Abstract
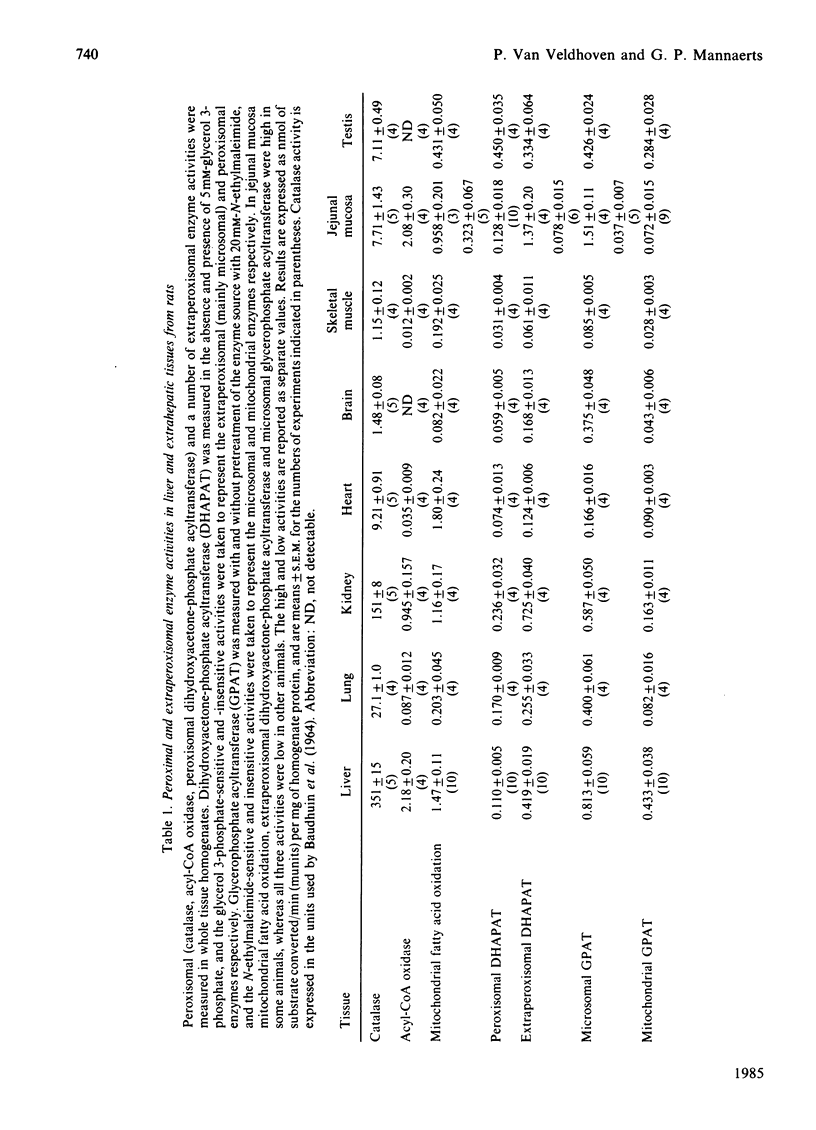
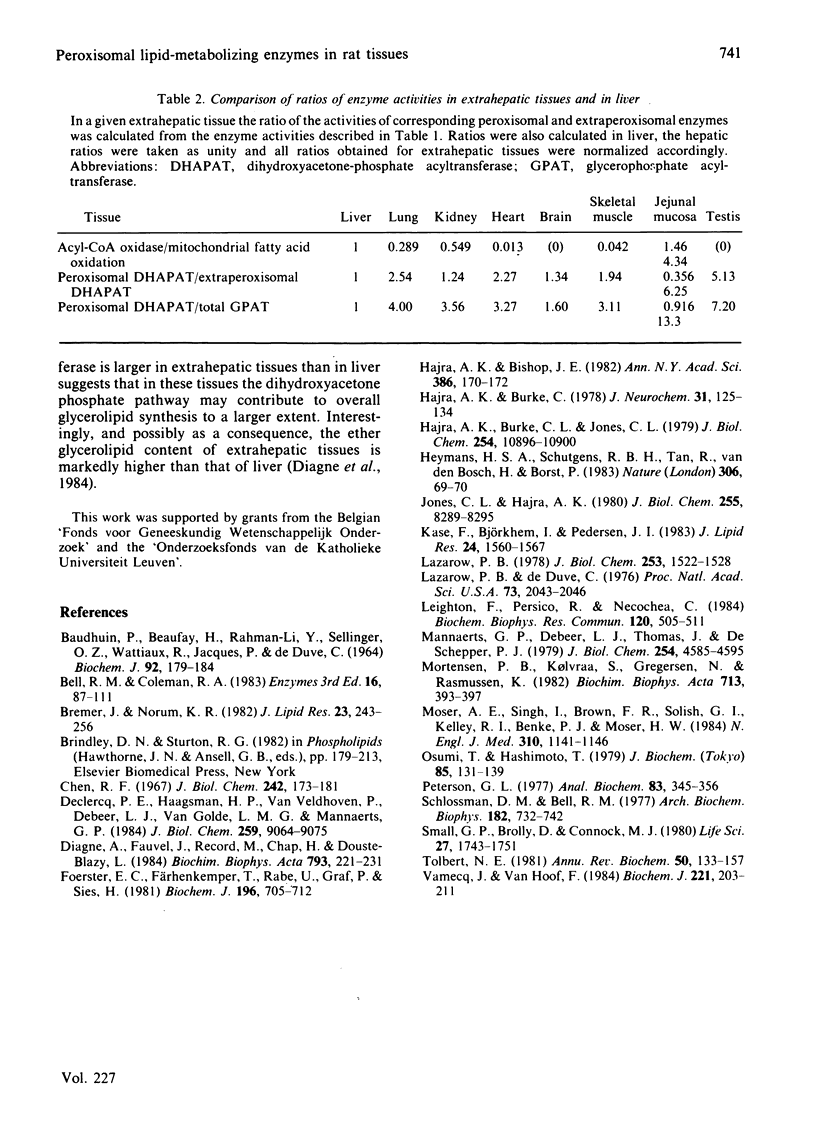
Peroxisomal (acyl-CoA oxidase and peroxisomal dihydroxyacetone-phosphate acyltransferase) and extraperoxisomal (mitochondrial fatty acid oxidation, extraperoxisomal dihydroxyacetone-phosphate acyltransferase, mitochondrial and microsomal glycerophosphate acyltransferases) lipid-metabolizing enzymes were measured in homogenates from rat liver and from seven extrahepatic tissues. Except for jejunal mucosa and kidney, extrahepatic tissues contained very little acyl-CoA oxidase activity. Peroxisomal dihydroxyacetone-phosphate acyltransferase, taken as the activity that was not inhibited by 5 mM-glycerol 3-phosphate, was present in all tissues examined, and its specific activity in liver and extrahepatic tissues was roughly of the same order of magnitude. Clofibrate treatment increased the activity of acyl-CoA oxidase in liver, and to a smaller extent also in kidney, but did not influence the activity of peroxisomal dihydroxyacetone-phosphate acyltransferase. Comparison of the activities of peroxisomal and extraperoxisomal lipid-metabolizing enzymes in extrahepatic tissues and in liver, an organ in which the contribution of peroxisomes to fatty acid oxidation and to glycerolipid synthesis has been estimated previously, suggests that, as in liver, peroxisomal long-chain fatty acid oxidation is of minor quantitative importance in extrahepatic tissues, but that in these tissues (micro)-peroxisomes are responsible for most of the dihydroxyacetone phosphate acylation and, consequently, for initiating ether glycerolipid synthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baudhuin P., Beaufay H., Rahman-Li Y., Sellinger O. Z., Wattiaux R., Jacques P., De Duve C. Tissue fractionation studies. 17. Intracellular distribution of monoamine oxidase, aspartate aminotransferase, alanine aminotransferase, D-amino acid oxidase and catalase in rat-liver tissue. Biochem J. 1964 Jul;92(1):179–184. doi: 10.1042/bj0920179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer J., Norum K. R. Metabolism of very long-chain monounsaturated fatty acids (22:1) and the adaptation to their presence in the diet. J Lipid Res. 1982 Feb;23(2):243–256. [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Declercq P. E., Haagsman H. P., Van Veldhoven P., Debeer L. J., Van Golde L. M., Mannaerts G. P. Rat liver dihydroxyacetone-phosphate acyltransferases and their contribution to glycerolipid synthesis. J Biol Chem. 1984 Jul 25;259(14):9064–9075. [PubMed] [Google Scholar]
- Diagne A., Fauvel J., Record M., Chap H., Douste-Blazy L. Studies on ether phospholipids. II. Comparative composition of various tissues from human, rat and guinea pig. Biochim Biophys Acta. 1984 Apr 18;793(2):221–231. doi: 10.1016/0005-2760(84)90324-2. [DOI] [PubMed] [Google Scholar]
- Foerster E. C., Fährenkemper T., Rabe U., Graf P., Sies H. Peroxisomal fatty acid oxidation as detected by H2O2 production in intact perfused rat liver. Biochem J. 1981 Jun 15;196(3):705–712. doi: 10.1042/bj1960705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajra A. K., Bishop J. E. Glycerolipid biosynthesis in peroxisomes via the acyl dihydroxyacetone phosphate pathway. Ann N Y Acad Sci. 1982;386:170–182. doi: 10.1111/j.1749-6632.1982.tb21415.x. [DOI] [PubMed] [Google Scholar]
- Hajra A. K., Burke C. Biosynthesis of phosphatidic acid in rat brain via acyl dihydroxyacetone phosphate. J Neurochem. 1978 Jul;31(1):125–134. doi: 10.1111/j.1471-4159.1978.tb12440.x. [DOI] [PubMed] [Google Scholar]
- Heymans H. S., Schutgens R. B., Tan R., van den Bosch H., Borst P. Severe plasmalogen deficiency in tissues of infants without peroxisomes (Zellweger syndrome). Nature. 1983 Nov 3;306(5938):69–70. doi: 10.1038/306069a0. [DOI] [PubMed] [Google Scholar]
- Jones C. L., Hajra A. K. Properties of guinea pig liver peroxisomal dihydroxyacetone phosphate acyltransferase. J Biol Chem. 1980 Sep 10;255(17):8289–8295. [PubMed] [Google Scholar]
- Kase F., Björkhem I., Pedersen J. I. Formation of cholic acid from 3 alpha, 7 alpha, 12 alpha-trihydroxy-5 beta-cholestanoic acid by rat liver peroxisomes. J Lipid Res. 1983 Dec;24(12):1560–1567. [PubMed] [Google Scholar]
- Lazarow P. B., De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow P. B. Rat liver peroxisomes catalyze the beta oxidation of fatty acids. J Biol Chem. 1978 Mar 10;253(5):1522–1528. [PubMed] [Google Scholar]
- Leighton F., Pérsico R., Necochea C. Peroxisomal fatty acid oxidation is selectively inhibited by phenothiazines in isolated hepatocytes. Biochem Biophys Res Commun. 1984 Apr 30;120(2):505–511. doi: 10.1016/0006-291x(84)91283-x. [DOI] [PubMed] [Google Scholar]
- Mannaerts G. P., Debeer L. J., Thomas J., De Schepper P. J. Mitochondrial and peroxisomal fatty acid oxidation in liver homogenates and isolated hepatocytes from control and clofibrate-treated rats. J Biol Chem. 1979 Jun 10;254(11):4585–4595. [PubMed] [Google Scholar]
- Mortensen P. B., Kølvraa S., Gregersen N., Rasmussen K. Cyanide-insensitive and clofibrate enhanced beta-oxidation of dodecanedioic acid in rat liver. An indication of peroxisomal beta-oxidation of N-dicarboxylic acids. Biochim Biophys Acta. 1982 Nov 12;713(2):393–397. doi: 10.1016/0005-2760(82)90258-2. [DOI] [PubMed] [Google Scholar]
- Moser A. E., Singh I., Brown F. R., 3rd, Solish G. I., Kelley R. I., Benke P. J., Moser H. W. The cerebrohepatorenal (Zellweger) syndrome. Increased levels and impaired degradation of very-long-chain fatty acids and their use in prenatal diagnosis. N Engl J Med. 1984 May 3;310(18):1141–1146. doi: 10.1056/NEJM198405033101802. [DOI] [PubMed] [Google Scholar]
- Osumi T., Hashimoto T. Subcellular distribution of the enzymes of the fatty acyl-CoA beta-oxidation system and their induction by di(2-ethylhexyl)phthalate in rat liver. J Biochem. 1979 Jan;85(1):131–139. doi: 10.1093/oxfordjournals.jbchem.a132302. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Schlossman D. M., Bell R. M. Microsomal sn-glycerol 3-phosphate and dihydroxyacetone phosphate acyltransferase activities from liver and other tissues. Evidence for a single enzyme catalizing both reactions. Arch Biochem Biophys. 1977 Aug;182(2):732–742. doi: 10.1016/0003-9861(77)90555-0. [DOI] [PubMed] [Google Scholar]
- Small G. M., Brolly D., Connock M. J. Palmityl-CoA oxidase: detection in several guinea pig tissues and peroxisomal localisation in mucosa, of small intestine. Life Sci. 1980 Nov 10;27(19):1743–1751. doi: 10.1016/0024-3205(80)90441-5. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E. Metabolic pathways in peroxisomes and glyoxysomes. Annu Rev Biochem. 1981;50:133–157. doi: 10.1146/annurev.bi.50.070181.001025. [DOI] [PubMed] [Google Scholar]
- Vamecq J., Van Hoof F. Implication of a peroxisomal enzyme in the catabolism of glutaryl-CoA. Biochem J. 1984 Jul 1;221(1):203–211. doi: 10.1042/bj2210203. [DOI] [PMC free article] [PubMed] [Google Scholar]


