragment (Fc), located at the carboxyl-terminal end of each chain. Both Fab and Fc fragments are linked by the hinge region, which provides conformational flexibility to the antibody [5]. In a given antibody, the two HCs are identical, as are the two LCs; therefore, an Ab is a bivalent molecule, having two identical combinations of VH and VL domains. Although most mammals have the conventional IgG subclass structure, which is composed of four polypeptide chains (2 HCs and 2 LCs), camelid species and sharks contain smaller Ig fractions lacking the LCs and the first HC constant domain (HC-only antibodies or HCAbs), accounting for 25–75% of their total serum IgG [6] (Fig. 1A). In this case, the term “VHH” is used to distinguish the variable domains of camelid from the conventional VH domains [7]. Due to its biological properties, IgG is the most widely used Ig as the basis for therapeutic antibody development, a topic that will be discussed in detail in this article.
Of the 76 TmAbs currently approved by the WLA, 49 are human IgG1, 3 are IgG2, and 19 are IgG4 [4]. The IgG HC constant region determines the antibody subclass. Human IgG1, IgG2, IgG3, and IgG4 carry the γ1, γ2, γ3, and γ4 chains, respectively. The Fc fragment of IgG (IgG-Fc) accounts for the antibody’s effector functions, which include complement-dependent cytotoxicity (CDC) [8], antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and antibody-dependent cellular trogocytosis (ADCT) [9–12] (Fig. 1B). These effector functions depend, respectively, on IgG-Fc binding to complement factor 1 (C1q), specific Fc-gamma receptors (FcγRs) on phagocytes, such as macrophages, FcγRs on Natural Killer (NK) cells, and FcγRs on neutrophils (Table 1). The IgG-Fc region can also bind to the neonatal Fc receptor (FcRn); this receptor promotes antibody recycling through rescue from normal lysosomal degradation by increasing the affinity of these molecules for the acidic pH of endosomes–lysosomes, which prolongs the antibody plasma half-life for more than 21 days for IgG1, IgG2, and IgG4, thus contributing to the pharmacokinetics of TmAbs [13, 14]. The binding of Fc regions to different receptors depends on allotypic variations on the FcγRIIa (CD32a), such as H131/R131, and FcγRIIIa (CD16a) such as F158/V158, with effect, respectively, on the ADCP and ADCC activities (Table 1). In addition, the type of post-translational modification to the Ab, such as N-glycosylation (addition of carbohydrates to the arginine 297 residue in the CH2 domain of IgG), also influences Fc-binding to specific receptors [5].
Table 1.
Structural, biological, and functional properties of IgG subclasses
| Characteristics | IgG1 | IgG2 | IgG3 | IgG4 |
|---|---|---|---|---|
| Structural | ||||
| Molecular weight (kDa) | 150 | 150 | 155–165 | 150 |
| Disulfide bond between heavy chains | 2 | 4 | 11 | 2 |
| H chain type | γ1 | γ2 | γ3 | γ4 |
| Ig H constant gene | IGHG1 | IGHG2 | IGHG3 | IGHG4 |
| Glycosylation site | 297 | 297 | 297 | 297 |
| N-linked glycans per H chain | 1 | 1 | 2 * | 1 |
| O-linked glycans per H chain | 0 | 0 | 3 | 0 |
| Biological | ||||
| Biological half-life (days) | 21–24 | 21–24 | 7–8 | 21–24 |
| Mean adult serum level (g/L) | 6.98 | 3.8 | 0.51 | 0.56 |
| Placental transfer | +++ | ++ | ++/+++* | ++ |
| Functional (binding—activation) | ||||
| C1q- CDC | ++ | + | +++ | – |
| FcγRI (CD64) on monocytes—ADCP | +++ | – | +++ | ++ |
| FcγRIIa (CD32a)-H131 on monocytes and neutrophils—ADCP (ADCT⇞)¨ | ++ | ++ | ++ | + |
| FcγRIIa (CD32a)-R131 on monocytes and neutrophils—ADCP (ADCT⇞)¨ | ++ | – | ++ | + |
| FcγRIIb (CD32b) on monocytes and neutrophils—ADCP | + | – | ++ | + |
| FcγRIIIa (CD16a)-F158 on NK cells—ADCC | ++ | – | +++ | – |
| FcγRIIIa (CD16a)-V158 on NK cells—ADCC | +++ | + | +++ | + |
| FcγRIIIb (CD16b)NA1 on neutrophils and monocytes—ADCP (ADCT⇞)¨ | +++ | – | +++ | – |
| FcγRIIIb (CD16b)NA2 on neutrophils and monocytes—ADCP (ADCT⇞)¨ | +++ | – | +++ | – |
| FcRn on epithelial, endothelial, macrophages/monocytes and dendritic cells—homeostasis—pH 7,4 / < 6,5 | +/+++ | +/+++ | +/+++ | +/+++ |
Number of ( +) symbols indicates the affinity/interaction magnitude of each one of the immunoglobulin subclasses. *IgG allotype-specific. ⇞Mechanism demonstrated for IgG1-TmAb. CDC: Complement-dependent cytotoxicity. ADCP: Antibody-dependent cellular phagocytosis. ADCC: Antibody-dependent cellular cytotoxicity. ADCT: Antibody-dependent cellular trogocytosis. FcRn: Neonatal Fc receptor. N-linked glycans connect the sugar to an asparagine residue in the glycosylation process, whereas O-linked glycans connect a serine or threonine residue
Development of TmAbs in oncology
Throughout history, the technology used for the production of mAbs has involved the application of techniques that seek to resolve the quantity and the design of essential fragments of Ab structure [15]. The hybridoma platform based on the fusion of murine B cells and myeloma cells, established by George Kohler and César Milstein in 1975 [16], allowed the in vitro production of a large number of mAbs. Muromonab, the first murine anti-CD3 TmAb, used to eliminate T lymphocytes, was approved in 1986 to treat acute graft rejection [17]. The only two therapeutic anti-cancer full-length murine mAbs were approved by the WLA in 2002 (ibritumomab tiuxetan, an anti-CD20 TmAb radioimmunoconjugate (RIC) with Itrium-90 (Y90), which was used for the treatment of non-Hodgkin's lymphoma [18]) and by the National Regulatory Authorities in Cuba (CECMED) in 2013 (racotumomab, a murine anti-GM3 mAb used for the treatment of non-small cell lung cancer). Nevertheless, the administration of murine antibodies to humans can evoke, in some patients, the production of human anti-mouse antibodies (HAMA); these are endogenous antibodies with specificity for antigenic determinants present in the Fc region of mouse Igs [19]. Production of HAMA accelerates mAb clearance, thus reducing its serum half-time; on the other hand, repeated mAb administration could result in undesirable allergic reactions [20, 21].
The limitations in using mAbs of murine origin have been overcome by the development of engineering techniques, such as the recombination of murine variables with human constant regions, thus resulting in the production of chimeric antibodies. In 1997, the Food and Drug Administration (FDA) agency of the USA approved the first chimeric TmAb, rituximab, an anti-CD20 mAb for treating non-Hodgkin’s lymphoma [22, 23]. Later, humanized antibodies were generated, which contained human framework sequences surrounded by murine antigen-binding CDRs on the light and heavy variable chains. In 1998, the first humanized TmAb, trastuzumab, a mAb targeting the human epidermal growth factor receptor 2 (HER2), was approved for the treatment of breast cancer [24]. Currently, there are 10 chimeric and 40 humanized TmAbs, with an expected significant growth in the development of antibodies with a greater proportion of human amino acid sequences (Supplementary Fig. 1A). In 2021, envafolimab, the first humanized anti-programmed death ligand 1 (PD-L1) single-variable domain camelid antibody (VHH), was approved in China for the treatment of adult patients with previously treated microsatellite instability-high (MSI-H) or deficient Mismatch Repair (dMMR) advanced solid tumors. Unlike other immune checkpoint inhibitors that are administered intravenously, which can be uncomfortable and lead to discontinuation of therapy protocols, envafolimab is injected subcutaneously (SC) to improve patient tolerance [25, 26].
Another strategy to avoid HAMA while using murine-derived antibodies that provide high specificity and affinity for antigens with low immunogenicity is the use of scFv antibodies [27]. These antibodies are fusion proteins of the variable regions of VH and VL chains connected with a short linker peptide of 10–25 amino acids [27, 28]. In 2014, the WLA approved blinatumomab, a bispecific molecule that includes the scFv structure [29] (Supplementary Fig. 1A and Fig. 2E).
In 1990, Gregory P. Winter introduced phage display, a groundbreaking technique that integrates human antibody genetic sequences into the genome of filamentous bacteriophages. These sequences are further expressed on the phage surface, enabling subsequent screening processes, and this technology also constitutes the basis for scFv production [30–32]. This opened new frontiers in the development of fully human antibodies. In 2002, the first fully human antibody, adalimumab, a phage display-derived mAb that neutralizes tumor necrosis factor-α (TNF-α), was approved for the treatment of rheumatoid arthritis. In addition, since 1994, transgenic mice engineered to carry human Ig genes have been used to develop fully human antibodies [33, 34]. In 2006, the first fully human antibody derived from a transgenic mouse platform, panitumumab, which inhibits epidermal growth factor receptor (EGFR) signaling, was approved for treating colorectal cancer [2, 22, 35]. Currently, there are 23 fully human TmAbs, most of which derive from transgenic mice. The use of the single B cell technique has allowed the isolation of antigen-specific B cells from human blood for the generation of human natural antibodies, which have been applied in the search for antiviral antibodies. More recently, the Next Generation Sequencing (NGS) technology has also been useful in discovering new antibodies and their optimization through glycoengineering [15].
The development of antineoplastic drug conjugation has also improved the efficacy of TmAbs; indeed, 19.7% of the antibodies approved in oncology are antibody–drug conjugates (or ADC) and chemical conjugates. In 2000, the first conjugated antibody was approved; this is gemtuzumab ozogamicin, an anti-CD33 mAb conjugated to the anti-tumor antibiotic calicheamicin. Over the last seven years, the WLA has approved 10 monospecific conjugated antibodies (Supplementary Fig. 1B). In September 2020, the Japanese government approved cetuximab sarotalocan, the first antibody-photosensitizer conjugate, to treat unresectable advanced or recurrent head and neck cancer [36].
The development of recombinant bispecific antibodies has strongly increased in recent years, which goes hand in hand with the increment in the number of uncovered molecular targets to which each therapeutic antibody is designed. These account for 13% of the total antibodies approved in oncology (Supplementary Fig. 1C); 80% of them were approved between 2022 and 2023. Most bispecific antibodies are full-length IgG that can target two different antigens on the same cell or on two different cells [37]. In 2021, amivantamab, a fully human, full-length bispecific antibody against EGFR and the cell survival-related tyrosine kinase receptor cMET, was approved for the treatment of non-small cell lung cancer (NSCLC). In 2022, tebentafusp, the first humanized T-cell receptor-fused scFv bispecific anti-gp-100 and CD3, expressed in E. coli bacteria, was approved for treating metastatic uveal melanoma.
Nomenclature of therapeutic antibodies
The first generation of TmAbs (murine, chimeric, humanized, and fully human) gave rise to the nomenclature used until 2017. With the generation of bispecific and conjugated TmAbs, a new nomenclature was adopted. The nomenclature of TmAbs is now standardized according to the International Nonproprietary Names (INN) assigned by the WHO. The names consist of a prefix (arbitrarily created by the registrant) followed by two infixes, and a suffix. The first infix indicates the target (e.g.: “tu'' for tumor, such as trastuzumab; “li'' when the antibody regulates the immune system, such as inebilizumab, a TmAb that targets CD19 [2]), while the second infix includes the source used for producing the antibody (e.g.: “u” for human or “o” for mouse, or their modifications: “xi” for chimeric, as cetuximab, “zu” for humanized, as gemtuzumab). However, the WHO discontinued the second infix for antibodies created after-mid 2017 to avoid inconsistencies [38]. For all the TmAb developed before 2022, the suffix used was “mab”; however, in 2021, four new suffixes were introduced to provide information about modifications to the Fc structure; these suffixes include: “tug,” for full-length unmodified Igs that recognize a single epitope (monospecific); “bart,” for full-length monospecific Igs with engineered constant regions or any point mutation introduced by engineering; “mig,” bispecific or multispecific Igs with any structure, and “ment” for monospecific Ig variable region fragments [39].
Classification of TmAbs targets in oncology
The current WLA-approved anti-cancer TmAbs were developed for different target molecules (Fig. 2). The most common target is programmed cell death protein 1 (PD-1) (15), followed by CD20 (9), PD-L1 (7), HER2 (7), and EGFR (6). The target molecules can be classified according to their main biological function, biochemical structure, site where they are expressed and release mechanisms, among others. In this review, we used the following target classification:
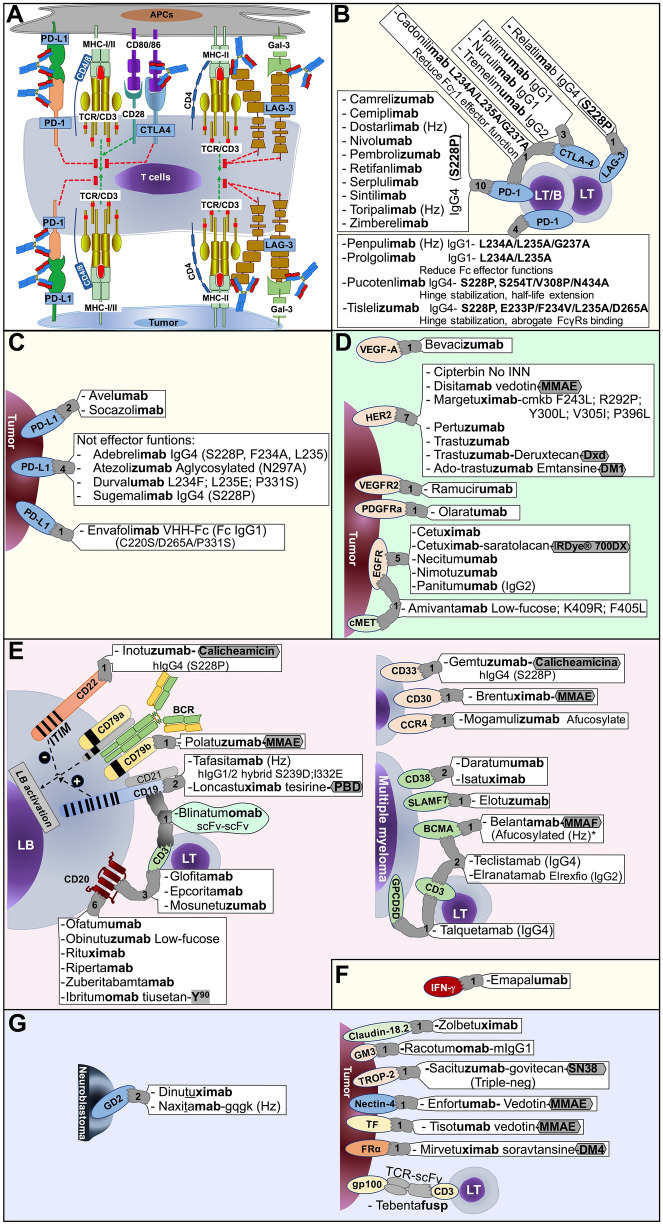
Fig. 2.
WLA-approved TmAbs and their targets for the treatment of solid and hematological tumors. A Cell-membrane molecules on T lymphocytes (LT), antigen-presenting cell (APC) and tumor cell associated with T cell activation (TCR, CD3, CD4/8, MHC-I/II, CD80/86), T cell inhibition (immune checkpoint molecules CTLA-4, PD-1, PD-L1, Gal-3, LAG-3), and therapeutic antibodies that block immune checkpoint. B and C Specific TmAbs that target immune checkpoint molecules that regulate T cell function. D TmAbs that bind to tyrosine kinase receptors of the ErbB family proteins. E TmAbs and their corresponding targets expressed on B lymphocytes (LB), multiple myeloma cells. Hodgkin lymphoma, systemic anaplastic large cell lymphoma (CD30), acute myeloid leukemia (CD33), and mycosis fungoides or Sézary syndrome (CCR4). F IFN-g, a soluble target involved in hemophagocytic lymphohistiocytosis (HLH) syndrome, can be targeted by a TmAb. G TmAbs that bind to cell surface molecules expressed on neuroblastoma cells and other types of solid tumors. The number in each target indicates the number of WLA-approved therapeutic antibodies for that target. The origin-dependent suffix letter is indicated in bold: Antibody is fully murine (-omab), chimeric (-ximab), humanized (-zumab), or fully human (-umab or -mab). IgG subclasses and modifications incorporated into TmAb design and their respective conjugated drugs are indicated. Afucosylated or low-fucose antibodies improve FcγRIIIa binding and ADCC; aglycosylated (N297A) block Fc effector functions; hinge stabilizing (S228P) is a IgG4 chain mutation to increase the binding between heavy chains (HCs), similar to IgG1. Antibody-conjugated molecules are indicated, including monomethyl auristatin E and F (MMAE and MMAF, respectively), calicheamicin, pyrrolobenzodiazepines (PBD), deruxtecan (DXd), emtansine (DM4), and govitecan (SN38). The radioactive compound yttrium-90 (Y90) is a radioimmunotherapeutic (RIT) drug. Bispecific antibodies, such as epcoritamab bind, concomitantly, to tumor cell targets and CD3 on T lymphocytes (LT). CD: Cluster of differentiation; PD-1: Programmed cell death protein 1, PD-L1: Programmed cell death ligand 1, CTLA-4: Cytotoxic T lymphocyte antigen 4, MHC: Major histocompatibility complex, Gal-3: Galectin-3 protein, LAG-3: Lymphocyte activation gene 3, VEGFA: Vascular endothelial growth factor A, HER2: Human epidermal growth factor receptor 2, VEGFR2: Vascular endothelial growth factor receptor 2, PDGFRα: Platelet-derived growth factor receptor α, BCMA: B cell maturation antigen, expressed primarily on B cells, but especially on multiple myeloma plasma cells; IFN-γ: interferon-gamma; SLAMF7: CD319 surface antigen. FRα: Folate receptor alpha; Hz: Humanized; scFv: Single-chain fragment variable; TCR: T cell receptor. Created with Microsoft® PowerPoint for Mac. Version 15.32 and Prism 6 for Mac OS X
(1) Molecules that modulate cells of the immune system, such as cytotoxic T lymphocyte antigen 4 (CTLA-4), PD-1, PD-L1, and lymphocyte activation gene 3 (LAG-3 or CD223) (Fig. 2E, F). The TmAbs directed toward these molecules block the inhibition of the immune system and are used in various types of tumors; these include ipilimumab and nurulimab, anti-CTLA-4 TmAbs for the treatment of melanoma; durvalumab and atezolizumab, two anti-PD-L1 TmAbs for bladder cancer treatment; avelumab, an anti-PD-L1 TmAb for the treatment of Merkel cell carcinoma; pembrolizumab and nivolumab, which are anti-PD-1 TmAbs used for patients with melanoma; cemiplimab, an anti-PD-1 TmAb used in advanced cutaneous squamous cell carcinoma (SCC); nivolumab and sintilimab, anti-PD-1 TmAbs against NSCLC; toripalimab and penpulimab, anti-PD-1 TmAbs used for nasopharyngeal carcinoma: tislelizumab, an anti-PD-1 TmAb used for esophageal squamous cell carcinoma, and dostarlimab, an anti-PD-1 TmAb for endometrial cancer. Retifanlimab, also an anti-PD-1 TmAb, as well as nivolumab, pembrolizumab, and avelumab, is used against anal canal carcinoma [40], while pembrolizumab, cemiplimab, durvalumab, atezolizumab, avelumab, nivolumab, and balstilimab (an anti-PD-1 TmAb) are also used for the treatment of cervical cancer [14, 41]. A LAG-3 blocking antibody, relatlimab, is indicated for melanoma treatment; it interferes with the binding of LAG-3 to major histocompatibility (MHC) class II molecules on antigen-presenting cells, an interaction that induces T cell exhaustion and prevents effector T cell function in the tumor microenvironment [42, 43]. Recently, it has been indicated for advanced melanoma protocols in combination with nivolumab [42].
(2) Tyrosine kinase receptors of the ErbB family proteins, which are overexpressed in solid cancers (Fig. 2D). ErbB receptors are pivotal for regulating cellular processes, such as proliferation, survival, differentiation, and migration; disruptions in their signaling are linked to cancer development [44, 45]. Some TmAbs bind to cell membrane receptors, thus altering their functions and potentially inducing receptor degradation [46]. This targeted approach offers improved therapeutic options for cancer patients. Combinations of tyrosine kinase inhibitors, which block receptor phosphorylation, can hinder cancer cell growth; therefore, antibodies are often employed to maximize treatment efficacy, addressing diverse aspects of cell signaling and survival [47, 48]. EGFR and HER2 (also known as the neu oncogene or ErbB2) were the first ErbB family receptors used as therapeutic targets, as they are involved in cell growth control and differentiation. TmAbs against EGFR include necitumumab, used in NSCLC, and cetuximab and panitumumab, used to treat colorectal cancer. On the other hand, trastuzumab, pertuzumab, and margetuximab, TmAbs that block HER2, are used in breast cancer, while ramucirumab, which blocks the vascular endothelial growth factor receptor 2 (VEGFR2), is used in gastric cancer [44]. Olaratumab blocks the platelet-derived growth factor receptor α (PDGFRα) and is used to treat soft tissue sarcoma [49]. The vascular endothelial growth factor (VEGF), a family of proteins involved in the regulation of angiogenesis, an essential step supporting tumor growth and metastasis [50, 51], is targeted by bevacizumab in colorectal, cervical, and ovarian cancers [41, 52, 53].
(3) Molecules that are preferentially expressed on the lymphoid and/or myeloid cell lineage, which are the targets for most of the currently approved TmAbs used for treating hematological cancers (Fig. 2E–G). One of these therapeutic targets is CD19, a cell membrane protein that is expressed on all B cell lineages and that plays two major roles in human B cells: On the one hand, it acts as an adaptor protein to recruit cytoplasmic signaling proteins to the membrane; on the other, it works within the CD19/CD21 complex to decrease the threshold for B cell receptor (BCR) signaling pathways [54]. Another significant target is CD20, a receptor involved in calcium transport [55–57]. It is a highly restricted cell surface antigen with abundant, stable, and highly characterized expression on B cells [58]. Additional targets are CD22, a receptor that regulates B lymphocyte survival and signal transduction [59], and CD79b, an Igβ transmembrane signaling subunit that, together with Igα, forms part of the multimeric BCR complex; both subunits are necessary for the expression and function of the BCR [60] (Fig. 2E).
In multiple myeloma (MM), the malignant clonal plasma cells overexpress the B cell maturation antigen (BCMA); it is expressed preferentially by mature B lymphocytes, with minimal expression on hematopoietic stem cells or non-hematopoietic cells, and is essential for the survival of long-lived bone marrow plasma cells, but not for overall B cell homeostasis [61–64]. The overexpression and activation of BCMA is associated with the progression of MM [65]; therefore, this molecule represents an interesting therapeutic target for the treatment of this disease. Another target is CD38, a cell surface glycoprotein that functions in cell adhesion, signal transduction, and calcium signaling. It is found at relatively low levels on normal lymphoid cells, including T cells, B lymphocytes, and NK cells, as well as myeloid cells, and some tissues of non-hematopoietic origin [66]. CD38 is highly and uniformly expressed on MM cells [66]. The G-protein-coupled receptor family C group 5 member D (GPRC5D) is a transmembrane receptor expressed in cells with a plasma-cell phenotype, including cells from patients with MM. However, aside from plasma cells and B cells, it is not found at appreciable levels on normal hematopoietic cells and bone marrow progenitors [67]. The signaling lymphocytic activation molecule family member 7 (SLAMF7) is a glycoprotein expressed on myeloma and NK cells but not normal tissues (Fig. 2E). The development of TmAbs targeting molecules associated with MM, whether used alone or in combination therapy, has shown promising results in the treatment of naive and relapsed/refractory MM, even in advanced-stage patients [68, 69]. One of the most revolutionary immunotherapy in MM is the use of ADCs, which will be reviewed below. Belantamab mafodotin was the first ADC approved for treating MM [70].
CD30, a member of the tumor necrosis factor receptor superfamily, is overexpressed on neoplastic cells and Reed-Sternberg cells typical of Hodgkin's lymphoma; therefore, this molecule also constitutes an interesting therapeutic target [71, 72]. CD33, a transmembrane receptor expressed on cells of the myeloid lineage, is implicated in the inhibition of cellular activity [73]; it is a target for gemtuzumab ozogamicin, used in the treatment of acute myeloid leukemia. Another target is C-Cg chemokine receptor type 4 (CCR4), as it is often expressed on leukemic cells in cutaneous T-cell lymphoma (CTCL), which encompasses a heterogeneous collection of non-Hodgkin lymphomas that arise from skin-tropic memory T lymphocytes. Among them, mycosis fungoides (MF) and Sézary syndrome (SS) are the most common malignancies [74] (Fig. 2E).
(4) Molecules that are released into the extracellular milieu in soluble forms. In hemophagocytic lymphohistiocytosis (HLH), an acute and rapidly progressive systemic inflammatory disorder that can be triggered by infection or malignancies, including leukemia and lymphoma, the excessive production of IFN-γ contributes to the life-threatening hyperinflammation that characterizes this condition [75, 76]. Emapalumab, approved for use in this syndrome [77], neutralizes IFN-γ by forming immune complexes, thus facilitating cytokine elimination and decreasing its serum concentration [78] (Fig. 2F).
(5) Miscellaneous targets. These include disialoganglioside (GD2), a target for high-risk neuroblastoma and refractory osteomedullary disease (naxitamab-gqgk in combination with granulocyte–macrophage colony-stimulating factor) [79]; GM3 ganglioside, a trophoblast cell surface antigen-2 (TROP-2) targeted for the treatment of triple-negative breast cancer (sacituzumab); Nectin-4, a target in urothelial cancer (enfortumab-vedotin linked to monomethyl auristatin E, MMAE); folate receptor α (FRα), a target in ovarian cancer (mirvetuximab); gp100 in metastatic uveal melanoma (tebentafusp scFv-TCR fusion protein) and Claudin 18.2, a tight-junction molecule, become accessible on the tumor cell surface in advanced-stage gastric cancer [80] (Fig. 2G).
Mechanisms of action of TmAbs
TmAbs can either block immunoregulatory molecules, cytokines or growth factors, tag cells to induce an immune response, or transport chemotherapeutics, toxins or radioactive elements into the tumor cells (Fig. 1B). TmAbs exert direct and indirect anti-tumor mechanisms of action (MoA) [81]. Most TmAbs can induce direct apoptosis, interfere with receptor signaling, neutralize soluble ligands, or block cell-surface receptors. TmAbs can also destroy tumor cells indirectly through the recruitment and activity of immune response mechanisms [82]. In this sense, once TmAbs bind to its target on the tumor cell, they can activate the classical pathway of the complement cascade and mediate CDC [8]; in addition, they can trigger ADCC, ADCP, ADCT, or a combination of these [10, 12].
Complement-dependent cytotoxicity (CDC)
The efficacy of TmAbs to induce CDC depends on several factors related to IgG subtype, antigen density [83, 84], antibody hexamerization [85], the specific orientation of the bound antibody molecules with each other, and the proximity of the epitope to the target cell membrane [84].
Under physiological conditions, complement molecules are continuously produced as inactive zymogens; these are cleaved into their active products by specific complement proteases, which generate effector molecules that ultimately promote cell lysis or phagocytosis [86]. To this end, the complement cascade can be activated through three distinct pathways that converge to generate the same set of effector molecules; these are the alternative, the lectin and the classical pathways. Distinct subclasses of IgG can activate the classical pathway: IgG1, IgG2, and IgG3, while IgG4 is unable to activate complement [87, 88].
Binding of IgG molecules to their specific target on the cell membrane is followed by aggregation and hexamerization of the antibodies, which permits the activation of the classical pathway of the complement cascade [8]. It is initiated by the C1 complex, a soluble multimolecular protease compromising a recognition subunit (C1q) and two modular serine proteases (C1r and C1s) [89]. The binding of C1q to the Fc region of TmAbs activates its associated proteases, which initiates the proteolytic cascade of complement [89–92]. Fc-bound C1q leads to the autoactivation of C1r for cleavage and activation of C1s; activated C1s cleaves soluble C4 into C4a and C4b; C4b fragments attach to the cell membrane and bind soluble zymogen C2, forming the C4bC2 proconvertase complex. Once associated with C4b, C2 is cleaved by C1s into C2a and C2b, generating the classical pathway C3 convertase C4bC2a, which binds and cleaves soluble C3 into C3a and C3b. The latter associates with C4bC2a, forming the C5 convertase C4bC2aC3b. C5 is further cleaved into C5a and C5b; C5b, attached to the cell membrane, binds C6, C7, C8, and multiple pore-forming C9 molecules, which polymerize and insert into the cell membrane, leading to complement-dependent cell lysis [93]. C3b alone can also deposit on the cell surface, leading to amplification of complement activation through the alternative pathway; in addition, C3b on the cell membrane can act as an opsonin, as it binds to specific receptors on the surface of phagocytes, thus promoting phagocytosis [88]. In turn, soluble fragments C3a and C5a are anaphylatoxins that induce chemotaxis of macrophages, neutrophils, and dendritic cells, among others, to the tumor microenvironment, which generates inflammation [94].
Several studies have reported classical pathway activation with therapeutic mAbs. One example is HuMab-7D8, a fully human IgG1 that binds to the extracellular loops of CD20, leading to potent complement-mediated B cell lysis [95]. Through cryo-electron microscopy analyses, Ugurlar and cols showed that the amino acid residues in IgG1 that are important for direct C1q binding are located on the two Fc-CH2 domains, which is near the Fab-Fc hinge [96]. Rituximab, a chimeric human-mouse IgG1 TmAb used for the treatment of non-Hodgkin's B cell lymphoma [97], binds to CD20 on malignant B cells, which triggers B cell lysis through the classical pathway [98]. Idusogie et al. demonstrated that the binding site of C1q on rituximab is centered around amino acid residues D270, K322, P329, and P331 of the Fc region [98]. Interestingly, it was further demonstrated that C1q binds a distinct site in each of the two CH2 domains of IgG1: One binding site includes the loop formed by residues 325–331, while the other binding site consists of two loops formed by residues 266–272 and residues 294–300 of the second CH2 domain [96, 98]. The addition of a positive charge at residues E269, E294, and Y300 abolished CDC, and mutations N297Q and S298K decreased CDC due to the absence of glycosylation; on the contrary, mutations of Y300D and G236D enhanced CDC [96, 99]. The knowledge of residues involved in the interaction between TmAbs and C1q is useful for designing therapeutic antibodies with improved CDC activity.
Antibody-dependent cellular cytotoxicity (ADCC)
A dominant component of the in vivo activity of antibodies against tumors involves ADCC, a mechanism that implies the binding of the Fc regions of target cell-bound TmAbs to functional activating Fc receptors for IgG (FcγR) on cytolytic cells, which triggers target cell death [100, 101]. The majority of antibodies, upon opsonizing target cells, exert their effector functions through FcγR on NK cells, mainly via FcγRIIIa (CD16a). These cells induce cytotoxicity through the exocytosis of perforin, a glycoprotein that forms pores on the membranes of target cells, and granzymes, a family of structurally related serine proteases. As perforin and granzymes are previously synthesized and stored in intracellular granules, they can rapidly trigger apoptosis of target cells [102]. The granule-exocytosis pathway potently activates cell-death mechanisms via the activation of apoptotic cysteine proteases (caspases) [103].
The IgG subclasses used in the design of TmAbs (IgG1, IgG2, and IgG4) differ from each other in their capacity to bind FcγR on immune cells [104], the strength of ADCC activity they trigger, the presence of N297-linked glycosylation on the antibody, and FcγR polymorphisms [105]. The FcγRIIIa (V158F) polymorphism has been reported to correlate with the response and efficacy of TmAbs, as its isoforms display higher affinity for the Fc region of IgG, resulting in a greater immunological response. These polymorphisms may even be useful as molecular markers to predict clinical outcomes in patients with metastatic colorectal cancer treated with cetuximab [106] or in patients bearing early ErbB2/HER2-positive breast cancer who receive treatment with trastuzumab [107].
Although ADCC has been studied mainly in NK cells, neutrophils can also participate in this mechanism in the presence of TmAbs. The antibody-related cytotoxic activity of neutrophils is associated especially with FcγRIIa [108, 109] and the presence of polymorphisms, such as the FcγRIIa-131H variant, which has shown higher killing capacity in comparison with the FcγRIIa-131R [110].
Antibody-dependent cellular phagocytosis (ADCP)
TmAbs can also promote tumor cell elimination through phagocytosis. The mechanism, named antibody-dependent cellular phagocytosis (ADCP), is mediated through the interaction of the Fc domain of target cell-bound IgG with FcγRs on phagocytic cells, such as macrophages [111, 112]. TmAbs can opsonize the tumor cell to promote its phagocytosis and degradation through phagosome acidification. Currently, some researchers have implemented novel in vitro models to demonstrate the internalization of antibody-coated target cells into macrophages [113, 114], or the capture of antigen-coated fluorescent beads bound to specific antibodies to measure antibody-dependent neutrophil phagocytosis [115]; such methodologies would certainly be useful tools to improve the development of therapeutic antibodies.
FcγRs that participate in ADCP preferentially interact with IgG1, which shows the best high binding affinity for activating FcγRs that trigger ADCC and/or ADCP [116]. On the other hand, FcγRs levels on the cell membrane can be modulated by cytokines. For instance, FcγRI expression can be upregulated on polymorphonuclear cells [117], macrophages [118], and monocytic cells [119] in response to IFN-γ [117], a cytokine released by activated NK cells [120], suggesting that the interplay of immune cells with cytokines may enhance the mechanisms of ADCC and/ or ADCP.
For some TmAbs, the ADCP contributes significantly to their anti-tumor activity [121]. This is the case for brentuximab vedotin, a chimeric IgG1-drug conjugate used for Hodgkin’s lymphoma and systemic anaplastic large cell lymphoma [122]. Not only have its effects been associated with the expected cytotoxicity triggered by mAb-conjugated drugs; its anti-tumor effect also relies on ADCP mediated by macrophages [121]. Similarly, rituximab is able to induce a potent ADCP response in in vitro and in vivo models, and depletion of macrophages results in the attenuation of the anti-tumor activity of this TmAb [123].
It has also been demonstrated that macrophage-mediated ADCP contributes to the anti-tumor activity of daratumumab, an IgG1 antibody against CD38 used for the treatment of MM [124]. ADCP may also be relevant for other hematological tumors [125]. Although some researchers have shown that target molecule expression is correlated with ADCP [126], this may not be the case for some TmAbs, including rituximab, as the expression level of CD20 on B cell-chronic lymphocytic leukemia (B-CLL) cells does not affect the efficacy of the antibody’s mediated ADCP [126]. This mechanism involves the participation of FcγRIIa (CD32a) expressed on macrophages (Table 1). Another TmAb that exerts ADCP is elotuzumab, a humanized IgG1 directed toward SLAMF7 in MM [112, 127]. This antibody induces activation of macrophages and mediates ADCP, thus contributing to its antitumor response [112].
Antibody-dependent cellular trogocytosis (ADCT)
A novel MoA of TmAbs, known as antibody-dependent cellular trogocytosis (ADCT), refers to the capacity of immune cells, such as monocytes, macrophages, and neutrophils, to remove cell surface proteins from the tumor cell membrane through an endocytic process [128]. This mechanism is enhanced by the Fc region of amivantamab [12]. This therapeutic antibody binds simultaneously to cMET and EGFR, cell surface receptors that promote tumor cell proliferation and survival [129]. Such binding leads to FcγRIIIa-mediated monocyte and macrophage-dependent downmodulation of these proteins by trogocytosis; deprivation of tumor cell membrane cMET and EGFR leads to an improved antibody-mediated tumor cell killing, both in vitro and in vivo [11, 130]. Neutrophils can also perform ADCT in the presence of TmAbs. Indeed, it has been demonstrated that rituximab can mediate trogocytosis of CLL B cells by neutrophils in vitro, as detected by CD20 downmodulation on these tumor cells. Although this response does not trigger tumor cell death, it will be important to uncover whether it has functional consequences in vivo, as downmodulation of CD20 has been associated with resistance to anti-CD20 treatment in CLL [131–133]. On the other hand, ADCT mediated by neutrophils can precede tumor cell lysis when associated with mechanical disruption of the tumor cell membrane, a process known as trogoptosis. After in vitro incubation of neutrophils with SKBR3 human breast cancer cells, previously labeled with the cytosolic dye calcein and opsonized with trastuzumab, tumor cells displayed significant loss of membrane integrity, which coincided with target cell lysis, as detected by 51Cr release assay. Therefore, these data provide evidence that neutrophil-mediated ADCT can directly cause tumor cell death [134].
Antibody–drug conjugate (ADC)
An antibody–drug conjugate (ADC) consists of a monoclonal antibody that targets a tumor-specific antigen and is covalently bound to a cytotoxic drug or payload through a chemical linker [135]. ADCs are designed to specifically deliver drugs or payloads into the vicinity of tumor cells, thereby mitigating the side effects of their cytotoxicity [135]. Currently, 13 monospecific ADCs have been approved by WLA (discussed later) (Supplementary Fig. 2A-1I). These ADCs are primarily used to treat hematological malignancies (6 out of 13) and breast cancer (3 out of 13). Additionally, ADC approvals extend to the treatment of ovarian, cervical, urothelial, and gastric cancer [4].
The primary advantage of ADCs in clinical use is their ability to extend the therapeutic window of cytotoxic drugs. This allows for higher local concentration at the target site while minimizing the risk of systemic toxicity compared to the cytotoxic drug without conjugate [135].
The efficacy of an ADC’s payload depends on various factors, including adequate cytotoxicity, low immunogenicity, high stability, and modifiable functional groups [136]. As a result, the drugs or payloads used in the conjugation process have undergone modifications over the years, leading to three generations of innovation fueled by emerging technologies [137]. They mainly include substances that disrupt the cellular cycle, such as tubulin inhibitors and DNA-damaging agents. Next, we describe the ADCs currently used in the clinic to treat cancer.
Payloads of the ADCs approved by the WLA
Monomethyl auristatin E (MMAE) and F (MMAF) are tubulin inhibitors that are linked to more than half of the approved ADCs, including belantamab mafodotin, an anti-BCMA antibody for MM treatment; brentuximab vedotin, an anti-CD30 antibody to treat Hodgkin lymphoma and systemic anaplastic large cell lymphoma treatment; disitamab vedotin, an anti-HER2 antibody for gastric cancer treatment; enfortumab vedotin, an anti-Nectin-4 antibody for urothelial cancer treatment; polatuzumab vedotin, an anti-CD79b antibody for diffuse large B-cell lymphoma treatment, and tisotumab vedotin, an anti-tissue factor antibody for cervical cancer treatment [4]. These substances are water-soluble synthetic analogs of dolastatin [138, 139], whose mechanisms of action involve binding to tubulin dimers and disrupting microtubule assembly, leading to cell cycle arrest and apoptosis [140].
Calicheamicins are a class of enediyne natural products that are isolated from the bacterium Micromonospora echinospora [141]. These natural compounds are among the most cytotoxic DNA-damaging products ever discovered, with unique molecular and cellular mechanisms of action [142]. Calicheamicin y11, a member of this family, was identified for its strong cellular activity, as it binds double-helical DNA within the minor groove, leading to site-specific double-stranded break [143–146]. Due to their robust antitumor activity, calicheamicins have been included in the clinical development of ADCs for treating hematological malignancies: gemtuzumab ozogamicin, which targets CD33 for acute myeloid leukemia (AML) treatment, and inotuzumab ozogamicin, which targets CD22 for acute lymphoblastic leukemia (ALL) treatment [147]. CD33 and CD22 are endocytic receptors ideal for delivering the cytotoxic drug into the cell [147].
Emtansine (DM1) and ravtansine (DM4) are maytansinoid drugs derived from maytansine, a compound originally isolated from the shrub Maytenus ovatus. They bind to the β-subunit of tubulin, inhibiting its polymerization and inducing its aggregation. Tubulin polymerization is crucial for spindle fiber formation and subsequent cell division. DM1 is a component of ado-trastuzumab emtansine (Supplementary Fig. 2F), a humanized anti-HER2 IgG1 used in breast cancer treatment. In turn, DM4 is utilized in mirvetuximab soravtansine, an antibody that targets folate receptor alpha (FRa), which is indicated for treating ovarian cancer [148]. DM1 exhibits both HER2-dependent and independent mechanisms of action. In the HER2-dependent pathway, upon internalization of the HER2-ACD complex, the payload activates caspase 3/7, inducing apoptosis; it also binds the microtubule to initiate mitotic arrest. In the HER2-independent pathway, when DM1 is conjugated to trastuzumab (T-DM1), it interacts with the cytoskeleton-associated protein 5 (CKAP5), causing cell membrane damage, increased calcium influx, and subsequent microtubule disruption [148, 149]. This cytotoxicity mechanism could also be extrapolated to DM4, but further studies are needed to unravel this possibility [150].
Camptothecin analogs, such as DXd, a derivative of exatecan, and SN-38, the active metabolite of irinotecan, are topoisomerase I inhibitors that interrupt DNA replication, resulting in cell death [151]. DXd has been reported to be more potent than SN-38 [152]. These components are conjugated to a humanized antibody targeting HER2 (fam-trastuzumab deruxtecan) (Supplementary Fig. 2D) [153] and to an antibody against the intracellular calcium signal transducer TROP-2 (sacituzumab govitecan), respectively [154]]; these TmAbs are indicated for HER2-positive metastatic breast cancer and triple-negative breast cancer treatment, respectively.
Pyrrolobenzodiazepine (PBD) dimers are powerful synthetic payloads developed for targeting low antigen expression and to overcome resistance to auristatins or maytansinoids [136]. An example of the dimeric derivative is SG3199, which is incorporated into loncastuximab tesirine, an anti-CD19 IgG1 humanized antibody for treating diffuse large B-cell lymphoma (Supplementary Fig. 2B). SG3199 produces covalent cytotoxic DNA interstrand cross-links mainly in human hematological tumor cell lines, but also in solid tumors. One of the main advantages of SG3199 over other DNA cross-linking drugs is its persistence in cells due to its ability to cause minimal distortion of the DNA. This property contributes to evading excision repair mechanisms that are initiated after repair enzymes detect distortion or perturbations in the DNA. Thus, PBD dimer avoids drug resistance [155].
Linkers of ADCs approved by WLA
The linker utilized in ADC conjugation plays a critical role in determining the efficacy and safety of these TmAbs. Ideally, the linker should exhibit stability in the bloodstream and facilitate the controlled release of the drug at the target site [156]. There are two main types of linkers used in ACD development: cleavable (chemically or enzymatically) and non-cleavable (thioether and maleimidocaproyl linkers) [157]. Most approved ADCs employ cleavable linkers, such as inotuzumab ozogamicin and brentuximab vedotin.
The chemically cleavable linkers can be pH-sensitive or reduction-sensitive types. An acid-labile hydrazone linker is stable in alkaline environments, but highly sensitive to acidic environments; thereby, it is hydrolyzed in the endosome and lysosome to release the payload. This chemical principle is applied to the conjugation of gemtuzumab ozogamicin or inotuzumab ozogamicin with calicheamicin (Supplementary Fig. 2I), which utilizes hydrozone as a linker; in turn, sacituzumab govitecan (Supplementary Fig. 2E) employs an ester linker enabling the conjugation of the antibody with SN-38 [136].
The group of enzymatically cleavable linkers used in the ADCs are peptide-based, also known as lysosomal protease-sensitive linkers. A prime example is valine-citrulline (Val-Cit), employed in conjugating brentuximab vedotin with MMAE (Supplementary Fig. 2A); this linker relies on cathepsin B, an intracellular protease, to cleave a dipeptide bond and release the payload. Loncastuximab tesirine (Supplementary Fig. 2B) also utilizes the same linker to conjugate PBD dimers. Another class of enzymatically cleavable linkers is based on phosphatase-cleavable, with enzyme targets pyrophosphatase and acid phosphatase in lysosomal compartments. Disitamab vedotin, an anti-HER2 antibody, enfortumab vedotin, an anti-Nectin-4 antibody, polatuzumab vedotin, an anti-CD79b antibody, and tisotumab vedotin, an anti-tissue factor antibody utilizes this strategy to conjugate MMAE through maleimidocaproyl-valine-citrulline-p-aminobenzyloxylcarbonyl (PABC) (Supplementary Fig. 2A). This latter, a self-cleavage spacer between the Val-Cit dipeptide and the drug, improves cathepsin B access for the cleavage site [158, 159].
Other cleavable linkers include mirvetuximab soravtansine, an anti-FRɑ antibody conjugated to DM4 through a cleavable sulfo-SPDB linker (Supplementary Fig. 2H), and fam-trastuzumab deruxtecan, an anti-HER2 antibody conjugated to DXd via Glycine-Glycine-Phenylalanine-Glycine (GGFC) (Supplementary Fig. 2D) [136].
Non-cleavable linkers can be grouped into thioether or maleimidocaproyl (MC) categories. ADCs that use this type of linker have greater plasma stability; in addition, drug release depends on lysosomal enzymatic degradation of the antibody upon internalization into the cell target. An example of the first group is ado-trastuzumab emtansine (Supplementary Fig. 2F), an anti-HER2 antibody in which trastuzumab-lysine is conjugated with thioether linker to DM1. On the other hand, the MC linker is used in the design of belantamab mafodotin (Supplementary Fig. 2C), an anti-BCMA antibody that conjugates with MMAF (described previously) [136].
Photoactivatable antibody–drug conjugates
Another innovative cancer treatment strategy is photoimmunotherapy, which involves an antibody conjugated with a light-sensitive compound. One example is cetuximab sarotalocan, a chimeric anti-EGFR IgG1 conjugated with the dye IRDye® 700DX. This antibody is specifically designed to treat head and neck cancer. Upon administration, the antibody is activated by laser illumination (690 nm), which damages the cell membrane, increases transmembrane water flux, and leads to tumor cell rupture and necrosis [160, 161].
Radioimmunoconjugate (RIC)
Radioimmunotherapy (RIT) is a highly effective modality in cancer therapy that uses radioisotope-conjugated mAbs to target cancer cells selectively. In 2002, the FDA approved the first radioimmunoconjugate (RIC). It was ibritumomab, a murine anti-CD20 IgG1 mAb that is bound to the chelator tiuxetan and covalently linked through a stable thiourea covalent bond to the β-emitting radionuclide 90 Y (Supplementary Fig. 2G). This RIC is specifically indicated for the treatment of non-Hodgkin lymphomas. Upon administration, the emitted radiation directly damages the DNA of cancer cells, ultimately destroying them [162].
Bispecific molecules
Bispecific molecules have dual binding sites that bind two different antigens; these molecules belong to either of three groups: bispecific antibodies (BsAs), scFv-TCR fusion proteins, or bispecific T-cell engagers (BiTEs).
Bispecific antibodies (BsAs)
Between 2021 and May 2024, the WLA approved 8 BsAs for cancer treatment [4, 163] (Supplementary Fig. 1B), as the biological features of these Abs are suitable for this type of clinical application [164, 165]. Such characteristics include increased specificity, as they target two distinct antigens, inhibition of activation of intracellular signals associated with drug resistance, activation of the immune system, and reduction of side effects [164–169]. They also have the potential to target various types of cancer, allow treatment customization, and may reduce disease recurrence [164].
Most BsAs simultaneously engage with an antigen present on the surface of tumor cells and CD3 on T cells, making them particularly effective in hematological cancers [170, 171] (Fig. 1B (vii, viii). The interaction between T cells and cancer cells promotes the immune response against the tumor [172]. Examples of BsAs include: elranatamab, an anti-BCMA and anti-CD3 antibody to treat patients with MM; epcoritamab, an anti-CD20 and anti-CD3 antibody for the treatment of relapsed or refractory diffuse large B-cell lymphoma; glofitamab, an anti-CD20 and anti-CD3 antibody for the treatment of diffuse large B-cell lymphoma or large B-cell lymphoma; mosunetuzumab, an anti-CD20 and anti-CD3 antibody for the treatment of follicular lymphoma; talquetamab, an anti-GPCR5D and anti-CD3 antibody for the treatment of MM; teclistamab-cqyv, an anti-BCMA and anti-CD3 antibody for the treatment of relapsed or refractory MM [4, 163].
On the other hand, only 2 approved BsAs are directed against tumor-expressed antigens (Fig. 2B,D): they are amivantamab, an anti-EGFR and anti-cMET antibody, and cadonilimab, an anti-PD-1 and anti-CTLA-4 antibody; these TmAbs are used to treat patients with NSCLC bearing EGFR exon 20 insertion mutations and with cervical cancer, respectively [4, 163].
scFv-TCR fusion proteins
Included in the roster of WLA-approved bispecific molecules is tebentafusp-tebn (approved in 2022), an anti-tumor-associated antigen gp100 and anti-CD3 scFv-TCR fusion protein for the treatment of metastatic uveal melanoma. This molecule is also known as the first immune-mobilizing monoclonal TCR against cancer (ImmTACs) due to its MoA and engineering. The design involves the fusion of two proteins. The first protein arm contains a soluble high-affinity TCR that recognizes a complex formed by a specific peptide of the gp100 protein presented by HLA-A*02:01, which is expressed in approximately 45% of patients with uveal melanoma in the USA and Europe. As a result, the therapeutic response to tebentafusp-tebn is allele-specific. The second protein arm comprises an anti-CD3 scFv to recruit killer T cells to the tumor microenvironment, where they can become activated [173, 174].
Bispecific T-cell engagers (BiTEs)
BiTEs are recombinant proteins that consist of two scFvs linked by a short flexible linker. They are a promising class of bispecific molecules directed to tumor antigens and CD3, thus establishing a bridge between tumor cells and T cells. In this platform, the activation of T cells is independent of MHC haplotype [175] or TCR recognition and costimulation [176]. This strategy is advantageous, as loss or downregulation of MHC is a common mechanism in tumor cells [177]. Moreover, BiTEs contribute to T cell proliferation, amplifying the T cell population and enhancing the efficacy of the treatment [178].
Blinatumomab was the first approved BiTE. It is composed of two fragments of scFv targeting CD19 and CD3; therefore, it is indicated for treating B cell malignancies, such as philadelphia chromosome-negative relapsed or refractory B-cell precursor acute lymphoblastic leukemia (Fig. 2E). The fragments are linked by a short linker composed mainly of serine/lysine (G4S) [179]. The proximity between both cells, provided by blinatumomab, structurally forms a typical cytolytic immune synapse, inducing the activation of T cells and triggering the migration of intracellular vesicles containing granzyme B and perforins into the synapse space. Perforins create pores on the membrane of tumor cells, allowing granzyme B to enter the target cell and induce its apoptosis [177, 180].
Rational design of TmAbs
The functions of TmAbs can be optimized by rational designs of different antibody subclasses used in the clinic, such as IgG1, IgG2, and IgG4. Although IgG3 binds with high affinity to most FcγRs, it is not usually chosen for therapeutic purposes because of its long hinge region, which is accessible to proteolysis, and polymorphisms, which may generate immunogenicity [116]. It is important to take into consideration that the variability in the flexibility and length of the hinge region between the different IgG subclasses affects the spatial arrangement of the Fab arms. This feature facilitates the ability of the Fab arms to bind to different targets, while allowing autonomous interactions of the Fc region with effector components of the immune system [181]. TmAbs can also be modified, radiolabeled, conjugated to drugs or toxins, or made bispecific.
IgG subclass selection
There are no strict criteria for selecting the IgG subclass for the design of TmAbs. The target cell type and aspects related to pharmacokinetics, such as molecular half-life, affinity for neonatal receptors and clearance, should be considered. In addition, the pharmacodynamic features, such as the ability of the therapeutic antibody to activate, reduce, or eliminate Fc effector functions, will also depend on its affinity for the different FcγRs expressed on immune cells and the glycosylation status in the Fc region (Table 1). To this end, it is essential to remember that each IgG subclass has different structural, functional, and biological characteristics [116]; therefore, the appropriate design of TmAbs must consider these characteristics to stimulate or inhibit immune cell functionality.
In general, IgG1 and IgG4 subclasses have been chosen for the design of most TmAbs (49 and 19, respectively) due to their functional characteristics and longer half-life in the serum, as they bind to FcRn [13, 182]. However, the instability of the γ4 chains of IgG4 due to the presence of S228 at the hinge region implies the need to introduce a mutation at this amino acid for proline to stabilize the binding between the two heavy chains, as it occurs at the junction between the γ1-γ1 chains in IgG1 [183]. The function and type of immune cells must be considered when selecting the appropriate IgG subclass. IgG4, in particular, does not trigger target cell lysis, making it a good candidate for blocking immune checkpoint molecules expressed on effector immune cells, such as PD-1 or LAG3, as maintaining the viability of these cells is crucial [184]. Additionally, IgG4 has been incorporated into the design of ADCs for drug delivery (Fig. 2). Structurally, both IgG1 and IgG4 have two disulfide bridges in their hinge region, serving as available sites for their conjugation with drugs. Although fewer sites than IgG2, this feature potentially enables more efficient drug conjugation to IgG1 and IgG4, as these sites are more readily reducible, rendering them favorable options for ADC design [185, 186].
On the other hand, IgG1 is more suitable for targets expressed on immunosuppressive cells, as IgG1 can trigger target cell lysis [116, 181]. For an immunostimulatory function, it is recommended to design IgG antibodies that favor more significant interaction with the FcγRIIB receptor and efficient recognition of specific epitopes [187].
Modifications to the IgG-Fc region
Among the five Fc gamma receptors expressed in humans, IgG1 and IgG3 display higher affinity for FcγRs present on effector cells compared to IgG2 and IgG4 (Table 1). IgG4 is known to have reduced affinity for FcγRI, attributed to differences in specific amino acids compared with IgG1 and IgG3. On the other hand, IgG2, in its monomeric form, binds solely to FcγRIIa, possibly due to its short hinge lacking G236 [181]. Although the precise mechanisms dictating these affinity differences are largely unknown, advancements in the X-ray crystal structures of mAbs have significantly contributed to understanding the influence of individual residues, as well as their combination, in their effector function, allowing for the selective elimination or enhancement of specific residues as needed [188].
The IgG-Fc region can be modified to improve their therapeutic parameters, such as efficacy, pharmacokinetics, and safety, which are rationally designed as extensively reviewed by Chiu et al. [5] Wang et al. [189], and Liu et al. [187]. In this section, we will review the major modifications that have been incorporated into WLA-approved TmAbs to modify TmAbs effector functions that influence their antitumor response.
To decrease effector functions of antibodies
The IgG-Fc-FcγRIII interaction is significantly affected by the glucan located in the conserved N-glycosylation site N297 in each of the CH2 domains (Fig. 1A). Mutations in the CH2 domains, which destroy this N-glycosylation motif, give rise to an aglycosylated IgG-Fc and result in complete loss of binding to most FcγR, except FcγRI [190]. An example is anti-PD-L1 antibodies used to treat bladder cancer, such as atezolizumab and durvalumab [191, 192]. The Fc functions of IgG1 were removed from these TmAbs through mutations of residues N297A in atezolizumab [193], and mutations in L234F, L235E, and P331S in durvalumab (Supplementary Fig. 3) [187].
Regarding most of the antibodies designed to block the inhibition of the immune response mediated by PD-1/PD-L1 axis, they lack the effector functions associated with the Fc region, since their mechanism of action is to block immune checkpoint molecules, thus maintaining the activation of immune cells, but not lysing them. Indeed, 19 out of the 76 TmAbs approved for oncology treatment are IgG4, which does not activate CDC or ADCC. The same occurs with ADCs, such as gemtuzumab and inotuzumab (Fig. 2E). As previously mentioned, this TmAb carries the chemotherapeutic agent calicheamicin; instead of inducing CDC or ADCC, this TmAb facilitates calicheamicin internalization and activity within the tumor cell [194]. The design of bispecifics directed to recognize an oncotarget and approximate T cells also involves the incorporation of mutations into IgG-Fc to reduce their effector functions. For instance, cadonilimab, a tetravalent PD-1/CTLA-4 bispecific antibody [195], decreased Fc-mediated ADCC and CDC effects after introducing L234A and L235A mutations, as well as the mutation G237A (Supplementary Fig. 3), which has also been reported to reduce TmAb binding to Fcγ receptors [196].
To optimize antibody effector functions and their antitumor efficacy
Several approaches have been used to increase the affinity between TmAbs and FcγRIIIa. These include engineering of the Fc region through amino acid mutations and glycoengineering of the N-glycan Fc to reduce core fucose, which is implicated in immune regulation. This strategy was applied to tafasitamab, an anti-CD19 TmAb used in diffuse large B-cell lymphoma. The increase in binding strength to FcγRIIIa, which increased ADCC capacity, was achieved through changes in residues S239D and I332E (Supplementary Fig. 3) [197, 198]. Genetically modified Chinese Hamster Ovary (CHO) cell lines or rat myeloma YB2/0 cells have also been used to produce recombinant afucosylated or low-fucose proteins. Obinutuzumab and ublituximab, two anti-CD20 TmAbs used for the treatment of chronic lymphocytic leukemia, are examples of these novel molecular modifications [199, 200] (Fig. 2E). Amivantamab, a bispecific full human IgG1 antibody against EGFR and cMET, includes modifications at residues K409R of cMET and F405L on EGFR, and both are produced with low fucose content to increase interaction with FcγRIIIa, thus improving ADCC [201]. Therefore, amivantamab induces a more potent ADCC than cetuximab, a bivalent anti-EGFR mAb with normal fucose content [11]. Other modifications that enhance binding to FcγRIII are the F243L, R292P, Y300L, V305I, and P396L mutants into the Fc domain of margetuximab, an anti-HER2 antibody (Supplementary Fig. 3). These mutations are strategically designed to promote ADCC while simultaneously reducing affinity for the low-affinity human inhibitory FcγRIIb receptor (CD32B), thereby augmenting its antitumoral activity [5, 202].
Conclusions and future perspectives
The therapeutic value of manipulating the immune system in oncology through immunotherapy, by either improving or suppressing its response through TmAbs, has been revolutionary, scientifically and clinically recognized.
The biotechnological development of TmAbs and the current scenario of their functional optimization through rational engineering have made it possible to considerably improve the effectiveness of oncological therapies, even positioning them as first-line therapies in some types of cancers. The novel modifications introduced to their design, which have improved their pharmacokinetics and pharmacodynamics, are crucial for optimizing therapeutic schemes and, simultaneously, reducing the adverse effects that were more severe in the first generations of therapeutic antibodies. In this context, the era of multispecific antibodies, designed to recognize multiple epitopes located on the same or distinct antigens, has transformed cancer immunotherapy. Indeed, VHH, scFv, and Fab-based technology platforms are helpful in their design due to their flexibility in building blocks against different molecules, thus facilitating the assembly of multispecific structures. Additionally, the affinity of these antibodies is carefully balanced for efficient binding to their respective antigens, which contributes to improving their pharmacokinetics and pharmacodynamics profiles. Several clinical trials are currently enrolling participants in phase I/II studies to evaluate the safety, tolerability, pharmacokinetics, and efficacy of trispecific antibodies in various types of cancer. Some examples of these new molecules include the GB263T antibody against EGFR and 2 distinct epitopes on cMET, which is being tested in advanced NSCLC and other solid tumors (NCT05332574), and the NM21-1480 antibody against PD-L1, 4-1BB, and human serum albumin (HSA), which is considered a new generation of immune checkpoint inhibitors that block PD-L1/PD-1 signaling and co-stimulates 4-1BB, a member of the tumor necrosis factor receptor superfamily expressed on tumor-infiltrating T cells (NCT04442126). Development of T cell-redirecting trispecific antibodies is also being tested in hematologic malignancies, including the JNJ-79635322 antibody targeting CD3 on T lymphocytes, as well as BCMA and GPRC5D on tumor cells; this TmAb is being evaluated in relapsed and/or refractory multiple myeloma (NCT05652335); the JNJ-80948543 antibody targeting CD3, CD79b, and CD20 in patients with non-Hodgkin lymphoma and CLL (NCT05424822); and the PIT565 antibody against CD3, CD2, and CD19 for relapsed and/or refractory B-cell non-Hodgkin lymphoma or B-ALL (NCT05397496).
TmAbs are currently used as transporters to target radioactive molecules, toxins, and antineoplastic drugs. They aim to improve the delivery of these substances, achieve a better antitumor response, and reduce unwanted toxicity in healthy cells and tissues. Their innovative design also considers directing therapies to more than one molecular target, aiming to enhance recognition of the tumor cell, thus favoring its elimination.
The understanding of the molecular basis of cancer development and progression currently goes hand in hand with the discovery and development of novel and more efficient therapeutic approaches to treat this disease. In this sense, we envision that the challenge for the future is to continue improving the effector functions of TmAbs to obtain the best clinical outcome.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
CR, KTS, MCM, YL, ST, DM, MLR, YG, FGH, IC, JR, CD, PN, MG, NF, GV, VV, and PM—searched literature regarding therapeutic monoclonal antibodies in oncology. MCM, CD, DM, VV, ST, and KTS—made the figures and Tables. CR, KTS, MCM, MLR, YL, and ST—wrote the manuscript. MCM, CR, KTS, FGH, PN, and CA—revised and checked the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by National Agency of Research and Development (ANID) FONDECYT GRANTS 1221031 (MCM), 3230454 (KT-S), 3220181 (YL), 3240175 (FG-H); ANID Scholarship 21221729 (IC), 21231772 (ST); Anillo Regular de Ciencia y/o Tecnología 2021 ACT210068 (CA).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Karen Toledo-Stuardo and Carolina H. Ribeiro have contributed equally to this work.
References
- 1.Kaplon H, Reichert JM (2021) Antibodies to watch in 2021. MAbs 13:1860476. 10.1080/19420862.2020.1860476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GLOBOCAN (2021) World Health Organization: Global cancer statistics 2020 report. (http://globocan.iarc.fr/)
- 3.Grillo-López AJ (2002) Monoclonal antibody therapy for B-cell lymphoma. Int J Hematol 76:385–393. 10.1007/bf02982803 [DOI] [PubMed] [Google Scholar]
- 4.The_Antibody_Society (2024) Therapeutic monoclonal antibodies approved or in regulatory review. www:antibodysociety.org/antibody-therapeutics-product-data. Accessed 15 May 2024
- 5.Chiu ML, Goulet DR, Teplyakov A, Gilliland GL (2019) Antibody structure and function: the basis for engineering therapeutics. Antibodies (Basel). 10.3390/antib8040055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alfaleh MA, Alsaab HO, Mahmoud AB, Alkayyal AA, Jones ML, Mahler SM, Hashem AM (2020) Phage display derived monoclonal antibodies: from bench to bedside. Front Immunol 11:1986. 10.3389/fimmu.2020.01986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arbabi-Ghahroudi M (2022) Camelid single-domain antibodies: promises and challenges as lifesaving treatments. Int J Mol Sci 23:5009. 10.3390/ijms23095009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golay J, Taylor RP (2020) The role of complement in the mechanism of action of therapeutic anti-cancer mAbs. Antibodies (Basel) 9:58. 10.3390/antib9040058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vyas AR, Singh SV (2014) Molecular targets and mechanisms of cancer prevention and treatment by withaferin a, a naturally occurring steroidal lactone. Aaps J 16:1–10. 10.1208/s12248-013-9531-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Meerten T, van Rijn RS, Hol S, Hagenbeek A, Ebeling SB (2006) Complement-induced cell death by rituximab depends on CD20 expression level and acts complementary to antibody-dependent cellular cytotoxicity. Clin Cancer Res 12:4027–4035. 10.1158/1078-0432.ccr-06-0066 [DOI] [PubMed] [Google Scholar]
- 11.Vijayaraghavan S, Lipfert L, Chevalier K, Bushey BS, Henley B, Lenhart R, Sendecki J et al (2020) Amivantamab (JNJ-61186372), an Fc enhanced EGFR/cMet bispecific antibody, induces receptor downmodulation and antitumor activity by monocyte/macrophage trogocytosis. Mol Cancer Ther 19:2044–2056. 10.1158/1535-7163.mct-20-0071 [DOI] [PubMed] [Google Scholar]
- 12.Cho BC, Simi A, Sabari J, Vijayaraghavan S, Moores S, Spira A (2023) Amivantamab, an epidermal growth factor receptor (EGFR) and mesenchymal-epithelial transition factor (MET) bispecific antibody, designed to enable multiple mechanisms of action and broad clinical applications. Clin Lung Cancer 24:89–97. 10.1016/j.cllc.2022.11.004 [DOI] [PubMed] [Google Scholar]
- 13.Datta-Mannan A, Witcher DR, Tang Y, Watkins J, Jiang W, Wroblewski VJ (2007) Humanized IgG1 variants with differential binding properties to the neonatal Fc receptor: relationship to pharmacokinetics in mice and primates. Drug Metab Dispos 35:86–94. 10.1124/dmd.106.011734 [DOI] [PubMed] [Google Scholar]
- 14.Shah A, Rauth S, Aithal A, Kaur S, Ganguly K, Orzechowski C, Varshney GC et al (2021) The current landscape of antibody-based therapies in solid malignancies. Theranostics 11:1493–1512. 10.7150/thno.52614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyu X, Zhao Q, Hui J, Wang T, Lin M, Wang K, Zhang J et al (2022) The global landscape of approved antibody therapies. Antib Ther 5:233–257. 10.1093/abt/tbac021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohler G, Milstein C (1975) Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495–497. 10.1038/256495a0 [DOI] [PubMed] [Google Scholar]
- 17.Hooks MA, Wade CS, Millikan WJ Jr (1991) Muromonab CD-3: a review of its pharmacology, pharmacokinetics, and clinical use in transplantation. Pharmacotherapy 11:26–37 [PubMed] [Google Scholar]
- 18.Grillo-López AJ (2002) Zevalin: the first radioimmunotherapy approved for the treatment of lymphoma. Expert Rev Anticancer Ther 2:485–493. 10.1586/14737140.2.5.485 [DOI] [PubMed] [Google Scholar]
- 19.Vaisman-Mentesh A, Gutierrez-Gonzalez M, DeKosky BJ, Wine Y (2020) The molecular mechanisms that underlie the immune biology of anti-drug antibody formation following treatment with monoclonal antibodies. Front Immunol 11:1951. 10.3389/fimmu.2020.01951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Legouffe E, Liautard J, Gaillard JP, Rossi JF, Wijdenes J, Bataille R, Klein B et al (1994) Human anti-mouse antibody response to the injection of murine monoclonal antibodies against IL-6. Clin Exp Immunol 98:323–329. 10.1111/j.1365-2249.1994.tb06145.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berger M, Shankar V, Vafai A (2002) Therapeutic applications of monoclonal antibodies. Am J Med Sci 324:14–30. 10.1097/00000441-200207000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aguillón JC, Contreras J, Dotte A, Cruzat A, Catalán D, Salazar L, Molina MC et al (2003) New immunological weapons for medicine in the 21st Century: biological therapy based on the use of the latest generation monoclonal antibodies. Rev Med Chil 131:1445–1453 [PubMed] [Google Scholar]
- 23.Salles G, Barrett M, Foà R, Maurer J, O’Brien S, Valente N, Wenger M et al (2017) Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience. Adv Ther 34:2232–2273. 10.1007/s12325-017-0612-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perry CM, Wiseman LR (1999) Trastuzumab. BioDrugs 12:129–135. 10.2165/00063030-199912020-00004 [DOI] [PubMed] [Google Scholar]
- 25.Markham A (2022) Envafolimab: first approval. Drugs 82:235–240. 10.1007/s40265-022-01671-w [DOI] [PubMed] [Google Scholar]
- 26.Chen M, Jiang M, Wang X, Shen L, Li J (2022) Envafolimab—first PD-1/PD-L1 antibody to be administered by subcutaneous injection for microsatellite instability-high or deficient mismatch repair advanced solid tumors. Expert Opin Biol Ther 22:1227–1232. 10.1080/14712598.2022.2125799 [DOI] [PubMed] [Google Scholar]
- 27.Muñoz-López P, Ribas-Aparicio RM, Becerra-Báez EI, Fraga-Pérez K, Flores-Martínez LF, Mateos-Chávez AA, Luria-Pérez R (2022) Single-chain fragment variable: recent progress in cancer diagnosis and therapy. Cancers (Basel) 14:4206. 10.3390/cancers14174206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huston JS, Levinson D, Mudgett-Hunter M, Tai MS, Novotný J, Margolies MN, Ridge RJ et al (1988) Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci U S A 85:5879–5883. 10.1073/pnas.85.16.5879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kantarjian H, Stein A, Gökbuget N, Fielding AK, Schuh AC, Ribera JM, Wei A et al (2017) Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med 376:836–847. 10.1056/NEJMoa1609783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCafferty J, Griffiths AD, Winter G, Chiswell DJ (1990) Phage antibodies: filamentous phage displaying antibody variable domains. Nature 348:552–554. 10.1038/348552a0 [DOI] [PubMed] [Google Scholar]
- 31.Smith GP (1985) Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science 228:1315–1317 [DOI] [PubMed] [Google Scholar]
- 32.Winter G, Milstein C (1991) Man-made antibodies. Nature 349:293–299. 10.1038/349293a0 [DOI] [PubMed] [Google Scholar]
- 33.Lonberg N, Taylor LD, Harding FA, Trounstine M, Higgins KM, Schramm SR, Kuo CC et al (1994) Antigen-specific human antibodies from mice comprising four distinct genetic modifications. Nature 368:856–859. 10.1038/368856a0 [DOI] [PubMed] [Google Scholar]
- 34.Lonberg N (2005) Human antibodies from transgenic animals. Nat Biotechnol 23:1117–1125. 10.1038/nbt1135 [DOI] [PubMed] [Google Scholar]
- 35.Gibson TB, Ranganathan A, Grothey A (2006) Randomized phase III trial results of panitumumab, a fully human anti-epidermal growth factor receptor monoclonal antibody, in metastatic colorectal cancer. Clin Colorectal Cancer 6:29–31. 10.3816/CCC.2006.n.01 [DOI] [PubMed] [Google Scholar]
- 36.Gomes-da-Silva LC, Kepp O, Kroemer G (2020) Regulatory approval of photoimmunotherapy: photodynamic therapy that induces immunogenic cell death. Oncoimmunology 9:1841393. 10.1080/2162402x.2020.1841393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Q, Chen Y, Park J, Liu X, Hu Y, Wang T, McFarland K et al (2019) Design and production of bispecific antibodies. Antibodies (Basel) 8:43. 10.3390/antib8030043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parren P, Carter PJ, Plückthun A (2017) Changes to international nonproprietary names for antibody therapeutics 2017 and beyond: of mice, men and more. MAbs 9:898–906. 10.1080/19420862.2017.1341029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization, Geneva (2022) New International Nonproprietary Names (INN) nomenclature scheme for monoclonal antibodies NN Working Doc. 22.542. https://cdn.who.int/media/docs/default-source/international-nonproprietary-names-(inn)/new_mab_nomenclature-_2021rev.pdf?sfvrsn=f3247646_9&download=true
- 40.Dhawan N, Afzal MZ, Amin M (2023) Immunotherapy in anal cancer. Curr Oncol 30:4538–4550. 10.3390/curroncol30050343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aghbash PS, Hemmat N, Fathi H, Baghi HB (2022) Monoclonal antibodies in cervical malignancy-related HPV. Front Oncol 12:904790. 10.3389/fonc.2022.904790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albrecht LJ, Livingstone E, Zimmer L, Schadendorf D (2023) The latest option: nivolumab and relatlimab in advanced melanoma. Curr Oncol Rep 25:647–657. 10.1007/s11912-023-01406-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huo JL, Wang YT, Fu WJ, Lu N, Liu ZS (2022) The promising immune checkpoint LAG-3 in cancer immunotherapy: from basic research to clinical application. Front Immunol 13:956090. 10.3389/fimmu.2022.956090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Appert-Collin A, Hubert P, Crémel G, Bennasroune A (2015) Role of ErbB receptors in cancer cell migration and invasion. Front Pharmacol 6:283. 10.3389/fphar.2015.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Androutsopoulos G, Styliara I, Zarogianni E, Lazurko N, Valasoulis G, Michail G, Adonakis G (2023) The ErbB signaling network and its potential role in endometrial cancer. Epigenomes 7:24. 10.3390/epigenomes7040024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.García-Merino A (2021) Bruton’s tyrosine kinase inhibitors: a new generation of promising agents for multiple sclerosis therapy. Cells. 10.3390/cells10102560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang Y, Zhang T, Zhang J (2020) Natural tyrosine kinase inhibitors acting on the epidermal growth factor receptor: their relevance for cancer therapy. Pharmacol Res 161:105164. 10.1016/j.phrs.2020.105164 [DOI] [PubMed] [Google Scholar]
- 48.Rassy E, Flippot R, Albiges L (2020) Tyrosine kinase inhibitors and immunotherapy combinations in renal cell carcinoma. Ther Adv Med Oncol 12:1758835920907504. 10.1177/1758835920907504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pender A, Jones RL (2017) Olaratumab: a platelet-derived growth factor receptor-α-blocking antibody for the treatment of soft tissue sarcoma. Clin Pharmacol 9:159–164. 10.2147/cpaa.s130178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Y, Cao Y (2022) The impact of VEGF on cancer metastasis and systemic disease. Semin Cancer Biol 86:251–261. 10.1016/j.semcancer.2022.03.011 [DOI] [PubMed] [Google Scholar]
- 51.Ahmad A, Nawaz MI (2022) Molecular mechanism of VEGF and its role in pathological angiogenesis. J Cell Biochem 123:1938–1965. 10.1002/jcb.30344 [DOI] [PubMed] [Google Scholar]
- 52.Ohishi T, Kaneko MK, Yoshida Y, Takashima A, Kato Y, Kawada M (2023) Current targeted therapy for metastatic colorectal cancer. Int J Mol Sci 24:1702. 10.3390/ijms24021702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tewari KS, Sill MW, Penson RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A et al (2017) Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet 390:1654–1663. 10.1016/s0140-6736(17)31607-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scheuermann RH, Racila E (1995) CD19 antigen in leukemia and lymphoma diagnosis and immunotherapy. Leuk Lymphoma 18:385–397. 10.3109/10428199509059636 [DOI] [PubMed] [Google Scholar]
- 55.Bubien JK, Zhou LJ, Bell PD, Frizzell RA, Tedder TF (1993) Transfection of the CD20 cell surface molecule into ectopic cell types generates a Ca2+ conductance found constitutively in B lymphocytes. J Cell Biol 121:1121–1132. 10.1083/jcb.121.5.1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tedder TF, Engel P (1994) CD20: a regulator of cell-cycle progression of B lymphocytes. Immunol Today 15:450–454. 10.1016/0167-5699(94)90276-3 [DOI] [PubMed] [Google Scholar]
- 57.Li H, Ayer LM, Lytton J, Deans JP (2003) Store-operated cation entry mediated by CD20 in membrane rafts. J Biol Chem 278:42427–42434. 10.1074/jbc.M308802200 [DOI] [PubMed] [Google Scholar]
- 58.Pavlasova G, Mraz M (2020) The regulation and function of CD20: an “enigma” of B-cell biology and targeted therapy. Haematologica 105:1494–1506. 10.3324/haematol.2019.243543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tedder TF, Poe JC, Haas KM (2005) CD22: a multifunctional receptor that regulates B lymphocyte survival and signal transduction. Adv Immunol 88:1–50. 10.1016/s0065-2776(05)88001-0 [DOI] [PubMed] [Google Scholar]
- 60.Van Noesel CJ, Brouns GS, van Schijndel GM, Bende RJ, Mason DY, Borst J, van Lier RA (1992) Comparison of human B cell antigen receptor complexes: membrane-expressed forms of immunoglobulin (Ig)M, IgD, and IgG are associated with structurally related heterodimers. J Exp Med 175:1511–1519. 10.1084/jem.175.6.1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laabi Y, Gras MP, Brouet JC, Berger R, Larsen CJ, Tsapis A (1994) The BCMA gene, preferentially expressed during B lymphoid maturation, is bidirectionally transcribed. Nucleic Acids Res 22:1147–1154. 10.1093/nar/22.7.1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu S, Lam KP (2001) B-cell maturation protein, which binds the tumor necrosis factor family members BAFF and APRIL, is dispensable for humoral immune responses. Mol Cell Biol 21:4067–4074. 10.1128/mcb.21.12.4067-4074.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Novak AJ, Darce JR, Arendt BK, Harder B, Henderson K, Kindsvogel W, Gross JA et al (2004) Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival. Blood 103:689–694. 10.1182/blood-2003-06-2043 [DOI] [PubMed] [Google Scholar]
- 64.O’Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, Lin LL et al (2004) BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med 199:91–98. 10.1084/jem.20031330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanchez E, Li M, Kitto A, Li J, Wang CS, Kirk DT, Yellin O et al (2012) Serum B-cell maturation antigen is elevated in multiple myeloma and correlates with disease status and survival. Br J Haematol 158:727–738. 10.1111/j.1365-2141.2012.09241.x [DOI] [PubMed] [Google Scholar]
- 66.van de Donk N, Richardson PG, Malavasi F (2018) CD38 antibodies in multiple myeloma: back to the future. Blood 131:13–29. 10.1182/blood-2017-06-740944 [DOI] [PubMed] [Google Scholar]
- 67.Kodama T, Kochi Y, Nakai W, Mizuno H, Baba T, Habu K, Sawada N et al (2019) Anti-GPRC5D/CD3 bispecific T-Cell-redirecting antibody for the treatment of multiple myeloma. Mol Cancer Ther 18:1555–1564. 10.1158/1535-7163.mct-18-1216 [DOI] [PubMed] [Google Scholar]
- 68.De Luca F, Allegra A, Di Chio C, Previti S, Zappalà M, Ettari R (2023) Monoclonal antibodies: the greatest resource to treat multiple myeloma. Int J Mol Sci 24:3136. 10.3390/ijms24043136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wudhikarn K, Wills B, Lesokhin AM (2020) Monoclonal antibodies in multiple myeloma: current and emerging targets and mechanisms of action. Best Pract Res Clin Haematol 33:101143. 10.1016/j.beha.2020.101143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hartley-Brown M, Richardson P (2022) Antibody-drug conjugate therapies in multiple myeloma-what’s next on the horizon? Explor Target Antitumor Ther 3:1–10. 10.37349/etat.2022.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, Ramchandren R et al (2012) Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol 30:2183–2189. 10.1200/jco.2011.38.0410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zinzani PL, Viviani S, Anastasia A, Vitolo U, Luminari S, Zaja F, Corradini P et al (2013) Brentuximab vedotin in relapsed/refractory Hodgkin’s lymphoma: the Italian experience and results of its use in daily clinical practice outside clinical trials. Haematologica 98:1232–1236. 10.3324/haematol.2012.083048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lambert J, Pautas C, Terré C, Raffoux E, Turlure P, Caillot D, Legrand O et al (2019) Gemtuzumab ozogamicin for de novo acute myeloid leukemia: final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica 104:113–119. 10.3324/haematol.2018.188888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Larocca C, Kupper T (2019) Mycosis fungoides and sézary syndrome: an update. Hematol Oncol Clin North Am 33:103–120. 10.1016/j.hoc.2018.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Merli P, Quintarelli C, Strocchio L, Locatelli F (2021) The role of interferon-gamma and its signaling pathway in pediatric hematological disorders. Pediatr Blood Cancer 68:e28900. 10.1002/pbc.28900 [DOI] [PubMed] [Google Scholar]
- 76.Prencipe G, Bracaglia C, Caiello I, Pascarella A, Francalanci P, Pardeo M, Meneghel A et al (2019) The interferon-gamma pathway is selectively up-regulated in the liver of patients with secondary hemophagocytic lymphohistiocytosis. PLoS ONE 14:e0226043. 10.1371/journal.pone.0226043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Al-Salama ZT (2019) Emapalumab: first global approval. Drugs 79:99–103. 10.1007/s40265-018-1046-8 [DOI] [PubMed] [Google Scholar]
- 78.Cheloff AZ, Al-Samkari H (2020) Emapalumab for the treatment of hemophagocytic lymphohistiocytosis. Drugs Today (Barc) 56:439–446. 10.1358/dot.2020.56.7.3145359 [DOI] [PubMed] [Google Scholar]
- 79.Markham A (2021) Naxitamab: first approval. Drugs 81:291–296. 10.1007/s40265-021-01467-4 [DOI] [PubMed] [Google Scholar]
- 80.Nakayama I, Qi C, Chen Y, Nakamura Y, Shen L, Shitara K (2024) Claudin 18.2 as a novel therapeutic target. Nat Rev Clin Oncol 21:354–369. 10.1038/s41571-024-00874-2 [DOI] [PubMed] [Google Scholar]
- 81.Gklinos P, Papadopoulou M, Stanulovic V, Mitsikostas DD, Papadopoulos D (2021) Monoclonal Antibodies as neurological therapeutics. Pharmaceuticals (Basel) 14:92. 10.3390/ph14020092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hubert P, Amigorena S (2012) Antibody-dependent cell cytotoxicity in monoclonal antibody-mediated tumor immunotherapy. Oncoimmunology 1:103–105. 10.4161/onci.1.1.17963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Diebolder CA, Beurskens FJ, de Jong RN, Koning RI, Strumane K, Lindorfer MA, Voorhorst M et al (2014) Complement is activated by IgG hexamers assembled at the cell surface. Science 343:1260–1263. 10.1126/science.1248943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cleary KLS, Chan HTC, James S, Glennie MJ, Cragg MS (2017) Antibody distance from the cell membrane regulates antibody effector mechanisms. J Immunol 198:3999–4011. 10.4049/jimmunol.1601473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weisser NE, Sanches M, Escobar-Cabrera E, O’Toole J, Whalen E, Chan PWY, Wickman G et al (2023) An anti-HER2 biparatopic antibody that induces unique HER2 clustering and complement-dependent cytotoxicity. Nat Commun 14:1394. 10.1038/s41467-023-37029-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Warwick CA, Keyes AL, Woodruff TM, Usachev YM (2021) The complement cascade in the regulation of neuroinflammation, nociceptive sensitization, and pain. J Biol Chem 297:101085. 10.1016/j.jbc.2021.101085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garred P, Michaelsen TE, Aase A (1989) The IgG subclass pattern of complement activation depends on epitope density and antibody and complement concentration. Scand J Immunol 30:379–382. 10.1111/j.1365-3083.1989.tb01225.x [DOI] [PubMed] [Google Scholar]
- 88.Michaelsen TE, Garred P, Aase A (1991) Human IgG subclass pattern of inducing complement-mediated cytolysis depends on antigen concentration and to a lesser extent on epitope patchiness, antibody affinity and complement concentration. Eur J Immunol 21:11–16. 10.1002/eji.1830210103 [DOI] [PubMed] [Google Scholar]
- 89.Arlaud GJ, Gaboriaud C, Thielens NM, Rossi V, Bersch B, Hernandez JF, Fontecilla-Camps JC (2001) Structural biology of C1: dissection of a complex molecular machinery. Immunol Rev 180:136–145. 10.1034/j.1600-065x.2001.1800112.x [DOI] [PubMed] [Google Scholar]
- 90.Hughes-Jones NC, Gardner B (1979) Reaction between the isolated globular sub-units of the complement component C1q and IgG-complexes. Mol Immunol 16:697–701. 10.1016/0161-5890(79)90010-5 [DOI] [PubMed] [Google Scholar]
- 91.Burton DR, Boyd J, Brampton AD, Easterbrook-Smith SB, Emanuel EJ, Novotny J, Rademacher TW et al (1980) The Clq receptor site on immunoglobulin G. Nature 288:338–344. 10.1038/288338a0 [DOI] [PubMed] [Google Scholar]
- 92.Roumenina LT, Ruseva MM, Zlatarova A, Ghai R, Kolev M, Olova N, Gadjeva M et al (2006) Interaction of C1q with IgG1, C-reactive protein and pentraxin 3: mutational studies using recombinant globular head modules of human C1q A, B, and C chains. Biochemistry 45:4093–4104. 10.1021/bi052646f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bajic G, Degn SE, Thiel S, Andersen GR (2015) Complement activation, regulation, and molecular basis for complement-related diseases. Embo J 34:2735–2757. 10.15252/embj.201591881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Laumonnier Y, Karsten CM, Köhl J (2017) Novel insights into the expression pattern of anaphylatoxin receptors in mice and men. Mol Immunol 89:44–58. 10.1016/j.molimm.2017.05.019 [DOI] [PubMed] [Google Scholar]
- 95.Teeling JL, Mackus WJ, Wiegman LJ, van den Brakel JH, Beers SA, French RR, van Meerten T et al (2006) The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol 177:362–371. 10.4049/jimmunol.177.1.362 [DOI] [PubMed] [Google Scholar]
- 96.Ugurlar D, Howes SC, de Kreuk BJ, Koning RI, de Jong RN, Beurskens FJ, Schuurman J et al (2018) Structures of C1-IgG1 provide insights into how danger pattern recognition activates complement. Science 359:794–797. 10.1126/science.aao4988 [DOI] [PubMed] [Google Scholar]
- 97.Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, Newman RA et al (1994) Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 83:435–445 [PubMed] [Google Scholar]
- 98.Idusogie EE, Presta LG, Gazzano-Santoro H, Totpal K, Wong PY, Ultsch M, Meng YG et al (2000) Mapping of the C1q binding site on rituxan, a chimeric antibody with a human IgG1 Fc. J Immunol 164:4178–4184. 10.4049/jimmunol.164.8.4178 [DOI] [PubMed] [Google Scholar]
- 99.Laursen NS, Pedersen DV, Gytz H, Zarantonello A, Bernth Jensen JM, Hansen AG, Thiel S et al (2020) Functional and structural characterization of a potent C1q inhibitor targeting the classical pathway of the complement system. Front Immunol 11:1504. 10.3389/fimmu.2020.01504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Clynes RA, Towers TL, Presta LG, Ravetch JV (2000) Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med 6:443–446. 10.1038/74704 [DOI] [PubMed] [Google Scholar]
- 101.Nimmerjahn F, Ravetch JV (2008) Fcgamma receptors as regulators of immune responses. Nat Rev Immunol 8:34–47. 10.1038/nri2206 [DOI] [PubMed] [Google Scholar]
- 102.Smyth MJ, Cretney E, Kelly JM, Westwood JA, Street SE, Yagita H, Takeda K et al (2005) Activation of NK cell cytotoxicity. Mol Immunol 42:501–510. 10.1016/j.molimm.2004.07.034 [DOI] [PubMed] [Google Scholar]
- 103.Behrens LM, van Egmond M, van den Berg TK (2023) Neutrophils as immune effector cells in antibody therapy in cancer. Immunol Rev 314:280–301. 10.1111/imr.13159 [DOI] [PubMed] [Google Scholar]
- 104.de Taeye SW, Bentlage AEH, Mebius MM, Meesters JI, Lissenberg-Thunnissen S, Falck D, Sénard T et al (2020) FcγR binding and ADCC activity of human IgG allotypes. Front Immunol 11:740. 10.3389/fimmu.2020.00740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mata-Molanes JJ, Rebollo-Liceaga J, Martínez-Navarro EM, Manzano RG, Brugarolas A, Juan M, Sureda M (2022) Relevance of Fc gamma receptor polymorphisms in cancer therapy with monoclonal antibodies. Front Oncol 12:926289. 10.3389/fonc.2022.926289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang W, Gordon M, Schultheis AM, Yang DY, Nagashima F, Azuma M, Chang HM et al (2007) FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol 25:3712–3718. 10.1200/jco.2006.08.8021 [DOI] [PubMed] [Google Scholar]
- 107.Gavin PG, Song N, Kim SR, Lipchik C, Johnson NL, Bandos H, Finnigan M et al (2017) Association of polymorphisms in FCGR2A and FCGR3A with degree of trastuzumab benefit in the adjuvant treatment of ERBB2/HER2-positive breast cancer: analysis of the NSABP B-31 trial. JAMA Oncol 3:335–341. 10.1001/jamaoncol.2016.4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ottonello L, Epstein AL, Mancini M, Tortolina G, Dapino P, Dallegri F (2001) Chimaeric Lym-1 monoclonal antibody-mediated cytolysis by neutrophils from G-CSF-treated patients: stimulation by GM-CSF and role of Fc gamma -receptors. Br J Cancer 85:463–469. 10.1054/bjoc.2001.1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schneider-Merck T, Lammerts van Bueren JJ, Berger S, Rossen K, van Berkel PH, Derer S, Beyer T et al (2010) Human IgG2 antibodies against epidermal growth factor receptor effectively trigger antibody-dependent cellular cytotoxicity but, in contrast to IgG1, only by cells of myeloid lineage. J Immunol 184:512–520. 10.4049/jimmunol.0900847 [DOI] [PubMed] [Google Scholar]
- 110.Treffers LW, Zhao XW, van der Heijden J, Nagelkerke SQ, van Rees DJ, Gonzalez P, Geissler J et al (2018) Genetic variation of human neutrophil Fcγ receptors and SIRPα in antibody-dependent cellular cytotoxicity towards cancer cells. Eur J Immunol 48:344–354. 10.1002/eji.201747215 [DOI] [PubMed] [Google Scholar]
- 111.Rodríguez-Nava C, Ortuño-Pineda C, Illades-Aguiar B, Flores-Alfaro E, Leyva-Vázquez MA, Parra-Rojas I, Del Moral-Hernández O et al (2023) Mechanisms of action and limitations of monoclonal antibodies and single chain fragment variable (scFv) in the treatment of cancer. Biomedicines 11:1610. 10.3390/biomedicines11061610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kurdi AT, Glavey SV, Bezman NA, Jhatakia A, Guerriero JL, Manier S, Moschetta M et al (2018) Antibody-dependent cellular phagocytosis by macrophages is a novel mechanism of action of elotuzumab. Mol Cancer Ther 17:1454–1463. 10.1158/1535-7163.mct-17-0998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kamen L, Myneni S, Langsdorf C, Kho E, Ordonia B, Thakurta T, Zheng K et al (2019) A novel method for determining antibody-dependent cellular phagocytosis. J Immunol Methods 468:55–60. 10.1016/j.jim.2019.03.001 [DOI] [PubMed] [Google Scholar]
- 114.Kamen L, Ordonia B, Myneni S, Chung S (2022) Method for measurement of antibody-dependent cellular phagocytosis. Methods Mol Biol 2313:305–312. 10.1007/978-1-0716-1450-1_19 [DOI] [PubMed] [Google Scholar]
- 115.Karsten CB, Mehta N, Shin SA, Diefenbach TJ, Slein MD, Karpinski W, Irvine EB et al (2019) A versatile high-throughput assay to characterize antibody-mediated neutrophil phagocytosis. J Immunol Methods 471:46–56. 10.1016/j.jim.2019.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yu J, Song Y, Tian W (2020) How to select IgG subclasses in developing anti-tumor therapeutic antibodies. J Hematol Oncol 13:45. 10.1186/s13045-020-00876-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hoffmeyer F, Witte K, Schmidt RE (1997) The high-affinity Fc gamma RI on PMN: regulation of expression and signal transduction. Immunology 92:544–552. 10.1046/j.1365-2567.1997.00381.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guyre PM, Morganelli PM, Miller R (1983) Recombinant immune interferon increases immunoglobulin G Fc receptors on cultured human mononuclear phagocytes. J Clin Invest 72:393–397. 10.1172/jci110980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kårehed K, Dimberg A, Dahl S, Nilsson K, Oberg F (2007) IFN-gamma-induced upregulation of Fcgamma-receptor-I during activation of monocytic cells requires the PKR and NFkappaB pathways. Mol Immunol 44:615–624. 10.1016/j.molimm.2006.01.013 [DOI] [PubMed] [Google Scholar]
- 120.Weiner GJ (2010) Rituximab: mechanism of action. Semin Hematol 47:115–123. 10.1053/j.seminhematol.2010.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Oflazoglu E, Stone IJ, Gordon KA, Grewal IS, van Rooijen N, Law CL, Gerber HP (2007) Macrophages contribute to the antitumor activity of the anti-CD30 antibody SGN-30. Blood 110:4370–4372. 10.1182/blood-2007-06-097014 [DOI] [PubMed] [Google Scholar]
- 122.Donato EM, Fernández-Zarzoso M, Hueso JA, de la Rubia J (2018) Brentuximab vedotin in Hodgkin lymphoma and anaplastic large-cell lymphoma: an evidence-based review. Onco Targets Ther 11:4583–4590. 10.2147/ott.s141053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lim SH, Beers SA, French RR, Johnson PW, Glennie MJ, Cragg MS (2010) Anti-CD20 monoclonal antibodies: historical and future perspectives. Haematologica 95:135–143. 10.3324/haematol.2008.001628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nooka AK, Kaufman JL, Hofmeister CC, Joseph NS, Heffner TL, Gupta VA, Sullivan HC et al (2019) Daratumumab in multiple myeloma. Cancer 125:2364–2382. 10.1002/cncr.32065 [DOI] [PubMed] [Google Scholar]
- 125.Overdijk MB, Verploegen S, Bögels M, van Egmond M, Lammerts van Bueren JJ, Mutis T, Groen RW et al (2015) Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs 7:311–321. 10.1080/19420862.2015.1007813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Leidi M, Gotti E, Bologna L, Miranda E, Rimoldi M, Sica A, Roncalli M et al (2009) M2 macrophages phagocytose rituximab-opsonized leukemic targets more efficiently than m1 cells in vitro. J Immunol 182:4415–4422. 10.4049/jimmunol.0713732 [DOI] [PubMed] [Google Scholar]
- 127.Wang Y, Sanchez L, Siegel DS, Wang ML (2016) Elotuzumab for the treatment of multiple myeloma. J Hematol Oncol 9:55. 10.1186/s13045-016-0284-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Joly E, Hudrisier D (2003) What is trogocytosis and what is its purpose? Nat Immunol 4:815. 10.1038/ni0903-815 [DOI] [PubMed] [Google Scholar]
- 129.Moores SL, Chiu ML, Bushey BS, Chevalier K, Luistro L, Dorn K, Brezski RJ et al (2016) A novel bispecific antibody targeting EGFR and cMet is effective against EGFR inhibitor-resistant lung tumors. Cancer Res 76:3942–3953. 10.1158/0008-5472.can-15-2833 [DOI] [PubMed] [Google Scholar]
- 130.Ochs J, Häusser-Kinzel S, Weber MS (2023) Trogocytosis challenges the cellular specificity of lineage markers and monoclonal antibodies. Nat Rev Immunol 23:539–540. 10.1038/s41577-023-00920-7 [DOI] [PubMed] [Google Scholar]
- 131.Valgardsdottir R, Cattaneo I, Klein C, Introna M, Figliuzzi M, Golay J (2017) Human neutrophils mediate trogocytosis rather than phagocytosis of CLL B cells opsonized with anti-CD20 antibodies. Blood 129:2636–2644. 10.1182/blood-2016-08-735605 [DOI] [PubMed] [Google Scholar]
- 132.Aue G, Lindorfer MA, Beum PV, Pawluczkowycz AW, Vire B, Hughes T, Taylor RP et al (2010) Fractionated subcutaneous rituximab is well-tolerated and preserves CD20 expression on tumor cells in patients with chronic lymphocytic leukemia. Haematologica 95:329–332. 10.3324/haematol.2009.012484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Taylor RP, Lindorfer MA (2015) Fcγ-receptor-mediated trogocytosis impacts mAb-based therapies: historical precedence and recent developments. Blood 125:762–766. 10.1182/blood-2014-10-569244 [DOI] [PubMed] [Google Scholar]
- 134.Matlung HL, Babes L, Zhao XW, van Houdt M, Treffers LW, van Rees DJ, Franke K et al (2018) Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Rep 23:3946-3959.e3946. 10.1016/j.celrep.2018.05.082 [DOI] [PubMed] [Google Scholar]
- 135.Fu Z, Li S, Han S, Shi C, Zhang Y (2022) Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct Target Ther 7:93. 10.1038/s41392-022-00947-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Joubert N, Beck A, Dumontet C, Denevault-Sabourin C (2020) Antibody-drug conjugates: the last decade. Pharmaceuticals (Basel) 13:245. 10.3390/ph13090245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang Z, Li H, Gou L, Li W, Wang Y (2023) Antibody-drug conjugates: recent advances in payloads. Acta Pharm Sin B 13:4025–4059. 10.1016/j.apsb.2023.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pettit GR, Srirangam JK, Barkoczy J, Williams MD, Boyd MR, Hamel E, Pettit RK et al (1998) Antineoplastic agents 365. Dolastatin 10 SAR probes. Anticancer Drug Des 13:243–277 [PubMed] [Google Scholar]
- 139.Gao G, Wang Y, Hua H, Li D, Tang C (2021) Marine antitumor peptide dolastatin 10: biological activity, structural modification and synthetic chemistry. Mar Drugs 19:363. 10.3390/md19070363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chang HP, Cheung YK, Shah DK (2021) Whole-body pharmacokinetics and physiologically based pharmacokinetic model for monomethyl auristatin E (MMAE). J Clin Med 10:1332. 10.3390/jcm10061332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fu Y, Ho M (2018) DNA damaging agent-based antibody-drug conjugates for cancer therapy. Antib Ther 1:33–43. 10.1093/abt/tby007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Adhikari A, Shen B, Rader C (2021) Challenges and opportunities to develop enediyne natural products as payloads for antibody-drug conjugates. Antib Ther 4:1–15. 10.1093/abt/tbab001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lode HN, Reisfeld RA, Handgretinger R, Nicolaou KC, Gaedicke G, Wrasidlo W (1998) Targeted therapy with a novel enediyene antibiotic calicheamicin theta(I)1 effectively suppresses growth and dissemination of liver metastases in a syngeneic model of murine neuroblastoma. Cancer Res 58:2925–2928 [PubMed] [Google Scholar]
- 144.Knoll K, Wrasidlo W, Scherberich JE, Gaedicke G, Fischer P (2000) Targeted therapy of experimental renal cell carcinoma with a novel conjugate of monoclonal antibody 138H11 and calicheamicin thetaI1. Cancer Res 60:6089–6094 [PubMed] [Google Scholar]
- 145.Zein N, Sinha AM, McGahren WJ, Ellestad GA (1988) Calicheamicin gamma 1I: an antitumor antibiotic that cleaves double-stranded DNA site specifically. Science 240:1198–1201. 10.1126/science.3240341 [DOI] [PubMed] [Google Scholar]
- 146.Zein N, Poncin M, Nilakantan R, Ellestad GA (1989) Calicheamicin gamma 1I and DNA: molecular recognition process responsible for site-specificity. Science 244:697–699. 10.1126/science.2717946 [DOI] [PubMed] [Google Scholar]
- 147.Ricart AD (2011) Antibody-drug conjugates of calicheamicin derivative: gemtuzumab ozogamicin and inotuzumab ozogamicin. Clin Cancer Res 17:6417–6427. 10.1158/1078-0432.ccr-11-0486 [DOI] [PubMed] [Google Scholar]
- 148.Zafar S, Armaghan M, Khan K, Hassan N, Sharifi-Rad J, Habtemariam S, Kieliszek M et al (2023) New insights into the anticancer therapeutic potential of maytansine and its derivatives. Biomed Pharmacother 165:115039. 10.1016/j.biopha.2023.115039 [DOI] [PubMed] [Google Scholar]
- 149.Endo Y, Takeda K, Mohan N, Shen Y, Jiang J, Rotstein D, Wu WJ (2018) Payload of T-DM1 binds to cell surface cytoskeleton-associated protein 5 to mediate cytotoxicity of hepatocytes. Oncotarget 9:37200–37215. 10.18632/oncotarget.26461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Endo Y, Mohan N, Dokmanovic M, Wu WJ (2021) Mechanisms contributing to ado-trastuzumab emtansine-induced toxicities: a gateway to better understanding of ADC-associated toxicities. Antib Ther 4:55–59. 10.1093/abt/tbab005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ewesuedo RB, Ratain MJ (1997) Topoisomerase I inhibitors. Oncologist 2:359–364 [PubMed] [Google Scholar]
- 152.Shastry M, Jacob S, Rugo HS, Hamilton E (2022) Antibody-drug conjugates targeting TROP-2: clinical development in metastatic breast cancer. Breast 66:169–177. 10.1016/j.breast.2022.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yver A, Agatsuma T, Soria JC (2020) The art of innovation: clinical development of trastuzumab deruxtecan and redefining how antibody-drug conjugates target HER2-positive cancers. Ann Oncol 31:430–434. 10.1016/j.annonc.2019.11.019 [DOI] [PubMed] [Google Scholar]
- 154.Schmitt S, Machui P, Mai I, Herterich S, Wunder S, Cyprys P, Gerlach M et al (2024) Design and evaluation of phosphonamidate-linked exatecan constructs for highly loaded, stable, and efficacious antibody-drug conjugates. Mol Cancer Ther 23:199–211. 10.1158/1535-7163.mct-23-0359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Su Z, Xiao D, Xie F, Liu L, Wang Y, Fan S, Zhou X et al (2021) Antibody-drug conjugates: recent advances in linker chemistry. Acta Pharm Sin B 11:3889–3907. 10.1016/j.apsb.2021.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hartley JA, Flynn MJ, Bingham JP, Corbett S, Reinert H, Tiberghien A, Masterson LA et al (2018) Pre-clinical pharmacology and mechanism of action of SG3199, the pyrrolobenzodiazepine (PBD) dimer warhead component of antibody-drug conjugate (ADC) payload tesirine. Sci Rep 8:10479. 10.1038/s41598-018-28533-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sheyi R, de la Torre BG, Albericio F (2022) Linkers: an assurance for controlled delivery of antibody-drug conjugate. Pharmaceutics 14:396. 10.3390/pharmaceutics14020396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Doronina SO, Bovee TD, Meyer DW, Miyamoto JB, Anderson ME, Morris-Tilden CA, Senter PD (2008) Novel peptide linkers for highly potent antibody-auristatin conjugate. Bioconjug Chem 19:1960–1963. 10.1021/bc800289a [DOI] [PubMed] [Google Scholar]
- 159.Balamkundu S, Liu CF (2023) Lysosomal-cleavable peptide linkers in antibody-drug conjugates. Biomedicines 11:3080. 10.3390/biomedicines11113080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shinozaki T, Matsuura K, Okano W, Tomioka T, Nishiya Y, Machida M, Hayashi R (2023) Eligibility for photoimmunotherapy in patients with unresectable advanced or recurrent head and neck cancer and changes before and after systemic therapy. Cancers (Basel) 15:3795. 10.3390/cancers15153795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kobayashi H, Griffiths GL, Choyke PL (2020) Near-infrared photoimmunotherapy: photoactivatable antibody-drug conjugates (ADCs). Bioconjug Chem 31:28–36. 10.1021/acs.bioconjchem.9b00546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cheson BD (2005) The role of radioimmunotherapy with yttrium-90 ibritumomab tiuxetan in the treatment of non-Hodgkin lymphoma. BioDrugs 19:309–322. 10.2165/00063030-200519050-00004 [DOI] [PubMed] [Google Scholar]
- 163.FDA (2024) U.S. Food & Drug administration. Bispecific Antibodies: An Area of Research and Clinical Applications. https://www.fda.gov/drugs/spotlight-cder-science/bispecific-antibodies-area-research-and-clinical-applications - :~:text=Bispecific%20Antibodies%3A%20An%20Area%20of%20Research%20and%20Clinical%20Applications,-Share&text=Bispecific%20antibodies%20(BsAbs)%20have%20two,of%20the%20same%20antigen%20simultaneously. Accessed 15 May 2024
- 164.Ordóñez-Reyes C, Garcia-Robledo JE, Chamorro DF, Mosquera A, Sussmann L, Ruiz-Patiño A, Arrieta O et al (2022) Bispecific antibodies in cancer immunotherapy: a novel response to an old question. Pharmaceutics 14:1243. 10.3390/pharmaceutics14061243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sun Y, Yu X, Wang X, Yuan K, Wang G, Hu L, Zhang G et al (2023) Bispecific antibodies in cancer therapy: target selection and regulatory requirements. Acta Pharm Sin B 13:3583–3597. 10.1016/j.apsb.2023.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang W, Qiu T, Li F, Ren S (2023) Current status and future perspectives of bispecific antibodies in the treatment of lung cancer. Chin Med J (Engl) 136:379–393. 10.1097/cm9.0000000000002460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Carrasco-Padilla C, Hernaiz-Esteban A, Álvarez-Vallina L, Aguilar-Sopeña O, Roda-Navarro P (2022) Bispecific antibody format and the organization of immunological synapses in T cell-redirecting strategies for cancer immunotherapy. Pharmaceutics 15:132. 10.3390/pharmaceutics15010132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Betts A, van der Graaf PH (2020) Mechanistic quantitative pharmacology strategies for the early clinical development of bispecific antibodies in oncology. Clin Pharmacol Ther 108:528–541. 10.1002/cpt.1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang S, Chen K, Lei Q, Ma P, Yuan AQ, Zhao Y, Jiang Y et al (2021) The state of the art of bispecific antibodies for treating human malignancies. EMBO Mol Med 13:e14291. 10.15252/emmm.202114291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Brinkmann U, Kontermann RE (2021) Bispecific antibodies. Science 372:916–917. 10.1126/science.abg1209 [DOI] [PubMed] [Google Scholar]
- 171.Ma J, Mo Y, Tang M, Shen J, Qi Y, Zhao W, Huang Y et al (2021) Bispecific antibodies: from research to clinical application. Front Immunol 12:626616. 10.3389/fimmu.2021.626616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Long M, Mims AS, Li Z (2022) Factors affecting the cancer immunotherapeutic efficacy of T cell bispecific antibodies and strategies for improvement. Immunol Invest 51:2176–2214. 10.1080/08820139.2022.2131569 [DOI] [PubMed] [Google Scholar]
- 173.Hua G, Carlson D, Starr JR (2022) Tebentafusp-tebn: a novel bispecific T-cell engager for metastatic uveal melanoma. J Adv Pract Oncol 13:717–723. 10.6004/jadpro.2022.13.7.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liddy N, Bossi G, Adams KJ, Lissina A, Mahon TM, Hassan NJ, Gavarret J et al (2012) Monoclonal TCR-redirected tumor cell killing. Nat Med 18:980–987. 10.1038/nm.2764 [DOI] [PubMed] [Google Scholar]
- 175.Klinger M, Benjamin J, Kischel R, Stienen S, Zugmaier G (2016) Harnessing T cells to fight cancer with BiTE® antibody constructs–past developments and future directions. Immunol Rev 270:193–208. 10.1111/imr.12393 [DOI] [PubMed] [Google Scholar]
- 176.Slaney CY, Wang P, Darcy PK, Kershaw MH (2018) CARs versus BiTEs: a comparison between T cell-redirection strategies for cancer treatment. Cancer Discov 8:924–934. 10.1158/2159-8290.cd-18-0297 [DOI] [PubMed] [Google Scholar]
- 177.Mocquot P, Mossazadeh Y, Lapierre L, Pineau F, Despas F (2022) The pharmacology of blinatumomab: state of the art on pharmacodynamics, pharmacokinetics, adverse drug reactions and evaluation in clinical trials. J Clin Pharm Ther 47:1337–1351. 10.1111/jcpt.13741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Einsele H, Borghaei H, Orlowski RZ, Subklewe M, Roboz GJ, Zugmaier G, Kufer P et al (2020) The BiTE (bispecific T-cell engager) platform: development and future potential of a targeted immuno-oncology therapy across tumor types. Cancer 126:3192–3201. 10.1002/cncr.32909 [DOI] [PubMed] [Google Scholar]
- 179.Brinkmann U, Kontermann RE (2017) The making of bispecific antibodies. MAbs 9:182–212. 10.1080/19420862.2016.1268307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Goebeler ME, Bargou R (2016) Blinatumomab: a CD19/CD3 bispecific T cell engager (BiTE) with unique anti-tumor efficacy. Leuk Lymphoma 57:1021–1032. 10.3109/10428194.2016.1161185 [DOI] [PubMed] [Google Scholar]
- 181.Damelang T, Brinkhaus M, van Osch TLJ, Schuurman J, Labrijn AF, Rispens T, Vidarsson G (2023) Impact of structural modifications of IgG antibodies on effector functions. Front Immunol 14:1304365. 10.3389/fimmu.2023.1304365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.McDonagh CF, Kim KM, Turcott E, Brown LL, Westendorf L, Feist T, Sussman D et al (2008) Engineered anti-CD70 antibody-drug conjugate with increased therapeutic index. Mol Cancer Ther 7:2913–2923. 10.1158/1535-7163.mct-08-0295 [DOI] [PubMed] [Google Scholar]
- 183.Silva JP, Vetterlein O, Jose J, Peters S, Kirby H (2015) The S228P mutation prevents in vivo and in vitro IgG4 Fab-arm exchange as demonstrated using a combination of novel quantitative immunoassays and physiological matrix preparation. J Biol Chem 290:5462–5469. 10.1074/jbc.M114.600973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rispens T, Huijbers MG (2023) The unique properties of IgG4 and its roles in health and disease. Nat Rev Immunol 23:763–778. 10.1038/s41577-023-00871-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wiggins B, Liu-Shin L, Yamaguchi H, Ratnaswamy G (2015) Characterization of cysteine-linked conjugation profiles of immunoglobulin G1 and immunoglobulin G2 antibody-drug conjugates. J Pharm Sci 104:1362–1372. 10.1002/jps.24338 [DOI] [PubMed] [Google Scholar]
- 186.Lefranc MP, Lefranc G (2020) Immunoglobulins or antibodies: IMGT(®) bridging genes structures and functions. Biomedicines 8:319. 10.3390/biomedicines8090319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liu R, Oldham RJ, Teal E, Beers SA, Cragg MS (2020) Fc-engineering for modulated effector functions-improving antibodies for cancer treatment. Antibodies (Basel) 9:64. 10.3390/antib9040064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Oganesyan V, Gao C, Shirinian L, Wu H, Dall’Acqua WF (2008) Structural characterization of a human Fc fragment engineered for lack of effector functions. Acta Crystallogr D Biol Crystallogr 64:700–704. 10.1107/s0907444908007877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang X, Mathieu M, Brezski RJ (2018) IgG Fc engineering to modulate antibody effector functions. Protein Cell 9:63–73. 10.1007/s13238-017-0473-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dashivets T, Thomann M, Rueger P, Knaupp A, Buchner J, Schlothauer T (2015) Multi-angle effector function analysis of human monoclonal IgG glycovariants. PLoS ONE 10:e0143520. 10.1371/journal.pone.0143520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Syn NL, Teng MWL, Mok TSK, Soo RA (2017) De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol 18:e731–e741. 10.1016/s1470-2045(17)30607-1 [DOI] [PubMed] [Google Scholar]
- 192.Nasser NJ, Gorenberg M, Agbarya A (2020) First line immunotherapy for non-small cell lung cancer. Pharmaceuticals (Basel) 13:373. 10.3390/ph13110373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Deng R, Bumbaca D, Pastuskovas CV, Boswell CA, West D, Cowan KJ, Chiu H et al (2016) Preclinical pharmacokinetics, pharmacodynamics, tissue distribution, and tumor penetration of anti-PD-L1 monoclonal antibody, an immune checkpoint inhibitor. MAbs 8:593–603. 10.1080/19420862.2015.1136043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Damle NK, Frost P (2003) Antibody-targeted chemotherapy with immunoconjugates of calicheamicin. Curr Opin Pharmacol 3:386–390. 10.1016/s1471-4892(03)00083-3 [DOI] [PubMed] [Google Scholar]
- 195.Pang X, Huang Z, Zhong T, Zhang P, Wang ZM, Xia M, Li B (2023) Cadonilimab, a tetravalent PD-1/CTLA-4 bispecific antibody with trans-binding and enhanced target binding avidity. MAbs 15:2180794. 10.1080/19420862.2023.2180794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wilkinson I, Anderson S, Fry J, Julien LA, Neville D, Qureshi O, Watts G et al (2021) Fc-engineered antibodies with immune effector functions completely abolished. PLoS ONE 16:e0260954. 10.1371/journal.pone.0260954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Horton HM, Bernett MJ, Pong E, Peipp M, Karki S, Chu SY, Richards JO et al (2008) Potent in vitro and in vivo activity of an Fc-engineered anti-CD19 monoclonal antibody against lymphoma and leukemia. Cancer Res 68:8049–8057. 10.1158/0008-5472.can-08-2268 [DOI] [PubMed] [Google Scholar]
- 198.Salles G, Długosz-Danecka M, Ghesquières H, Jurczak W (2021) Tafasitamab for the treatment of relapsed or refractory diffuse large B-cell lymphoma. Expert Opin Biol Ther 21:455–463. 10.1080/14712598.2021.1884677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sawas A, Farber CM, Schreeder MT, Khalil MY, Mahadevan D, Deng C, Amengual JE et al (2017) A phase 1/2 trial of ublituximab, a novel anti-CD20 monoclonal antibody, in patients with B-cell non-Hodgkin lymphoma or chronic lymphocytic leukaemia previously exposed to rituximab. Br J Haematol 177:243–253. 10.1111/bjh.14534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cartron G, de Guibert S, Dilhuydy MS, Morschhauser F, Leblond V, Dupuis J, Mahe B et al (2014) Obinutuzumab (GA101) in relapsed/refractory chronic lymphocytic leukemia: final data from the phase 1/2 GAUGUIN study. Blood 124:2196–2202. 10.1182/blood-2014-07-586610 [DOI] [PubMed] [Google Scholar]
- 201.Neijssen J, Cardoso RMF, Chevalier KM, Wiegman L, Valerius T, Anderson GM, Moores SL et al (2021) Discovery of amivantamab (JNJ-61186372), a bispecific antibody targeting EGFR and MET. J Biol Chem 296:100641. 10.1016/j.jbc.2021.100641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Alasmari MM (2022) A review of margetuximab-based therapies in patients with HER2-positive metastatic breast cancer. Cancers (Basel) 15:38. 10.3390/cancers15010038 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.