Abstract
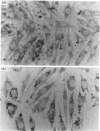
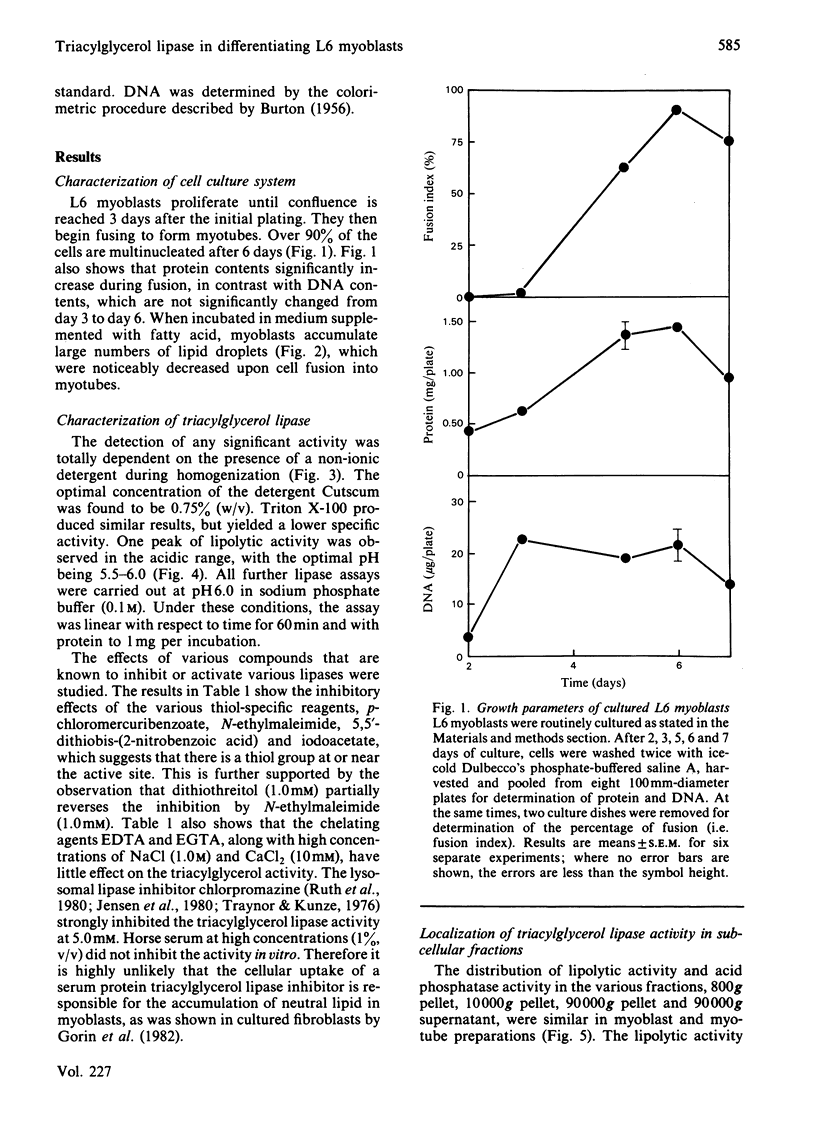
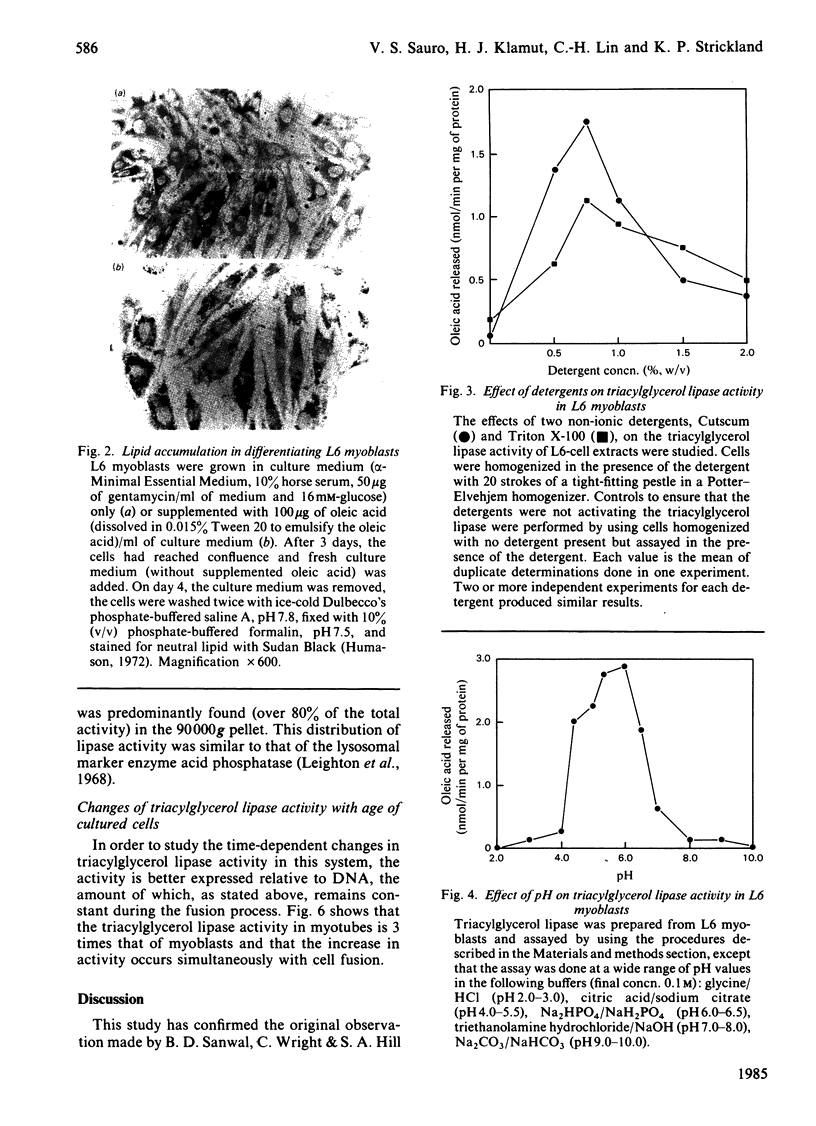
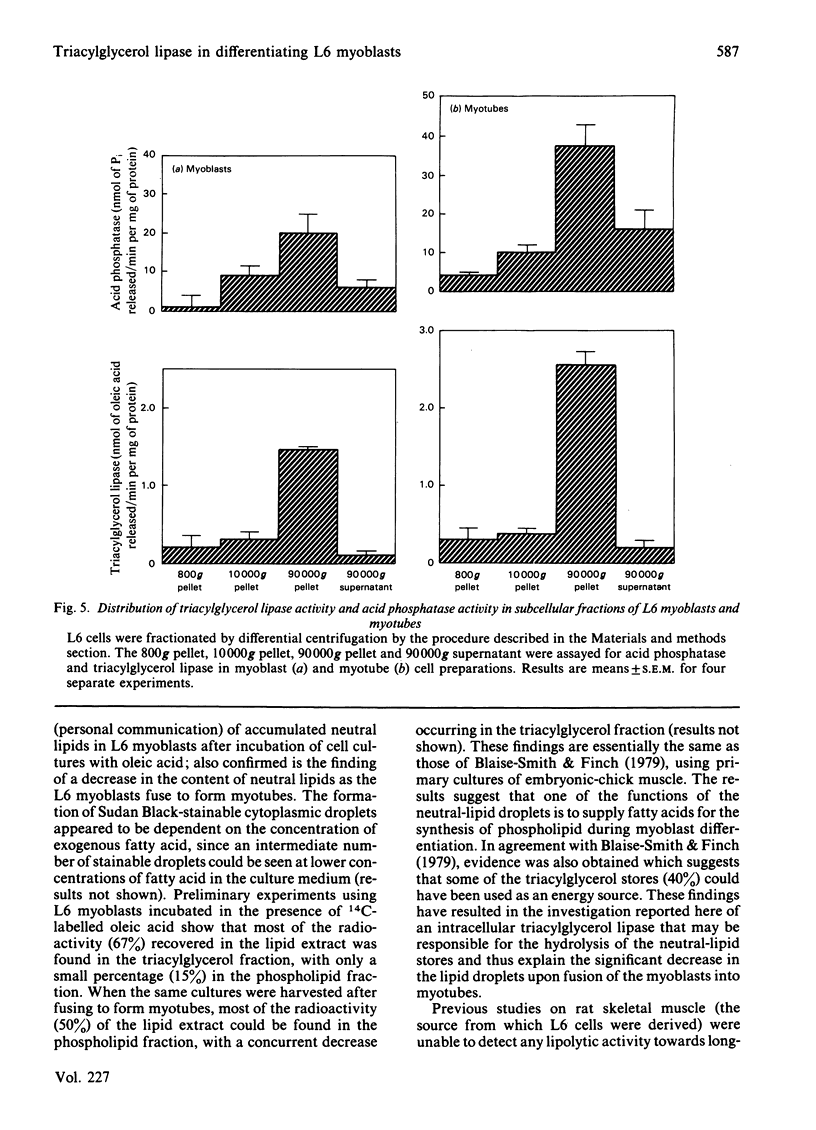
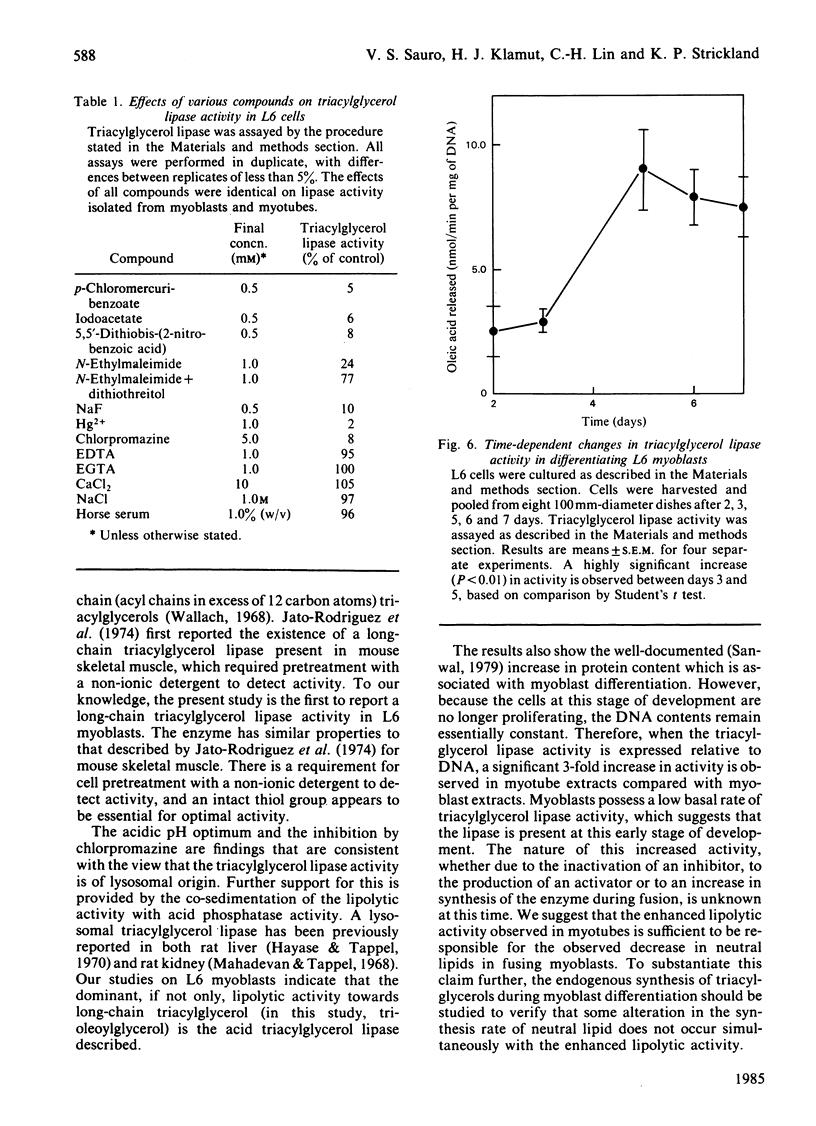
L6 myoblasts, before fusion, accumulate large stores of neutral lipid when cultured in medium supplemented with fatty acid. Upon fusion to terminally differentiated myotubes, a noticeable decrease in these neutral-lipid stores was observed. Triacylglycerol lipase activity was examined in L6 myoblasts at various stages of cell differentiation to assess a possible role for this enzyme in the above phenomenon. In this first study to demonstrate lipolytic activity in cultured muscle cells, the activity was found to be totally dependent on the presence of a detergent, either Cutscum or Triton X-100, during homogenization. The inhibition by many thiol-specific reagents [N-ethylmaleimide, p-chloromercuribenzoate, iodoacetate, 5,5'-dithiobis-(2-nitrobenzoic acid)] suggest that a thiol group is at or near the active site. The observed acidic pH optimum (5.5-6.0), the acute inhibition by chlorpromazine (a lysosomal lipase inhibitor) and the distribution of lipolytic activity upon cell fractionation (which co-sediments with acid phosphatase, a lysosomal marker enzyme) suggest that the lipase may be of lysosomal origin. Under the optimal conditions described, the triacylglycerol lipase activity of L6 myoblasts was determined to be 2.9 +/- 0.4 nmol of oleic acid released/min per mg of DNA. This activity increased 3-fold, to 9.0 +/- 1.6 nmol/min per mg, in the myotube phase. This increase in lipolytic activity may be responsible for the observed decrease in neutral-lipid stores of differentiating myoblasts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfrage P., Vaughan M. Simple liquid-liquid partition system for isolation of labeled oleic acid from mixtures with glycerides. J Lipid Res. 1969 May;10(3):341–344. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Gorin E., Gonen H., Dickbuch S. A serum protein inhibitor of acid lipase and its possible role in lipid accumulation in cultured fibroblasts. Biochem J. 1982 Apr 15;204(1):221–227. doi: 10.1042/bj2040221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayase K., Tappel A. L. Specificity and other properties of lysosomal lipase of rat liver. J Biol Chem. 1970 Jan 10;245(1):169–175. [PubMed] [Google Scholar]
- Hochachka P. W., Neely J. R., Driedzic W. R. Integration of lipid utilization with Krebs cycle activity in muscle. Fed Proc. 1977 Jun;36(7):2009–2014. [PubMed] [Google Scholar]
- Jato-Rodriguez J. J., Hudson A. J., Strickland K. P. Triglyceride metabolism in skeletal muscle from normal and dystrophic mice. Biochim Biophys Acta. 1974 Apr 26;348(1):1–13. doi: 10.1016/0005-2760(74)90087-3. [DOI] [PubMed] [Google Scholar]
- Jensen G. L., Baly D. L., Brannon P. M., Bensadoun A. Synthesis and secretion of lipolytic enzymes by cultured chicken hepatocytes. J Biol Chem. 1980 Dec 10;255(23):11141–11148. [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan S., Tappel A. L. Lysosomal lipases of rat liver and kidney. J Biol Chem. 1968 Jun 10;243(11):2849–2854. [PubMed] [Google Scholar]
- Morris G. E., Cole R. J. Cell fusion and differentiation in cultured chick muscle cells. Exp Cell Res. 1972 Nov;75(1):191–199. doi: 10.1016/0014-4827(72)90536-8. [DOI] [PubMed] [Google Scholar]
- Nilsson-Ehle P., Schotz M. C. A stable, radioactive substrate emulsion for assay of lipoprotein lipase. J Lipid Res. 1976 Sep;17(5):536–541. [PubMed] [Google Scholar]
- Odusote K., Karpati G., Carpenter S. An experimental morphometric study of neutral lipid accumulation in skeletal muscles. Muscle Nerve. 1981 Jan-Feb;4(1):3–9. doi: 10.1002/mus.880040103. [DOI] [PubMed] [Google Scholar]
- Ruth R. C., Owens K., Weglicki W. B. Inhibition of lysosomal lipases by chlorpromazine: a possible mechanism of stabilization. J Pharmacol Exp Ther. 1980 Mar;212(3):361–367. [PubMed] [Google Scholar]
- Slavin G., Wills E. J., Richmond J. E., Chanarin I., Andrews T., Stewart G. Morphological features in a neutral lipid storage disease. J Clin Pathol. 1975 Sep;28(9):701–710. doi: 10.1136/jcp.28.9.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. B., Finch R. A. Alterations in lipid metabolism of developing muscle cells in culture. Biochim Biophys Acta. 1979 Jan 29;572(1):139–145. doi: 10.1016/0005-2760(79)90208-x. [DOI] [PubMed] [Google Scholar]
- Stearns S. B., Tepperman H. M., Tepperman J. Studies on the utilization and mobilization of lipid in skeletal muscles from streptozotocin-diabetic and control rats. J Lipid Res. 1979 Jul;20(5):654–662. [PubMed] [Google Scholar]
- Traynor J. R., Kunze H. Effects of local anaesthetics on the lipase of Rhizopus arrhizus. Biochim Biophys Acta. 1976 Feb 23;424(2):246–252. doi: 10.1016/0005-2760(76)90192-2. [DOI] [PubMed] [Google Scholar]
- Wallach D. P. Isolation and characterization of four lipolytic preparations from rat skeletal muscle. J Lipid Res. 1968 Mar;9(2):200–206. [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]