Abstract
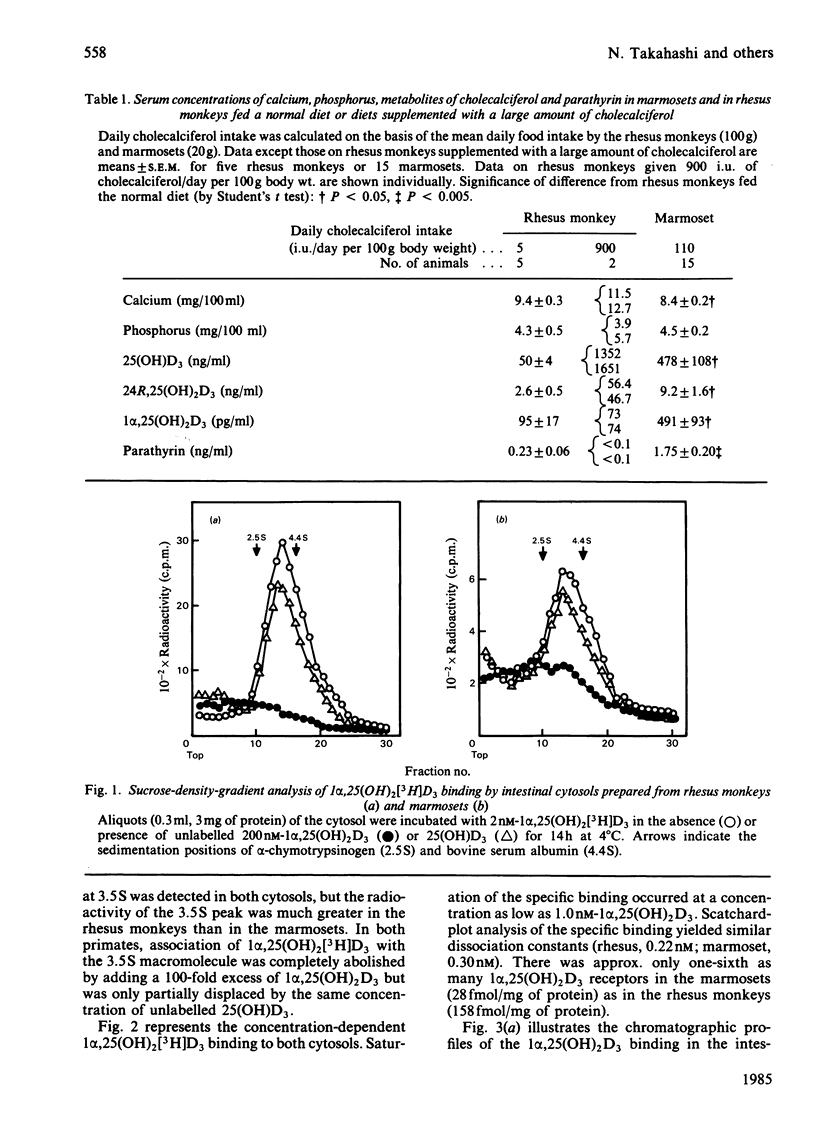
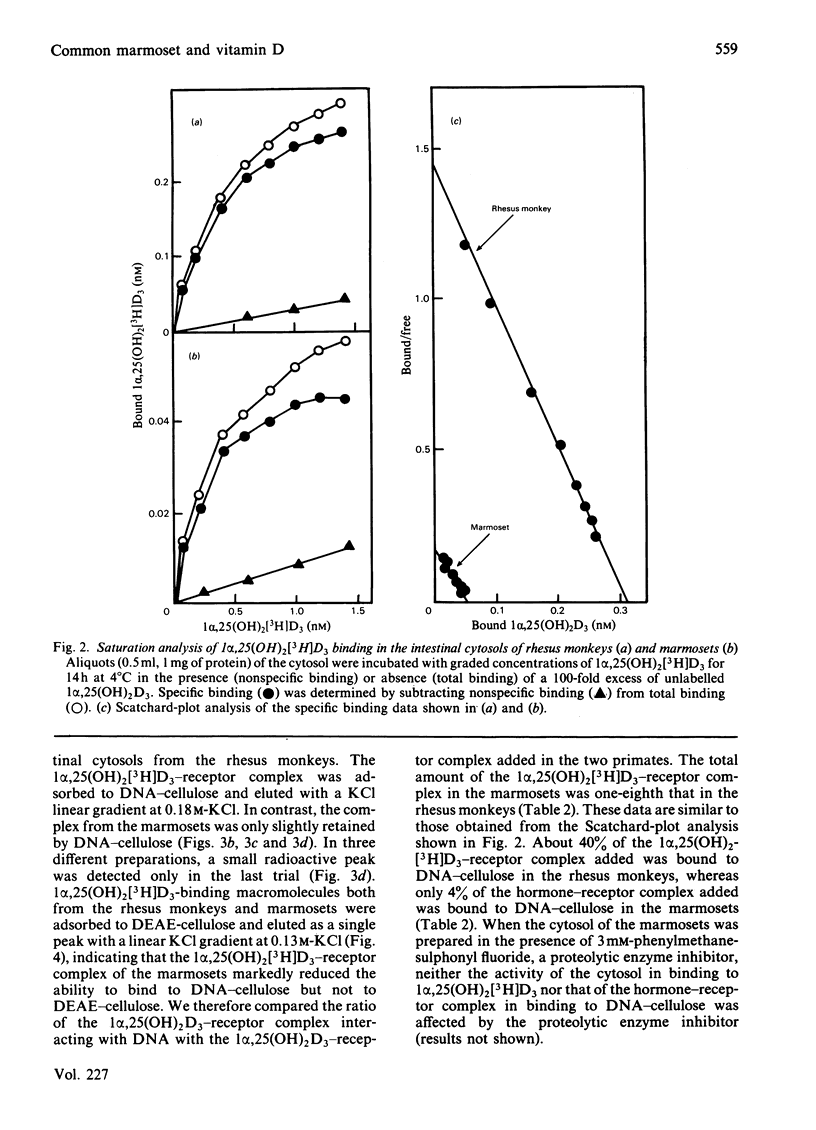
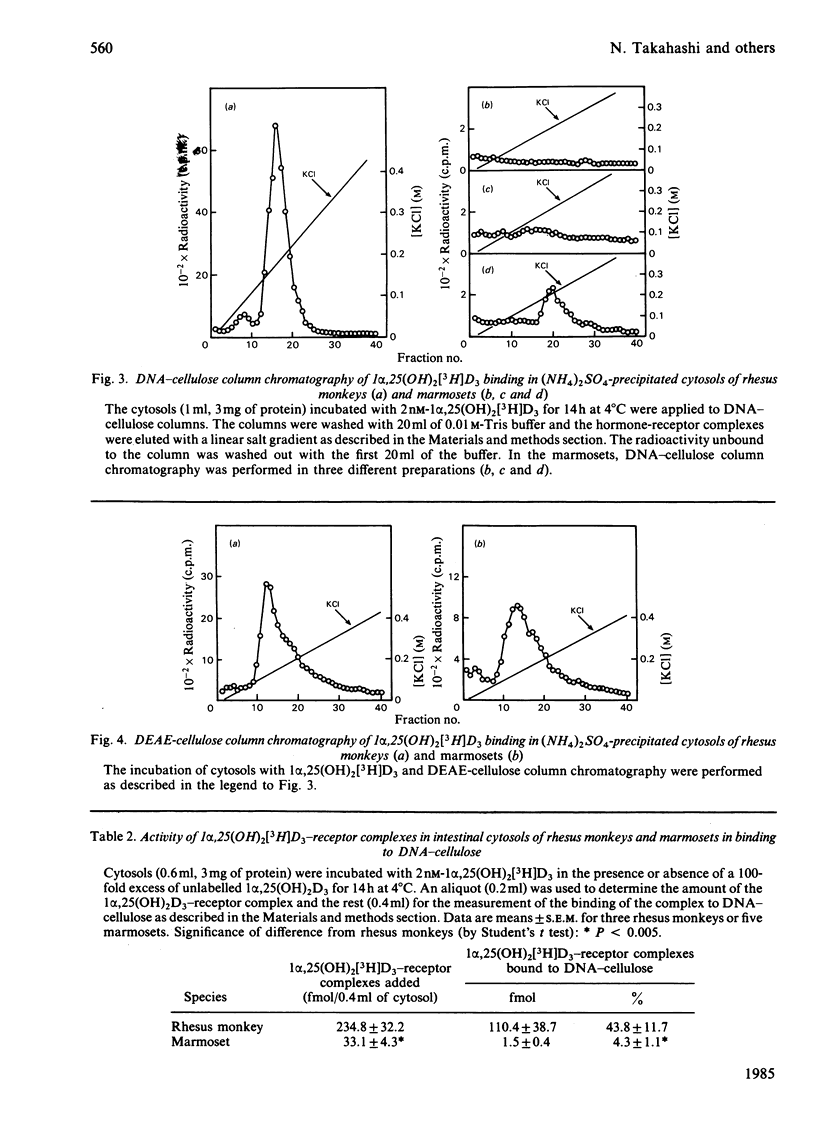
The common marmoset, a New World monkey, requires a large amount of cholecalciferol (110 i.u./day per 100g body wt.) to maintain its normal growth. In a previous report, we demonstrated that the circulating levels of 1 alpha, 25-dihydroxycholecalciferol [1 alpha,25(OH)2D3] in the marmosets are much higher than those in rhesus monkeys and humans, but the marmosets are not hypercalcaemic [Shinki, Shiina, Takahashi, Tanioka, Koizumi & Suda (1983) Biochem. Biophys. Res. Commun. 14, 452-457]. To compare the effect of the daily intake of cholecalciferol, two rhesus monkeys were given a large amount of cholecalciferol (900 i.u./day per 100g body wt). Their serum levels of calcium, 25-hydroxycholecalciferol and 24R,25-dihydroxycholecalciferol were markedly elevated, but the serum 1 alpha,25(OH)2D3 levels remained within a range similar to those in the rhesus monkeys fed the normal diet (intake of cholecalciferol 5 i.u./day per 100g body wt). Intestinal cytosols prepared from both monkeys contained similar 3.5 S macromolecules to which 1 alpha,25(OH)2D3 was bound specifically. However, the cytosols from the marmosets contained only one-sixth as many 1 alpha,25(OH)2D3 receptors as those from the rhesus monkeys. Furthermore, the activity of the 1 alpha,25(OH)2D3-receptor complex in binding to DNA-cellulose was very low in the marmosets. These results suggest that the marmoset possesses an end-organ resistance to 1 alpha,25(OH)2D3 and is a useful animal model for studying the mechanism of vitamin D-dependent rickets, type II.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott D. H., Hearn J. P. Physical, hormonal and behavioural aspects of sexual development in the marmoset monkey, Callithrix jacchus. J Reprod Fertil. 1978 May;53(1):155–166. doi: 10.1530/jrf.0.0530155. [DOI] [PubMed] [Google Scholar]
- Baksi S. N., Kenny A. D. Vitamim D3 metabolism in immature Japanese quail: effects of ovarian hormones. Endocrinology. 1977 Oct;101(4):1216–1220. doi: 10.1210/endo-101-4-1216. [DOI] [PubMed] [Google Scholar]
- Bonney R. C., Dixson A. F., Fleming D. Cyclic changes in the circulating and urinary levels of ovarian steroids in the adult female owl monkey (Aotus trivirgatus). J Reprod Fertil. 1979 May;56(1):271–280. doi: 10.1530/jrf.0.0560271. [DOI] [PubMed] [Google Scholar]
- Boyle I. T., Gray R. W., DeLuca H. F. Regulation by calcium of in vivo synthesis of 1,25-dihydroxycholecalciferol and 21,25-dihydroxycholecalciferol. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2131–2134. doi: 10.1073/pnas.68.9.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brumbaugh P. F., Haussler M. R. 1 Alpha,25-dihydroxycholecalciferol receptors in intestine. II. Temperature-dependent transfer of the hormone to chromatin via a specific cytosol receptor. J Biol Chem. 1974 Feb 25;249(4):1258–1262. [PubMed] [Google Scholar]
- Brumbaugh P. F., Hughes M. R., Haussler M. R. Cytoplasmic and nuclear binding components for 1alpha25-dihydroxyvitamin D3 in chick parathyroid glands. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4871–4875. doi: 10.1073/pnas.72.12.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos G. P., Brandon D., Renquist D. M., Tomita M., Johnson E., Loriaux D. L., Lipsett M. B. Uterine estrogen and progesterone receptors in an estrogen- and progesterone- "resistant" primate. J Clin Endocrinol Metab. 1984 Mar;58(3):516–520. doi: 10.1210/jcem-58-3-516. [DOI] [PubMed] [Google Scholar]
- Chrousos G. P., Renquist D., Brandon D., Barnard D., Fowler D., Loriaux D. L., Lipsett M. B. The squirrel monkey: receptor-mediated end-organ resistance to progesterone? J Clin Endocrinol Metab. 1982 Aug;55(2):364–368. doi: 10.1210/jcem-55-2-364. [DOI] [PubMed] [Google Scholar]
- Clemens T. L., Adams J. S., Horiuchi N., Gilchrest B. A., Cho H., Tsuchiya Y., Matsuo N., Suda T., Holick M. F. Interaction of 1,25-dihydroxyvitamin-D3 with keratinocytes and fibroblasts from skin of normal subjects and a subject with vitamin-D-dependent rickets, type II: a model for study of the mode of action of 1,25-dihydroxyvitamin D3. J Clin Endocrinol Metab. 1983 Apr;56(4):824–830. doi: 10.1210/jcem-56-4-824. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F. Vitamin D: the vitamin and the hormone. Fed Proc. 1974 Nov;33(11):2211–2219. [PubMed] [Google Scholar]
- Eil C., Liberman U. A., Rosen J. F., Marx S. J. A cellular defect in hereditary vitamin-D-dependent rickets type II: defective nuclear uptake of 1,25-dihydroxyvitamin D in cultured skin fibroblasts. N Engl J Med. 1981 Jun 25;304(26):1588–1591. doi: 10.1056/NEJM198106253042608. [DOI] [PubMed] [Google Scholar]
- Feldman D., Chen T., Cone C., Hirst M., Shani S., Benderli A., Hochberg Z. Vitamin D resistant rickets with alopecia: cultured skin fibroblasts exhibit defective cytoplasmic receptors and unresponsiveness to 1,25(OH)2D3. J Clin Endocrinol Metab. 1982 Nov;55(5):1020–1022. doi: 10.1210/jcem-55-5-1020. [DOI] [PubMed] [Google Scholar]
- Fraser D. R. Regulation of the metabolism of vitamin D. Physiol Rev. 1980 Apr;60(2):551–613. doi: 10.1152/physrev.1980.60.2.551. [DOI] [PubMed] [Google Scholar]
- Fraser D., Kooh S. W., Kind H. P., Holick M. F., Tanaka Y., DeLuca H. F. Pathogenesis of hereditary vitamin-D-dependent rickets. An inborn error of vitamin D metabolism involving defective conversion of 25-hydroxyvitamin D to 1 alpha,25-dihydroxyvitamin D. N Engl J Med. 1973 Oct 18;289(16):817–822. doi: 10.1056/NEJM197310182891601. [DOI] [PubMed] [Google Scholar]
- Garabedian M., Holick M. F., Deluca H. F., Boyle I. T. Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1673–1676. doi: 10.1073/pnas.69.7.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grody W. W., Schrader W. T., O'Malley B. W. Activation, transformation, and subunit structure of steroid hormone receptors. Endocr Rev. 1982 Spring;3(2):141–163. doi: 10.1210/edrv-3-2-141. [DOI] [PubMed] [Google Scholar]
- Hearn J. P., Abbott D. H., Chambers P. C., Hodges J. K., Lunn S. F. Use of the common marmoset, Callithrix jacchus, in reproductive research. Primates Med. 1978;10:40–49. [PubMed] [Google Scholar]
- Hunziker W., Walters M. R., Norman A. W. 1,25-dihydroxyvitamin D3 receptors. Differential quantitation of endogenously occupied and unoccupied sites. J Biol Chem. 1980 Oct 25;255(20):9534–9537. [PubMed] [Google Scholar]
- Jones P. G., Haussler M. R. Scintillation autoradiographic localization of 1,25-dihydroxyvitamin D3 in chick intestine. Endocrinology. 1979 Feb;104(2):313–321. doi: 10.1210/endo-104-2-313. [DOI] [PubMed] [Google Scholar]
- Kano K., Yoshida H., Yata J., Abe E., Tanabe R., Suda T. An assay method for separately measuring metabolites of vitamin D3 and those presumed to be derived from vitamin D2. J Nutr Sci Vitaminol (Tokyo) 1979;25(5):351–360. doi: 10.3177/jnsv.25.351. [DOI] [PubMed] [Google Scholar]
- Kream B. E., Jose M., Yamada S., DeLuca H. F. A specific high-affinity binding macromolecule for 1,25-dihydroxyvitamin D3 in fetal bone. Science. 1977 Sep 9;197(4308):1086–1088. doi: 10.1126/science.887939. [DOI] [PubMed] [Google Scholar]
- Lawson D. E., Wilson P. W. Intranuclear localization and receptor proteins for 1,25-dihydroxycholecalciferol in chick intestine. Biochem J. 1974 Dec;144(3):573–583. doi: 10.1042/bj1440573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman U. A., Eil C., Marx S. J. Resistance to 1,25-dihydroxyvitamin D. Association with heterogeneous defects in cultured skin fibroblasts. J Clin Invest. 1983 Feb;71(2):192–200. doi: 10.1172/JCI110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman U. A., Samuel R., Halabe A., Kauli R., Edelstein S., Weisman Y., Papapoulos S. E., Clemens T. L., Fraher L. J., O'Riordan J. L. End-organ resistance to 1,25-dihydroxycholecalciferol. Lancet. 1980 Mar 8;1(8167):504–506. doi: 10.1016/s0140-6736(80)92763-4. [DOI] [PubMed] [Google Scholar]
- Marx S. J., Spiegel A. M., Brown E. M., Gardner D. G., Downs R. W., Jr, Attie M., Hamstra A. J., DeLuca H. F. A familial syndrome of decrease in sensitivity to 1,25-dihydroxyvitamin D. J Clin Endocrinol Metab. 1978 Dec;47(6):1303–1310. doi: 10.1210/jcem-47-6-1303. [DOI] [PubMed] [Google Scholar]
- Mason J. B., Hay R. W., Leresche J., Peel S., Darley S. The story of vitamin D: from vitamin to hormone. Lancet. 1974 Mar 2;1(7853):325–329. [PubMed] [Google Scholar]
- Massaro E. R., Simpson R. U., DeLuca H. F. Quantitation of endogenously occupied and unoccupied binding sites for 1,25-dihydroxyvitamin D3 in rat intestine. Proc Natl Acad Sci U S A. 1983 May;80(9):2549–2553. doi: 10.1073/pnas.80.9.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preslock J. P., Hampton S. H., Hampton J. K., Jr Cyclic variations of serum progestins and immunoreactive estrogens in marmosets. Endocrinology. 1973 Apr;92(4):1096–1101. doi: 10.1210/endo-92-4-1096. [DOI] [PubMed] [Google Scholar]
- Scriver C. R., Reade T. M., DeLuca H. F., Hamstra A. J. Serum 1,25-dihydroxyvitamin D levels in normal subjects and in patients with hereditary rickets or bone disease. N Engl J Med. 1978 Nov 2;299(18):976–979. doi: 10.1056/NEJM197811022991803. [DOI] [PubMed] [Google Scholar]
- Seino Y., Yamaoka K., Ishida M., Yabuuchi H., Ichikawa M., Ishige H., Yoshino H., Avioli L. V. Biochemical characterization of 1,25(OH)2D3 receptors in chick embryonal duodenal cytosol. Calcif Tissue Int. 1982 May;34(3):265–269. doi: 10.1007/BF02411248. [DOI] [PubMed] [Google Scholar]
- Shinki T., Shiina Y., Takahashi N., Tanioka Y., Koizumi H., Suda T. Extremely high circulating levels of 1 alpha,25-dihydroxyvitamin D3 in the marmoset, a new world monkey. Biochem Biophys Res Commun. 1983 Jul 29;114(2):452–457. doi: 10.1016/0006-291x(83)90801-x. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Castillo L., DeLuca H. F. Control of renal vitamin D hydroxylases in birds by sex hormones. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2701–2705. doi: 10.1073/pnas.73.8.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., DeLuca H. F. Stimulation of 24,25-dihydroxyvitamin D3 production by 1,25-dihydroxyvitamin D3. Science. 1974 Mar;183(130):1198–1200. doi: 10.1126/science.183.4130.1198. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Deluca H. F. The control of 25-hydroxyvitamin D metabolism by inorganic phosphorus. Arch Biochem Biophys. 1973 Feb;154(2):566–574. doi: 10.1016/0003-9861(73)90010-6. [DOI] [PubMed] [Google Scholar]
- Tsai H. C., Wong R. G., Norman A. W. Studies on calciferol metabolism. IV. Subcellular localization of 1,25-dihydroxy-vitamin D 3 in intestinal mucosa and correlation with increased calcium transport. J Biol Chem. 1972 Sep 10;247(17):5511–5519. [PubMed] [Google Scholar]
- Tsuchiya Y., Matsuo N., Cho H., Kumagai M., Yasaka A., Suda T., Orimo H., Shiraki M. An unusual form of vitamin D-dependent rickets in a child: alopecia and marked end-organ hyposensitivity to biologically active vitamin D. J Clin Endocrinol Metab. 1980 Oct;51(4):685–690. doi: 10.1210/jcem-51-4-685. [DOI] [PubMed] [Google Scholar]
- Wecksler W. R., Norman A. W. An hydroxylapatite batch assay for the quantitation of 1alpha,25-dihydroxyvitamin D3-receptor complexes. Anal Biochem. 1979 Jan 15;92(2):314–323. doi: 10.1016/0003-2697(79)90664-x. [DOI] [PubMed] [Google Scholar]
- Wilson M. I., Brown G. M., Wilson D. Annual and diurnal changes in plasma androgen and cortisol in adult male Squirrel monkeys (Saimiri sciureus) studied longitudinally. Acta Endocrinol (Copenh) 1978 Feb;87(2):424–433. doi: 10.1530/acta.0.0870424. [DOI] [PubMed] [Google Scholar]
- Wolf R. C., O'Connor R. F., Robinson J. A. Cyclic changes in plasma progestins and estrogens in squirrel monkeys. Biol Reprod. 1977 Sep;17(2):228–231. doi: 10.1095/biolreprod17.2.228. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Utsu S., Tanioka Y., Ohsawa N. Extremely high levels of corticosteroids and low levels of corticosteroid binding macromolecule in plasma of marmoset monkeys. Acta Endocrinol (Copenh) 1977 Jun;85(2):398–405. doi: 10.1530/acta.0.0850398. [DOI] [PubMed] [Google Scholar]
- Zile M., Bunge E. C., Barsness L., Yamada S., Schnoes H. K., DeLuca H. F. Localization of 1,25-dihydroxyvitamin D3 in intestinal nuclei in vivo. Arch Biochem Biophys. 1978 Feb;186(1):15–24. doi: 10.1016/0003-9861(78)90458-7. [DOI] [PubMed] [Google Scholar]


