Abstract
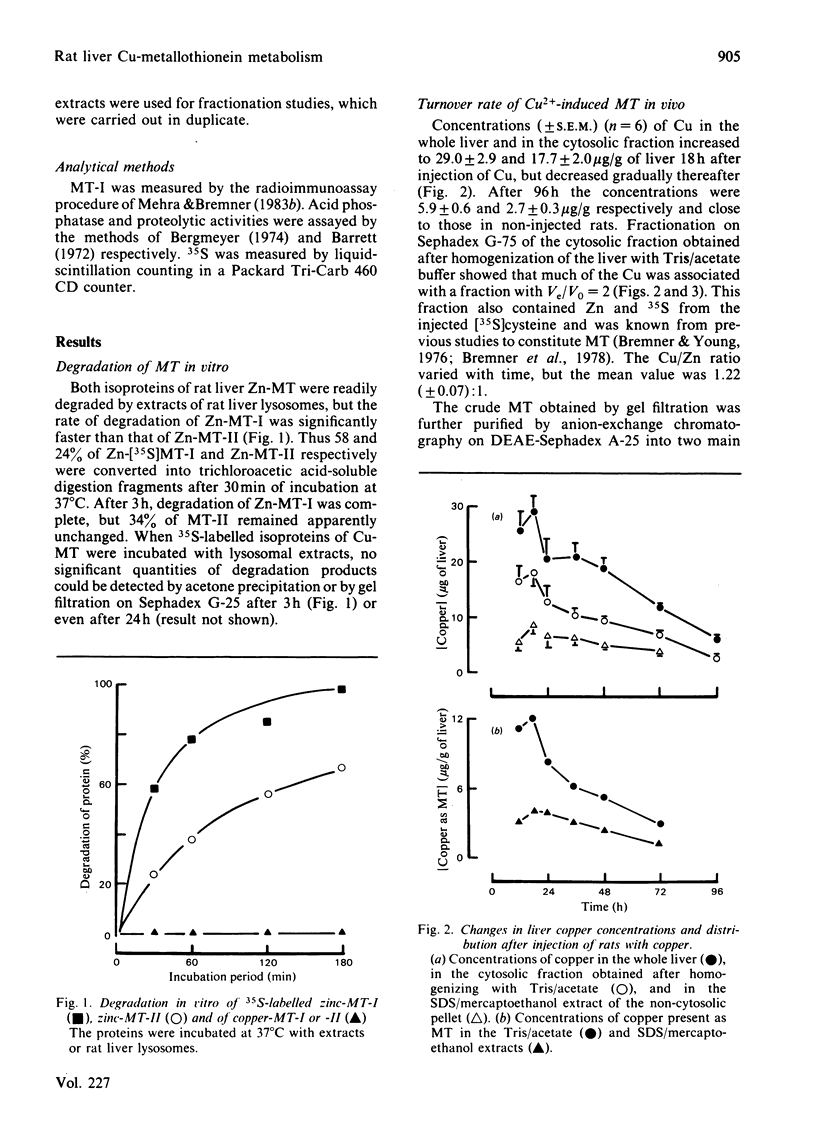
The degradation of purified 35S-labelled rat liver isometallothioneins (MT) by lysosomal extracts was studied. Zn-MT-I was more readily hydrolysed than Zn-MT-II, but no significant degradation of the Cu-containing metallothioneins could be detected, even after 24 h incubation. The susceptibility of MT to degradation in vitro may be related to the strength of the metal-thiolate bonds. However, the turnover rates of cytosolic MT in vivo, as established by pulse-labelling techniques, are apparently subject to different controls. The half-lives of MT-I and -II in the liver cytosol of Cu2+-injected rats were only 15.4 +/- 1.5 and 18.2 +/- 1.1 h respectively. Approx. 25% of the total liver MT was present in particulate fractions (probably in lysosomes) of the liver and had a half-life of 25.1 +/- 4.1 h.
Full text
PDF

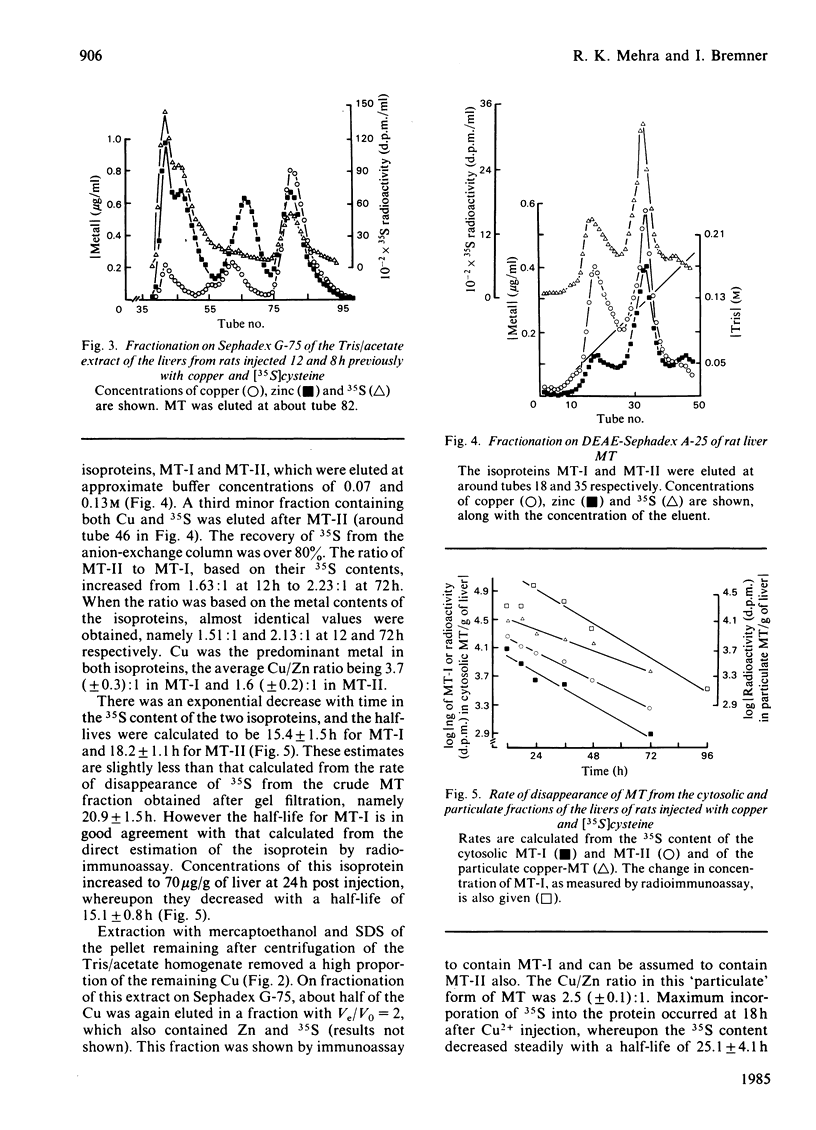


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen R. D., Winter W. P., Maher J. J., Bernstein I. A. Turnover of metallothioneins in rat liver. Biochem J. 1978 Jul 15;174(1):327–338. doi: 10.1042/bj1740327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner I., Hoekstra G., Davies N. T., Young B. W. Effect of zinc status of rats on the synthesis and degradation of copper-induced metallothioneins. Biochem J. 1978 Sep 15;174(3):883–892. doi: 10.1042/bj1740883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner I., Marshall R. B. Hepatic copper- and zinc-binding proteins in ruminants. 2. Relationship between Cu and Zn concentrations and the occurrence of a metallothionein-like fraction. Br J Nutr. 1974 Sep;32(2):293–300. doi: 10.1079/bjn19740082. [DOI] [PubMed] [Google Scholar]
- Bremner I., Young B. W. Isolation of (copper, zinc)-thioneins from the livers of copper-injected rats. Biochem J. 1976 Aug 1;157(2):517–520. doi: 10.1042/bj1570517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain K., Griffiths B. L. A comparison of isometallothionein synthesis in rat liver after partial hepatectomy and parenteral zinc injection. Biochem J. 1984 Jan 1;217(1):85–92. doi: 10.1042/bj2170085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain K., Holt D. E. Metallothionein degradation: metal composition as a controlling factor. Chem Biol Interact. 1979;28(1):91–106. doi: 10.1016/0009-2797(79)90117-0. [DOI] [PubMed] [Google Scholar]
- Dean R. T., Barrett A. J. Lysosomes. Essays Biochem. 1976;12:1–40. [PubMed] [Google Scholar]
- Feldman S. L., Cousins R. J. Degradation of hepatic zinc-thionein after parenteral zinc administration. Biochem J. 1976 Dec 15;160(3):583–588. doi: 10.1042/bj1600583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman S. L., Failla M. L., Cousins R. J. Degradation of rat liver metallothioneins in vitro. Biochim Biophys Acta. 1978 Dec 18;544(3):638–646. doi: 10.1016/0304-4165(78)90338-0. [DOI] [PubMed] [Google Scholar]
- KAGI J. H., VALLEE B. L. Metallothionein: a cadmium and zinc-containign protein from equine renal cortex. II. Physico-chemical properties. J Biol Chem. 1961 Sep;236:2435–2442. [PubMed] [Google Scholar]
- Mehra R. K., Bremner I. Development of a radioimmunoassay for rat liver metallothionein-I and its application to the analysis of rat plasma and kidneys. Biochem J. 1983 Aug 1;213(2):459–465. doi: 10.1042/bj2130459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra R. K., Bremner I. Species differences in the occurrence of copper-metallothionein in the particulate fractions of the liver of copper-loaded animals. Biochem J. 1984 Apr 15;219(2):539–546. doi: 10.1042/bj2190539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson J. K., Sadler P. J., Cain K., Holt D. E., Webb M., Hawkes G. E. 88MHz 113Cd-n.m.r. studies of native rat liver metallothioneins. Biochem J. 1983 Apr 1;211(1):251–255. doi: 10.1042/bj2110251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panem S., Reynolds J. T. Retrovirus expression in normal and pathogenic processes of man. Fed Proc. 1979 Dec;38(13):2674–2678. [PubMed] [Google Scholar]
- Panemangalore M., Banerjee D., Onosaka S., Cherian M. G. Changes in the intracellular accumulation and distribution of metallothionein in rat liver and kidney during postnatal development. Dev Biol. 1983 May;97(1):95–102. doi: 10.1016/0012-1606(83)90067-2. [DOI] [PubMed] [Google Scholar]
- Porter H. The particulate half-cystine-rich copper protein of newborn liver. Relationship to metallothionein and subcellular localization in non-mitochondrial particles possibly representing heavy lysosomes. Biochem Biophys Res Commun. 1974 Feb 4;56(3):661–668. doi: 10.1016/0006-291x(74)90656-1. [DOI] [PubMed] [Google Scholar]
- Ragab H., Beck C., Dillard C., Tappel A. L. Preparation of rat liver lysosomes. Biochim Biophys Acta. 1967 Nov 28;148(2):501–505. doi: 10.1016/0304-4165(67)90148-1. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Richards V. Human fetal liver contains both zinc- and copper-rich forms of metallothionein. J Biol Chem. 1980 Jun 10;255(11):5380–5383. [PubMed] [Google Scholar]
- Sato M., Bremner I. Biliary excretion of metallothionein and a possible degradation product in rats injected with copper and zinc. Biochem J. 1984 Oct 15;223(2):475–479. doi: 10.1042/bj2230475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh Z. A., Smith J. C. The biosynthesis of metallothionein rat liver and kidney after administration of cadmium. Chem Biol Interact. 1976 Dec;15(4):327–336. doi: 10.1016/0009-2797(76)90138-1. [DOI] [PubMed] [Google Scholar]
- Vasák M., Galdes A., Hill H. A., Kägi J. H., Bremner I., Young B. W. Investigation of the structure of metallothioneins by proton nuclear magnetic resonance spectroscopy. Biochemistry. 1980 Feb 5;19(3):416–425. doi: 10.1021/bi00544a003. [DOI] [PubMed] [Google Scholar]
- Winge D. R., Miklossy K. A. Differences in the polymorphic forms of metallothionein. Arch Biochem Biophys. 1982 Mar;214(1):80–88. doi: 10.1016/0003-9861(82)90010-8. [DOI] [PubMed] [Google Scholar]
- Winge D. R., Miklossy K. A. Domain nature of metallothionein. J Biol Chem. 1982 Apr 10;257(7):3471–3476. [PubMed] [Google Scholar]


