Abstract
Spinal cord injury (SCI) is a grievous neurology-related disorder that causes many devastating symptoms. Emerging roles of long non-coding RNAs (lncRNA) have been shown to play critical roles in multiple neurological diseases. This research planned to dig the function and latent molecular mechanisms of the lncRNA CCAT1 on OGD/R-disposed injury in astrocytes. We observed that CCAT1 expression was diminished and miR-218 expression was elevated in astrocytes during OGD/R. Additionally, an abundance of CCAT1 obviously amplified cell viability and restrained OGD/R-triggered apoptosis in astrocytes, as characterized by reduced levels of pro-apoptotic proteins Bax and C-caspase-3, concomitant with elevated level of anti-apoptotic Bcl-2 protein. Furthermore, administration of CCAT1 remarkably mitigated OGD/R injury-induced neuro-inflammatory responses, reflected in a reduction of inflammatory cytokines including TNF-α, IL-1β, and IL-6. In action, CCAT1 served as an endogenous sponge effectively downregulating miR-218 expression by binding directly to it, and a negative regulatory relationship between miR-218 and NFAT5. Mechanistically, introduction of miR-218 reversed the inhibitory effects of CCAT1 on OGD/R-induced apoptosis and inflammation damage, which directly resulted from the inhibition of miR-218 and its targeting of NFAT5. Collectively, our study illuminated a new CCAT1/miR-218/NFAT5 regulatory axis in which CCAT1 served as a competing endogenous RNA by sponging miR-218, effectively upregulating NFAT5 expression, thereby alleviating apoptosis and inflammation damage under OGD/R condition. CCAT1 is, therefore, a putative therapeutic target for SCI, based on the results of this study and the potential application of CCAT1 as a neuroprotective agent.
Keywords: LncRNA CCAT1, Apoptosis, Inflammatory responses, miR-218/NFAT5 axis
Introduction
SCI is a serious central nervous system (CNS) trauma and commonly results in permanent and lifelong disabilities that reduce the quality of life and increase morbidity and mortality (Varma et al. 2013). To date, no effective treatments for SCI exist (Silva et al. 2014). Thus, research into the mechanisms involved in SCI must be conducted to develop new therapeutic strategies to alleviate this harmful condition.
A number of research are increasingly focused on the secondary cascade of injuries that follow SCI. Despite that the pathogenesis of SCI is not clearly understood, it is generally believed that oxidative damage, inflammatory response, neuron apoptosis, and necrosis are caused by SCI (David and Kroner 2011). After SCI, inflammation and oxidative stress cause axonal destruction, necrotic cell death, neuronal loss and demyelination during the secondary cascade of injury (Mortezaee et al. 2018). Neuron apoptosis is the main pathological feature of highest concern as it leads to a certain degree of neurological damage, necrosis, or autonomic nervous system dysfunction (Zhang et al. 2018). Astrocytes are the most abundant cell type in the central nervous system (CNS), and are among the first to respond to SCI. Astrocytes appear to be remarkably effective at activating cellular responses to SCI, therefore, identifying the procedures that increase the protective functions of astrocytes and maintain their reactive status could lead to effective therapies for improving patients functional outcomes and reducing secondary tissue degeneration after SCI (Xia et al. 2016). Recently, astrocytes have been recognized as having high potential as a pathway for treating neuronal dysfunction-related diseases including SCI. Previous studies have shown that astrocytes protect neurons against oxidative stress and affect neuronal survival in some neurodegenerative diseases (Maragakis and Rothstein 2006). Astrocytes also play important roles in regulating inflammatory responses to ischemic injury (Hirayama and Koizumi 2018).
Long noncoding RNAs (lncRNAs) are transcripts longer than 200 nucleotides without a protein-coding capacity, and they exert their physiological and pathological functions by interacting with genomic DNA, microRNAs, mRNAs, and proteins (Hu et al. 2018). LncRNAs have been identified as essential regulators in numerous biological processes, and their aberrant expression is apparent in many human diseases (Lalevee and Feil 2015). Currently, an increasing number of lncRNAs that are involved in SCI progression have been identified (Li et al. 2019a; Riva et al. 2016; Wu et al. 2019). Recent evidences have been demonstrated that lncRNAs were upregulated or downregulated in neuropathic pain models, which support the potential role of lncRNAs as a novel group of targets for the treatment of neuropathic pain (Jia et al. 2019; Li et al. 2019b; Zhang et al. 2018). For example, the lncRNA colon cancer-associated transcript-1 (CCAT1) was a novel lncRNA which was indicated to be upregulated in multiple cancers (Dou et al. 2017a; Xin et al. 2016). However, a previous study indicate that CCAT1 expression was decreased in the spinal dorsal horn, dorsal root ganglion (DRG), hippocampus, and anterior cingulate cortex (ACC) of rats with bilateral sciatic nerve chronic constriction injury (BCCI) injury, and CCAT1 overexpression alleviated the pain thresholds (Dou et al. 2017b). However, the role of CCAT1 in OGD/R-evoked injury and its potential mechanisms are still unclear and need to be investigated further. In this study, we used primary astrocytes under in vitro OGD/R conditions to mimic ischemic reperfusion insult to reveal the effects and molecular mechanisms of CCAT1 in the OGD/R-induced astrocytes injury and figure out innovative therapeutic targets for treating neuropathic pain after SCI.
Materials and Methods
Astrocytes Culture
Astrocytes were prepared from neonatal SD rats. Briefly, the spinal cords were minced and digested with 0.25% trypsin for 10 min, before being cultured in DMEM with 10% FBS, and 1% penicillin/streptomycin in an incubator at conditions of 37 °C with 5% CO2. All experiments were authorized by the Institutional Animal Ethics Committee of the First Affiliated Hospital of Chengdu Medical College, China, and all procedures complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering.
Cell Transfection
The pcDNA-CCAT1 vector and its negative control pcDNA were obtained from GenePharma (Shanghai, China). Twenty-four hours before OGD/R stimulation, these plasmids were transfected into the cells using the Lipofectamine 3000 reagent (Life Technologies Corporation, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Establishment of OGD/R Model
To mimic the impairment of astrocytes in ischemic injury in vivo, we established the OGD/R model in vitro as described previously with modifications (Liu et al. 2019). Briefly, 24 h after transfection, the cells were washed with PBS and cultured in a glucose-free DMEM medium in a closed hypoxic chamber (Thermo Forma, Marietta, OH, USA) with 95% N2 and 5% CO2. After 6 h of OGD, cultures were restored to fresh DMEM-containing 10% FBS at 37 °C with 95% O2/5% CO2 for 24-h reoxygenation and glucose restoration. The controls were incubated with high-glucose DMEM in a normal oxygen incubator.
qRT-PCR Assay
Total RNA was extracted from cells with Trizol Reagent (Life Technologies Corporation, Carlsbad, CA, USA). The RNA concentration was measured using a NanoDrop ND-1000 system (NanoDrop Technologies, Wilmington, DE, USA). For CCAT1, miR-218, TNF-α, IL-1β, IL-6 and NFAT5 expression detection, the RNA was reverse transcribed into cDNA using the RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, MA, USA). qRT-PCR analysis was performed with SYBR Green (Applied Biosystems, Foster City, CA, USA) with β-actin as an internal control on an ABI Prism 7500 Sequence Detection System (Applied Biosystems). The result was measured following to the comparative 2−ΔΔCT method (Livak and Schmittgen 2001). Each sample was repeated in triplicate. Primer sequences is shown in Table 1.
Table 1.
Primer sequences for qRT-PCR
| Gene | Primer sequence |
|---|---|
| CCAT1 | Forward 5′-GCAGGCAGAAAGCCGTATCT-3′ |
| Reverse 5′-TCCCAGGTCCTAGTCTGCTT-3′ | |
| miR-218 | Forward 5′-CGGGATCCGACCAGTCGCTGCGGGGCTTTCCTTTGTGCTT GATCTAACCATGTGGTGGAACGATGGAAA-3′ |
| Reverse 5′-CCCAAGCTTTGCAGGAGAGCACGGTGCTTTCCGCGGTGC TTGACAGAACCATGTTCCGTTTCCATCGTTC-3′ | |
| TNF-α | Forward 5′-GAGTGAGAGATGTAGAGG-3′ |
| Reverse 5′-ATACATTAGGGAGAACAACGA-3′ | |
| IL-1β | Forward 5′-GAAGAGACGAGAGATCGA-3′ |
| Reverse 5′-CACACAGATCTCCTCAAGGCA-3′ | |
| IL-6 | Forward 5′-ATCACGAACTAGAGCAGCAG-3′ |
| Reverse 5′-TCATACACAGCCACAGTCACCAC-3′ | |
| NFAT5 | Forward 5′-CGACAGTGCCAAAGCACCTC-3′ |
| Reverse 5′-AACCGGATACTGTCCACACAACATA-3′ | |
| U6 | Forward 5′-CTCGCTTCGGCAGCACA-3′ |
| Reverse 5′-AACGCTTCACGAATTTGCGT-3′ | |
| β-actin | Forward 5′-GCACTGTGTTGGCATAGAGGTC-3′ |
| Reverse 5′-ACGGTCAGGTCATCACTATCGG-3′ |
Western Blot
The total protein from primary astrocytes was extracted using RIPA buffer (Beyotime Biotechnology, China) containing protease inhibitor cocktail (Applygen, China). The protein concentration was measured with the BCA Protein Assay kit (Thermo Fisher Scientific). Equal amounts of protein extracts were separated by SDS-PAGE and transferred onto PVDF membranes (Millipore, Billerica, MA, USA). After being blocked for 1 h in TBST buffer containing 5% nonfat milk at room temperature, the membrane was incubated with the appropriate primary antibodies at 4 °C overnight. The primary antibodies were obtained from the following sources: anti-Bcl-2 (1:1000 dilution), anti-Bax (1:1000 dilution), and anti-C-caspase-3 (1:500 dilution), anti-NFAT5 (1:1000 dilution) and anti-β-actin (1:2000 dilution) antibodies (Abcam, Cambridge, MA, USA). After washing with TBST buffer, membranes were incubated with HRP-conjugated secondary antibodies (1:1000 dilution, Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 2 h at 37 °C. The positive bands were visualized using the enhanced chemiluminescence agent (ECL kit; Amersham Biosciences, Piscataway, NJ, USA), and then analyzed using the ImageJ software (NIH, Bethesda, MD, USA).
Cell Viability Assessment
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays were performed to assess cell survival. Briefly, cells (5 × 103) were seeded into 96-well culture plates and incubated overnight. MTT solution (5 mg/mL; Sigma-Aldrich, St Louis, MO, USA) was pipetted into each well, and incubated for 4 h at 37 °C. The resulting purple formazan crystals were dissolved in 150 μL dimethyl sulfoxide (DMSO, Sigma-Aldrich). The absorbance at 570 nm was measured using a microplate reader (Bio-Rad, Hercules, CA, USA). All experiments were repeated in triplicate.
Lactate Dehydrogenase (LDH) Assay
The release of LDH from intracellular to extracellular matrix is an indicator of cell damage. Therefore, LDH activity in the culture medium was measured by the LDH cytotoxicity detection kit (Cayman Chemical, Ann Arbor, MI, USA) in accordance with the manufacturer’s supplied protocol. The absorbance at 490 nm was measured using a microplate reader (Bio-Rad, Hercules, CA, USA). All experiments were repeated in triplicate.
Detection of Cell Apoptosis
Apoptosis was measured by detecting fragmented DNA using the Cell Death Detection ELISAPLUS Assay Kit (Roche Diagnostics, Indianapolis, IN) in accordance with manufacturer’s instructions. All experiments were repeated in triplicate.
Inflammatory Cytokine Release Measurement
Production of inflammatory cytokines, including TNF-α, IL-1β, and IL-6 in the supernatant of the astrocytes was detected with ELISA assays (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s recommendation. All experiments were repeated in triplicate.
Dual luciferase Reporter Analysis
The CCAT1 fragment and NFAT5 3′-UTR fragment containing the putative target sites or mutated target sites for miR-218 were amplified and subcloned into a pmirGLO vector to generate wild-type CCAT1 (CCAT1-WT) and NFAT5 (NFAT5-WT) or mutant-type CCAT1 (CCAT1-Mut) and NFAT5 (NFAT5-Mut) vectors. Then, the constructed vectors were respectively co-transfected with the miR-218 mimic or Negative Control (NC) into cells using Lipofectamine 3000 following the manufacturer’s procedures. The luciferase activities were measured by the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) in accordance with the manufacturer’s protocols. Data were exhibited as the ratio of Renilla luciferase activity to firefly luciferase activity.
RNA Immunoprecipitation (RIP) Assay
RIP assay was conducted using a Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore, Boston, MA, USA). Briefly, cells were lysed in RIP lysis buffer and the cell lysate was incubated with magnetic beads conjugated with Ago2 or IgG antibody (Millipore). qRT-PCR was performed to detect the expression of CCAT1 and miR-218 in the precipitates. Immunoprecipitation-western blotting was conducted to measure the Ago2 protein level.
RNA Pull-Down Assay
RNA pull-down assay was performed with the Magnetic RNA–Protein Pull-Down Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. In brief, the biotin-labelled RNAs were transcribed with the Biotin RNA Labeling Mix (Roche, Basel, Switzerland) and purified with the RNeasy Mini Kit (Qiagen, Inc., Valencia, CA, USA). Subsequently, the streptavidin agarose beads (Invitrogen) were added to each binding reaction and incubated at room temperature. Ago2 was determined by western blot, and miR-218 expression in the precipitates was detected by qRT-PCR.
Statistical Analysis
Data are presented as means ± SD calculated from at least three independent determinations. All statistical analyses were performed with the GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA, USA) and SPSS 21.0 (SPSS, Inc., Chicago, IL, USA). A t test was used for the comparison of differences between two groups, and a one-way analysis of variance (ANOVA) to compare differences among multiple groups. P < 0.05 was defined as statistically significant.
Results
LncRNA CCAT1 and miR-218 were Abnormally Expressed in OGD/R-Induced Astrocytes
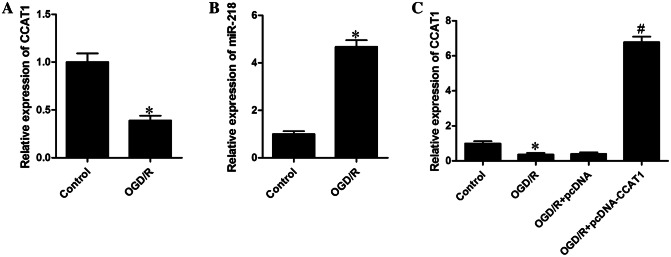
To explore effects and mechanisms of CCAT1 on the neuroprotection of astrocytes, we used primary astrocytes under OGD/R in vitro to mimic ischemic reperfusion insult. Using qRT-PCR examination, we observed that the level of CCAT1 was gradually downregulated (0.39 ± 0.05, 0.39-fold, Fig. 1a), while the expression of miR-218 was remarkably enhanced (4.68 ± 0.27, 4.68-fold; Fig. 1b) in astrocytes treated with OGD/R. Collectively, these data indicate that CCAT1 expression was declined and miR-218 was heightened in astrocytes subjected to OGD/R. Therefore, these data indicate that CCAT1 has a potentially important role within astrocytes after OGD/R injury. To identify the effect of CCAT1 on OGD/R-induced injury, we conducted indicator experiments. First, pcDNA-CCAT1 was transfected into astrocytes, and transfection efficiency was tested by qRT-PCR. The results as indicated in Fig. 1c, the expression of CCAT1 was dramatically elevated by pcDNA-CCAT1 transfection (6.78 ± 0.31, 6.78-fold), suggesting that CCAT1 was overexpressed successfully after transfection.
Fig. 1.
CCAT1 was declined and miR-218 was elevated in astrocytes under OGD/R conditions. a The expression of CCAT1 was determined using qRT-PCR analysis. b qRT-PCR was performed to detect miR-218 expression. c The transfection efficiency was validated using qRT-PCR assays. The data are presented as mean ± SD of three independent experiments (n = 3). *P < 0.05 vs. Control; #P < 0.05 vs. OGD/R + pcDNA
LncRNA CCAT1 Ameliorated OGD/R-Disrupted Astrocytes Apoptosis
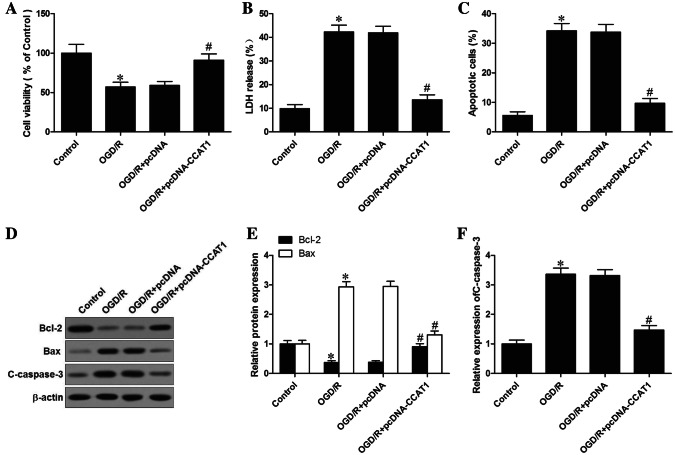
To investigate the effect of CCAT1 on OGD/R-stimulated astrocyte apoptosis, astrocyte viability and damage were determined using MTT assay and LDH assay. As illustrated in Fig. 2a, b, an abundance of CCAT1 markedly promoted cell viability (91.2 ± 8.3, ~ 1.60-fold; Fig. 2a) and reduced LDH release in astrocytes subjected to OGD/R (13.6 ± 2.1, ~ 0.32-fold; Fig. 2b). These observations indicated that CCAT1 obviously accelerated cell viability and hampered cell damage under OGD/R conditions. For assessment of cell apoptosis, ELISA assays were performed. As described in Fig. 2c, OGD/R-triggered apoptosis was prevented by CCAT1 overexpression (9.7 ± 1.6, ~ 0.28-fold). Simultaneously, forced expression of CCAT1 downregulated Bax expression (1.31 ± 0.13, ~ 0.45-fold) and cleaved caspase-3 (c-caspase-3) (1.47 ± 0.15, ~ 0.44-fold) and upregulated Bcl-2 expression (0.91 ± 0.11, ~ 2.46-fold) (Fig. 2d–f). Based on these results, we conclude that CCAT1 protected astrocytes against OGD/R-triggered damage, implying that CCAT1 is a potential anti-apoptosis molecule for astrocytes subjected to OGD/R. Collectively, these findings suggest that CCAT1 deserved further evaluation as a potential therapeutic agent for protection against OGD/R-induced injury.
Fig. 2.
CCAT1 repressed OGD/R-triggered apoptosis in astrocytes. a MTT assays were conducted to measure cell viability. b Cell damage was assessed by LDH assay. c ELISA assays were applied to evaluate cell apoptosis. d–f Western blot analysis and quantitative analyses show the protein levels of the apoptosis-associated markers Bax, Bcl-2, and C-caspase-3. Data represent the mean ± SD from three separate experiments (n = 3). *P < 0.05 vs. Control; #P < 0.05 vs. OGD/R + pcDNA
LncRNA CCAT1 Alleviated OGD/R-Injured Inflammatory Response in Astrocytes
Inflammatory responses are closely associated with SCI. To elucidate the influence of CCAT1 on OGD/R-mediated inflammatory injury, we designed to assess the production of some inflammation-related genes (TNF-α, IL-1β, and IL-6) in response to OGD/R. Not surprisingly, OGD/R injury led to a remarkable enhance in the level of TNF-α, IL-1β, and IL-6 in astrocytes (246 ± 24, ~ 4.24-fold, 72 ± 6, ~ 4.5-fold, and 67 ± 6, ~ 5.15-fold, respectively). However, the release of these inflammatory factors stimulated by OGD/R was also markedly attenuated by CCAT1 overexpression (113 ± 16, ~ 0.46-fold, 31 ± 4, ~ 0.43-fold, and 22 ± 4, ~ 0.34-fold, respectively) (Fig. 3a–c). Furthermore, RT-PCT assay demonstrated that the increased mRNA expression of these inflammatory mediators in OGD/R-treated astrocytes was effectively reduced (1.62 ± 0.27, ~ 0.35-fold, 1.51 ± 0.18, ~ 0.41-fold, and 1.43 ± 0.13, ~ 0.47-fold, respectively) (Fig. 3d–f), implying that CCAT1 exerted an anti-inflammatory influence in OGD/R-exposed astrocytes. Taken together, these results demonstrated that administration of CCAT1 alleviated OGD/R-evoked injury by decreasing pro-inflammatory cytokine release and suppressing cell apoptosis.
Fig. 3.
Effects of CCAT1 on the expression of inflammatory cytokines in OGD/R-injured astrocytes. a The expression of TNF-α, IL-1β, and IL-6 was carried out with ELISA assays. b qRT-PCR assays were executed for the detection of pro-inflammatory cytokine expression. All results are expressed as mean ± SD of three independent experiments (n = 3). *P < 0.05 vs. Control; #P < 0.05 vs. OGD/R + pcDNA
LncRNA CCAT1 Functioned as a Sponge for miR-218
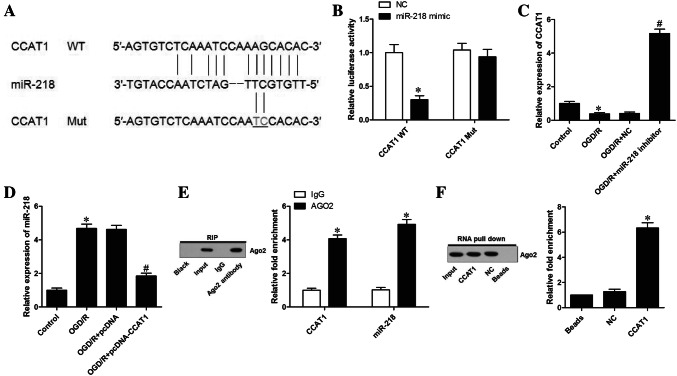
According to above findings, we can see that CCAT1 regulated the OGD/R injury in astrocytes. To further illuminate the regulatory mechanism of CCAT1 under OGD/R condition, we examined the relationship between CCAT1 and miR-218. A bioinformatics analysis of miRNA recognition sequences on CCAT1 using the Starbase v2.0 (https://starbase.sysu.edu.cn/starbase2/index.php) database revealed that miR-218 can bind to the complementary sequence in CCAT1 (Fig. 4a). Furthermore, luciferase reporter assay confirmed that CCAT1 directly interacted with miR-218. The results depicted in Fig. 4b show that the introduction of miR-218 obviously attenuated CCAT1-WT luciferase activity (0.31 ± 0.06, ~ 0.31-fold), not CCAT1-Mut, indicating that miR-218 directly bind with CCAT1. Then, we performed qRT-PCR assay to evaluate the effect of CCAT1 on miR-218 expression in astrocytes subjected to OGD/R. In addition, qRT-PCR analysis also revealed that inhibition of miR-218 obviously enhanced CCAT1 expression (5.17 ± 0.26, ~ 13.26-fold; Fig. 4c). Results in Fig. 4d displayed that forced expression of CCAT1 dramatically diminished miR-218 expression (1.84 ± 0.18, ~ 0.39-fold), suggesting that miR-218 served as a direct microRNA target of CCAT1. Ago2 protein is the key component of functional RNA-induced silencing complex (RISC) assembly by binding to miRNA. RIP assay was performed to observe whether both CCAT1 and miR-218 exist in the Ago2 protein complex and thus to determine possible interaction between CCAT1 and miR-218. We found that both CCAT1 and miR-218 accumulated in the Ago2 antibody precipitates (Fig. 4e). To explore the interaction between CCAT1 and miR-218, RNA pull-down assay was performed. The data showed that miR-218 were enriched in the complex pulled down by the biotinylated CCAT1 (Fig. 4f), suggesting the specificity of the association between CCAT1 and miR-218. Collectively, these findings indicated that CCAT1 can function as a competing endogenous RNAs to sponge miR-218.
Fig. 4.
CCAT1 sponged miR-218 and negatively regulated its expression. a Diagram of bioinformatic prediction of the binding site of miR-218 by CCAT1. b Dual luciferase reporter assays were conducted to validate the binding effect between miR-218 and CCAT1. c CCAT1 expression was evaluated by qRT-PCR. d qRT-PCR assays were performed to estimate miR-218 expression. e The association of CCAT1 and miR-218 with Ago2 was performed by RIP assay. f RNA pull-down assay was designed to explore the interaction between CCAT1 and miR-218. Data are presented as the mean ± SD of three independent experiments (n = 3). *P < 0.05 vs. Control; #P < 0.05 vs. OGD/R + pcDNA
Identification of NFAT5 as a Direct Target of miR-218
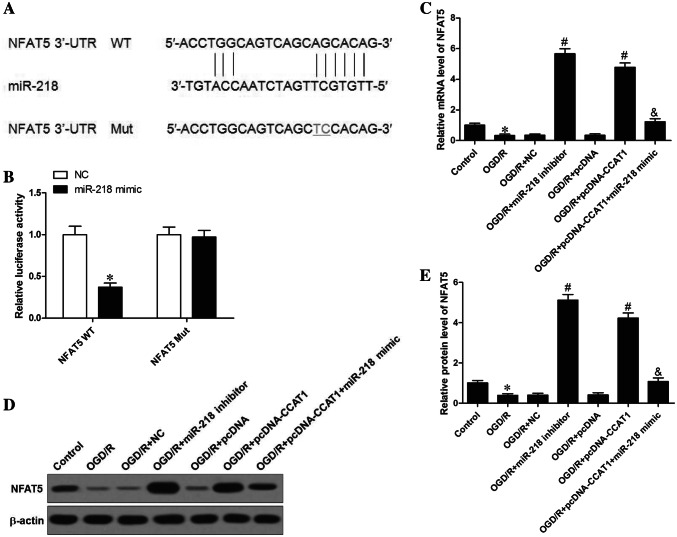
It is well known that miRNAs achieve their biological function via multiple target genes. In the next step of our work, we tried to explore a potential target gene of miR-218. Bioinformatic analysis using three different miRNA target-predicting algorithms (TargetScan, microRNA, and PicTar) were used to predict a putative target of miR-218. The putative binding sites, displayed in Fig. 5a, revealed that NFAT5 was predicted as miR-218 target. A dual luciferase reporter system was constructed to validate the computational prediction and, as displayed in Fig. 5b, deletion of miR-218 effectively diminished the luciferase reporter activity of NFAT5-WT (3.37 ± 0.05, ~ 0.37-fold), but had no influence on that of NFAT5-Mut, confirming that NFAT5 is a target of miR-218. We further investigated the regulation of miR-218 on the expression of NFAT5 in astrocytes subjected to OGD/R. Notably, the mRNA and protein expression levels of NFAT5 were downregulated in astrocytes under OGD/R conditions (0.33 ± 0.09, ~ 0.33-fold, and 0.39 ± 0.08, ~ 0.39-fold, respectively). Importantly, depleting of miR-218 obviously augmented the mRNA expression of NFAT5 (5.67 ± 0.32, ~ 17.18-fold; Fig. 5c). Similar results were observed using Western blot assays to measure the NFAT5 protein (5.12 ± 0.28, ~ 13.13-fold, Fig. 5d, e). Collectively, miR-218 directly bound to the NFAT5 3′-UTR to regulate its expression in astrocytes under OGD/R conditions. In consideration of the inhibitory effect of CCAT1 on miR-218 expression in OGD/R-disposed astrocytes, we further investigated whether CCAT1 effectively regulated NFAT5 expression. As anticipated, overexpression of CCAT1 prominently enhanced NFAT5 mRNA and protein expression (4.78 ± 0.29, ~ 14.06-fold, and 4.23 ± 0.25, ~ 10.32-fold, respectively), while introduction of miR-218 reduced the expression level of NFAT5 mRNA and protein (1.23 ± 0.19, ~ 0.26-fold, and 1.08 ± 0.18, ~ 0.25-fold; Fig. 5c–e). These data, therefore, indicate that CCAT1 heightened NFAT5 expression by binding to miR-218.
Fig. 5.
NFAT5 was identified as a direct target of miR-218. a The predicted binding site between the 3′-UTR of NFAT5 and miR-218. b The relative luciferase activity was tested by dual-luciferase reporter assay. c The mRNA expression of NFAT5 was estimated by qRT-PCR assays. d Western blot analysis was utilized for detection of NFAT5 protein levels. e Quantification of the protein levels of NFAT5. The data are presented as mean ± SD of three independent experiments (n = 3). *P < 0.05 vs. Control; #P < 0.05 vs. OGD/R + NC or OGD/R + pcDNA; &P < 0.05 vs. OGD/R + pcDNA-CCAT1
miR-218/NFAT5 axis was Involved in the Neuroprotective Effect of CCAT1 on OGD/R-Induced Injury
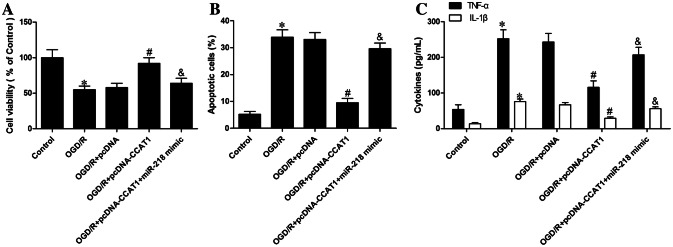
To disclose whether CCAT1 prevented OGD/R-evoked damage by binding miR-218, we performed a rescue experiment. As can be seen in Fig. 6a, administration of miR-218 profoundly counteracted CCAT1-mediated cell viability in astrocytes subjected to OGD/R. Meanwhile, introduction of miR-218 largely eliminated the ability of CCAT1 to ameliorate OGD/R-triggered apoptosis (Fig. 6b). In Fig. 6c, it was manifested that the restoration of miR-218 also observably reversed the salutary effects of CCAT1 on the accumulated inflammatory factors TNF-α and IL-1β, indicating that the neuroprotective impacts of CCAT1 may be due to the inhibition of miR-218 expression. Considering the above results, we concluded that CCAT1-protected astrocytes against apoptotic and inflammatory injury stimulated by OGD/R, and that this protective effect results from an interaction with miR-218/NFAT5 signaling axis.
Fig. 6.
MiR-218/NFAT5 axis contributed to the beneficial effects of CCAT1 on OGD/R-induced astrocyte injury. a MTT assays were applied to evaluate cell viability. b ELISA assays were utilized to identify apoptosis. c Levels of TNF-α and IL-1β were measured using ELISA assays. (n = 3) *P < 0.05 vs. Control; #P < 0.05 vs. OGD/R + pcDNA; &P < 0.05 vs. OGD/R + pcDNA-CCAT1
Discussion
SCI is a serious neurological challenge characterized by loss of nervous tissue and the subsequent deficit of sensory and motor functions, and effective treatment for SCI remains elusive. It is important to understand the molecular mechanism to enable treatment of neuropathic pain after SCI. At present, an increasing number of studies have recognized that lncRNAs are key regulators of gene expression in many cellular processes. In the case of SCI progression, the dysregulation of lncRNAs has been shown to play a pivotal role. However, the function of lncRNAs in SCI is not well-characterized and further studies are needed to pinpoint potential lncRNAs related to neuropathic pain caused by SCI. Herein, we aimed to assess the detailed regulatory roles of lncRNAs in OGD/R-induced ischemia-like injury in vitro and explore the underlying mechanism, hoping to get a better understanding of the pathomechanism of SCI with the goal of finding potential therapeutic targets and directions for treatment. We discovered that CCAT1 was manifestly downregulated in astrocytes during the OGD/R insult, which was consistent with a previous study (Zhou et al. 2018). On the basis of the above observations, CCAT1 was considered promising as a novel avenue for therapy during OGD/R-induced astrocyte injury.
There is a growing body of evidence that indicates astrocyte apoptosis is an important contributor to SCI (Chi et al. 2013). Owing to that CCAT1 has been reported to regulate cellular apoptosis in OGD/R-induced hepatocyte injury (Zhou et al. 2018), we explored the effect of CCAT1 on OGD/R-evoked astrocyte injury. Currently, in our study, upon inspection of the involvement of CCAT1 in mediating astrocyte apoptosis and its potential neuroprotective action, we observed that OGD/R conditions resulted in a notable decrease in cell viability, whereas abundance of CCAT1 rescued this phenomenon. In addition to increasing cell viability, CCAT1 exhibited an inhibitory effect on OGD/R-stimulated LDH release, indicating that CCAT1 mitigated damage to astrocytes. Concurrently, forced expression of CCAT1 led to a dramatic prevention of OGD/R-triggered apoptosis. This was accompanied by downregulation of Bax and C-caspase-3 and an upregulation of Bcl-2, indicating that CCAT1 participated in ameliorating OGD/R-induced astrocyte apoptosis. On the basis of the findings described above, CCAT1 was initially discovered to be a crucial anti-apoptotic transcript, which may be effective in protecting astrocytes against OGD/R-induced injury.
Neuroinflammation is another key component of the secondary injury mechanisms associated with SCI (Alizadeh et al. 2019; Mortezaee et al. 2018). Moreover, inflammation induced by SCI can exacerbate the neuron dysfunction and neuron death (Donnelly and Popovich 2008). The levels of inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, can reach the highest level in a few hours after SCI (Zhao et al. 2017). Therefore, quick action to control these cytokines and inhibit neuroinflammation, and the subsequent cell death, will be highly beneficial in reducing OGD/R-induced injury. Based on this reason, we are interested in investigating the necessary role of CCAT1 in OGD/R-induced inflammatory injury. As can been see, overexpression of CCAT1 tempered the OGD/R-induced upregulation of the pro-inflammatory cytokines TNF-α, IL-1β, and IL-6. Therefore, we conclude that CCAT1 contributed to the OGD/R injury by affecting regulating the inflammatory responses associated with OGD/R injury. Altogether, these results provide strong evidence that CCAT1 exerted multiple protective effects on OGD/R-stimulated astrocytes injury. These protective effects potentially occur via inhibiting apoptosis and neuro-inflammatory responses, subsequently resulting in alleviated neuronal damage. This study revealed the regulatory effect of CCAT1 in OGD/R-induced astrocytes injury for the first time, offering a novel perspective on SCI pathological study and therapy development.
Locally clustered enhancers form super enhancers, which recruit a large number of transcription factors and cofactors, confer particularly strong transcriptional regulation. Super-enhancers play prominent roles in driving expression of cell-type-specific genes through interacting with master transcription factors (TFs), cofactors, RNA polymerase II as well as non-coding RNAs (Whyte et al. 2013). CCAT1 generally acts as a coactivator of several transcription factors. Previous reports studying gastric, colon and live cancers demonstrated that c-MYC could directly bind to the promoter region of CCAT1 to enhance expression of this lncRNA, facilitating tumor progression (He et al. 2014; Yang et al. 2013; Zhu et al. 2015). A recent study demonstrated that TP63 and SOX2 co-occupy the promoter and super-enhancers of CCAT1, and contributed to its transcription activity specifically in SCCs, but not other cancer types, indicating that tissue-specific regulations of CCAT1 transcription is consistent with the lineage-specific nature of TP63 in SCC cells (Jiang et al. 2018). Growing evidence has confirmed that, by regulating the expression of miRNAs, lncRNAs have important roles in multiple neurological diseases (Wei et al. 2018). Previous studies have demonstrated that lncRNAs can act as competitive endogenous RNAs (ceRNA) to inhibit miRNA expression (Cao et al. 2017). Moreover, studies have shown that miRNAs are involved in the pathological responses following SCI (Deng et al. 2018; Li and Zhou 2019). In the current study, using bioinformatics software, it was predicted that CCAT1 could sponge miR-218 due to their complementary binding sites. The dual-luciferase reporter assay confirmed that the binding interaction between CCAT1 and miR-218 exists. It was apparent that there was a negative regulation of miR-218 by CCAT1, and they can bind with each other, implying that CCAT1 acted as a molecular sponge for miR-218. Furthermore, NFAT5 was identified as a direct target of miR-218, which was then validated by a dual-luciferase reporter assay, confirming that NFAT5 is negatively regulated by miR-218. It is interesting to notice that overexpression of CCAT1 augmented NFAT5 expression, but that effect was neutralized by the delivery of the miR-218 mimic. In light of the above findings, we therefore, concluded that CCAT1 functions as a ceRNA that regulated NFAT5 expression by sponging miR-218. More importantly, the investigation into the mechanism revealed that the beneficial effects of CCAT1 in regulating OGD/R-induced cell apoptosis and inflammation response were largely eliminated by the administration of miR-218. This implied that CCAT1 exerted its neuroprotective effect by binding miR-218 directly and negatively regulating it. This marks the first time miR-218 was identified as the direct target of CCAT1, devoting itself to modulating OGD/R-induced damage. Our previous study demonstrated that NFAT5-protected astrocytes against OGD/R-induced damage via the SIRT1/Nrf2 pathway (Xia et al. 2017). This study goes further by identifying a potential avenue for treatment of SCI, using the lncRNA-miRNA regulation network. Our results lead us to speculate that CCAT1 can prevent OGD/R-induced apoptosis and inflammatory responses by regulating the miR-218/NFAT5-signaling axis, potentially relieving OGD/R-induced astrocyte injury resulting from SCI.
Overall, as a summary of our findings in this study, CCAT1 contributed to the OGD/R-induced astrocyte injury and the neuro-inflammation damage via miR-218/NFAT5-signaling axis, suggesting that CCAT1 may act as a novel therapeutic target for SCI treatments. This study clarifies the targeting relationship between CCAT1 and miR-218, and emphasizes the influence of CCAT1 on SCI pathological development in preventing astrocyte apoptosis and neuro-inflammation damage, providing novel targets and theoretic foundation for the prevention of secondary injuries following SCI and their treatment. This study highlights the potential for treating SCI using the CCAT1/miR-218/NFAT5 regulation network, but further studies using animal models will be needed to illustrate the value of the lncRNA CCAT1 in SCI.
Author Contributions
XX, HN, YM, JWG and GL: Conception, design, and development of methodology; XX, HN, BQ, MJH, KY, ERW and GL: Acquisition of data, analysis, and interpretation of data; LZ and JWG: Contribution new reagents or analytic tools; XX, HN, YM, BQ, MJH and JWG: Study supervision, manuscript writing, and manuscript revision. All authors read and approved the final version.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Research Involving Human and/or Animals Rights
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xun Xia and Hao Niu are coauthors and contributed equally to this work.
References
- Alizadeh A, Dyck SM, Karimi-Abdolrezaee S (2019) Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front Neurol 10:282. 10.3389/fneur.2019.00282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao MX, Jiang YP, Tang YL, Liang XH (2017) The crosstalk between lncRNA and microRNA in cancer metastasis: orchestrating the epithelial-mesenchymal plasticity. Oncotarget 8:12472–12483. 10.18632/oncotarget.13957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Z, Ma X, Cui G, Li M, Li F (2013) Cinnamtannin B-1 regulates cell proliferation of spinal cord astrocytes and protects the cell from oxygen-glucose-serum deprivation/reoxygenation-induced apoptosis. Int J Mol Sci 14:15827–15837. 10.3390/ijms140815827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Kroner A (2011) Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 12:388–399. 10.1038/nrn3053 [DOI] [PubMed] [Google Scholar]
- Deng G, Gao Y, Cen Z, He J, Cao B, Zeng G, Zong S (2018) miR-136-5p regulates the inflammatory response by targeting the IKKbeta/NF-kappaB/A20 pathway after spinal cord injury cellular physiology and biochemistry. Int J Exp Cell Physiol Biochem Pharmacol 50:512–524. 10.1159/000494165 [DOI] [PubMed] [Google Scholar]
- Donnelly DJ, Popovich PG (2008) Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol 209:378–388. 10.1016/j.expneurol.2007.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou C, Sun L, Jin X, Han M, Zhang B, Li T (2017a) Long non-coding RNA colon cancer-associated transcript 1 functions as a competing endogenous RNA to regulate cyclin-dependent kinase 1 expression by sponging miR-490-3p in hepatocellular carcinoma progression. Tumour Biol 39:1010428317697572. 10.1177/1010428317697572 [DOI] [PubMed] [Google Scholar]
- Dou L, Lin H, Wang K, Zhu G, Zou X, Chang E, Zhu Y (2017b) Long non-coding RNA CCAT1 modulates neuropathic pain progression through sponging miR-155. Oncotarget 8:89949–89957. 10.18632/oncotarget.21192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X et al (2014) C-Myc-activated long noncoding RNA CCAT1 promotes colon cancer cell proliferation and invasion. Tumour Biol 35:12181–12188. 10.1007/s13277-014-2526-4 [DOI] [PubMed] [Google Scholar]
- Hirayama Y, Koizumi S (2018) Astrocytes and ischemic tolerance. Neurosci Res 126:53–59. 10.1016/j.neures.2017.11.013 [DOI] [PubMed] [Google Scholar]
- Hu G et al (2018) Molecular mechanisms of long noncoding RNAs and their role in disease pathogenesis. Oncotarget 9:18648–18663. 10.18632/oncotarget.24307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H et al (2019) Downregulation of LncRNA TUG1 inhibited TLR4 signaling pathway-mediated inflammatory damage after spinal cord ischemia reperfusion in rats via suppressing TRIL expression. J Neuropathol Exp Neurol 78:268–282. 10.1093/jnen/nly126 [DOI] [PubMed] [Google Scholar]
- Jiang Y et al (2018) Co-activation of super-enhancer-driven CCAT1 by TP63 and SOX2 promotes squamous cancer progression. Nat Commun 9:3619. 10.1038/s41467-018-06081-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalevee S, Feil R (2015) Long noncoding RNAs in human disease: emerging mechanisms and therapeutic strategies. Epigenomics 7:877–879. 10.2217/epi.15.55 [DOI] [PubMed] [Google Scholar]
- Li F, Zhou MW (2019) MicroRNAs in contusion spinal cord injury: pathophysiology and clinical utility. Acta Neurol Belg 119:21–27. 10.1007/s13760-019-01076-9 [DOI] [PubMed] [Google Scholar]
- Li Z et al (2019a) Long non-coding RNAs in the spinal cord injury: novel spotlight. J Cell Mol Med 23:4883–4890. 10.1111/jcmm.14422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z et al (2019b) Emerging roles of long non-coding RNAs in neuropathic pain. Cell Prolif 52:e12528. 10.1111/cpr.12528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhao Z, Yin Q, Zhang X (2019) TTB protects astrocytes against oxygen-glucose deprivation/reoxygenation-induced injury via activation of Nrf2/HO-1 signaling pathway. Front Pharmacol 10:792. 10.3389/fphar.2019.00792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Maragakis NJ, Rothstein JD (2006) Mechanisms of disease: astrocytes in neurodegenerative disease. Nat Clin Neurol 2:679–689. 10.1038/ncpneuro0355 [DOI] [PubMed] [Google Scholar]
- Mortezaee K, Khanlarkhani N, Beyer C, Zendedel A (2018) Inflammasome: its role in traumatic brain and spinal cord injury. J Cell Physiol 233:5160–5169. 10.1002/jcp.26287 [DOI] [PubMed] [Google Scholar]
- Riva P, Ratti A, Venturin M (2016) The long non-coding RNAs in neurodegenerative diseases: novel mechanisms of pathogenesis. Curr Alzheimer Res 13:1219–1231 [DOI] [PubMed] [Google Scholar]
- Silva NA, Sousa N, Reis RL, Salgado AJ (2014) From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol 114:25–57. 10.1016/j.pneurobio.2013.11.002 [DOI] [PubMed] [Google Scholar]
- Varma AK, Das A, Gt W, Barry J, Vertegel AA, Ray SK, Banik NL (2013) Spinal cord injury: a review of current therapy, future treatments, and basic science frontiers. Neurochem Res 38:895–905. 10.1007/s11064-013-0991-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CW, Luo T, Zou SS, Wu AS (2018) The role of long noncoding RNAs in central nervous system and neurodegenerative diseases. Front Behav Neurosci 12:175. 10.3389/fnbeh.2018.00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte WA et al (2013) Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153:307–319. 10.1016/j.cell.2013.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Bono J, Tao YX (2019) Long noncoding RNA (lncRNA): a target in neuropathic pain. Exp Opin Therap Targets 23:15–20. 10.1080/14728222.2019.1550075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X et al (2016) Impact of heat shock protein A 12B overexpression on spinal astrocyte survival against oxygen-glucose-serum deprivation/restoration in primary cultured astrocytes. J Mol Neurosci 59:511–520. 10.1007/s12031-016-0768-x [DOI] [PubMed] [Google Scholar]
- Xia X et al (2017) NFAT5 protects astrocytes against oxygen-glucose-serum deprivation/restoration damage via the SIRT1/Nrf2 pathway. J Mol Neurosci 61:96–104. 10.1007/s12031-016-0849-x [DOI] [PubMed] [Google Scholar]
- Xin Y, Li Z, Shen J, Chan MT, Wu WK (2016) CCAT1: a pivotal oncogenic long non-coding RNA in human cancers. Cell Prolif 49:255–260. 10.1111/cpr.12252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Xue X, Bi J, Zheng L, Zhi K, Gu Y, Fang G (2013) Long noncoding RNA CCAT1, which could be activated by c-Myc, promotes the progression of gastric carcinoma. J Cancer Res Clin Oncol 139:437–445. 10.1007/s00432-012-1324-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H et al (2018) LncRNA DGCR5 suppresses neuronal apoptosis to improve acute spinal cord injury through targeting PRDM5. Cell Cycle 17:1992–2000. 10.1080/15384101.2018.1509622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Wang L, Li Y (2017) Electroacupuncture alleviates the inflammatory response via effects on M1 and M2 macrophages after spinal cord injury. Acupunct Med 35:224–230. 10.1136/acupmed-2016-011107 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Chen Q, Wan L, Zheng D, Li Z, Wu Z (2018) Dexmedetomidine protects hepatic cells against oxygen-glucose deprivation/reperfusion injury via lncRNA CCAT1. Cell Biol Int 42:1250–1258. 10.1002/cbin.10996 [DOI] [PubMed] [Google Scholar]
- Zhu HQ, Zhou X, Chang H, Li HG, Liu FF, Ma CQ, Lu J (2015) Aberrant expression of CCAT1 regulated by c-Myc predicts the prognosis of hepatocellular carcinoma. Asian Pac J Cancer Prev 16:5181–5185. 10.7314/apjcp.2015.16.13.5181 [DOI] [PubMed] [Google Scholar]