Abstract
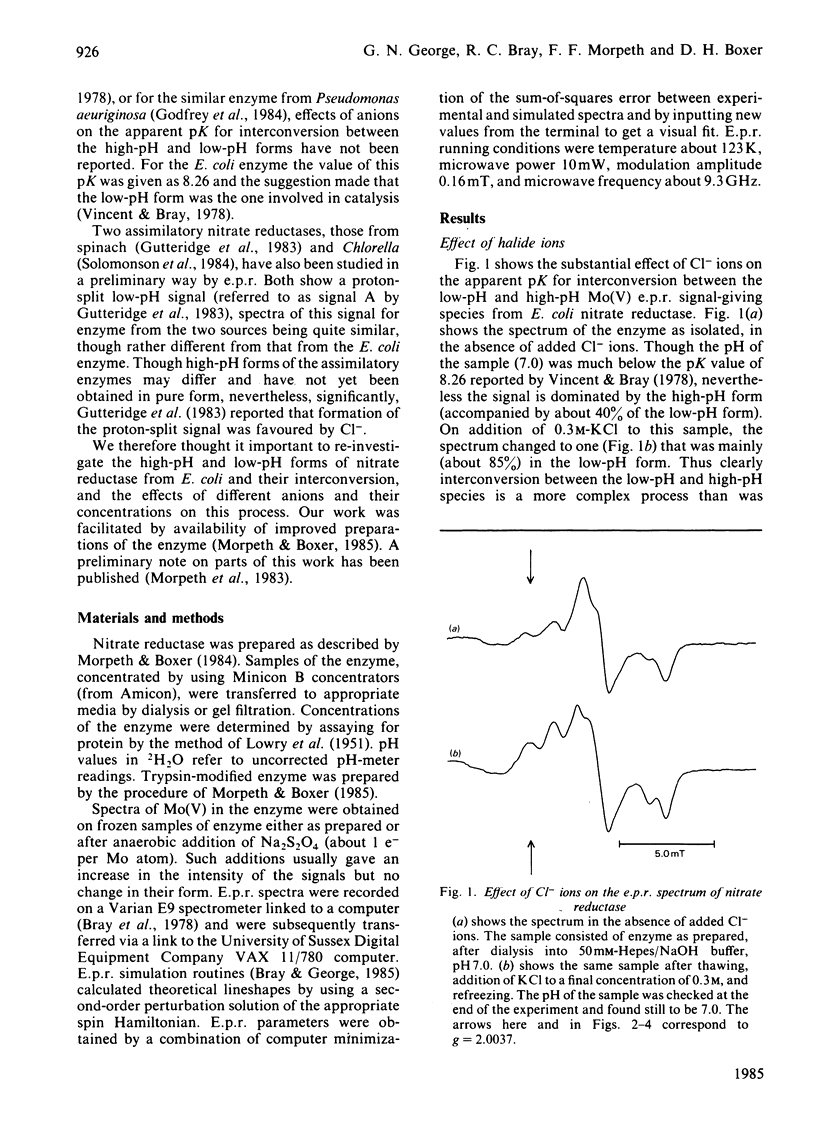
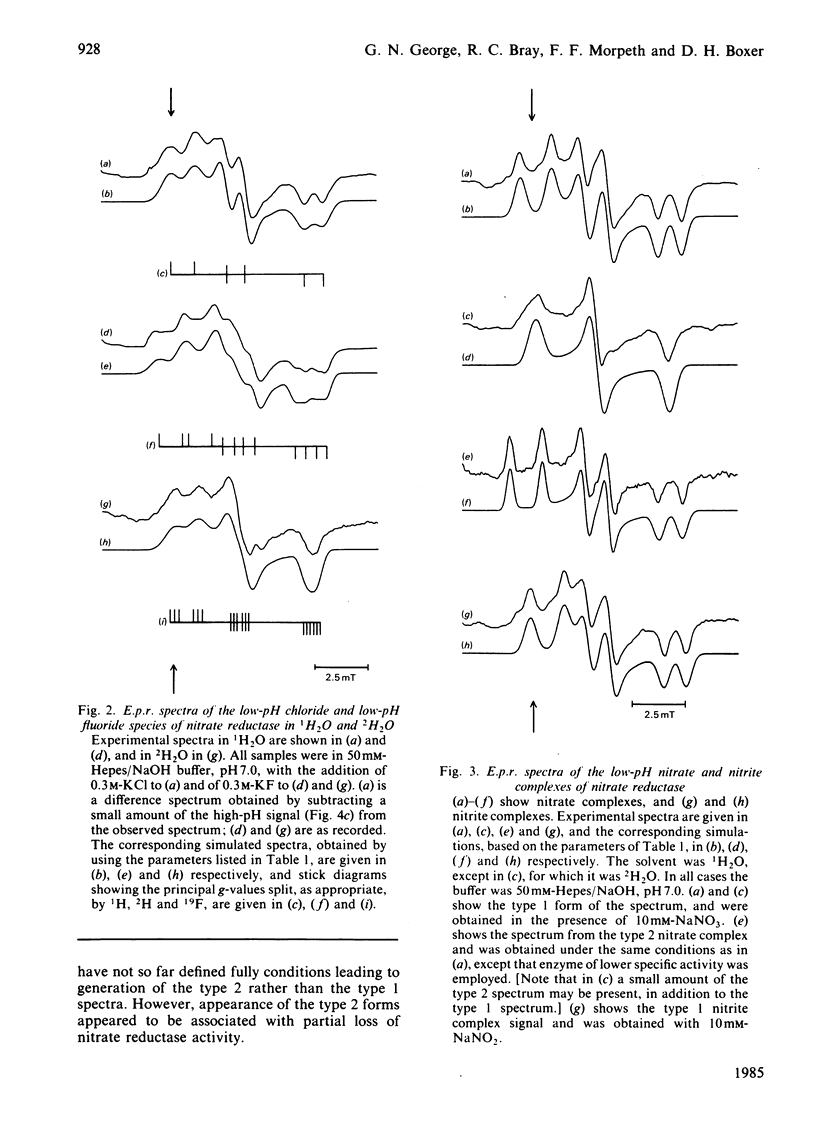
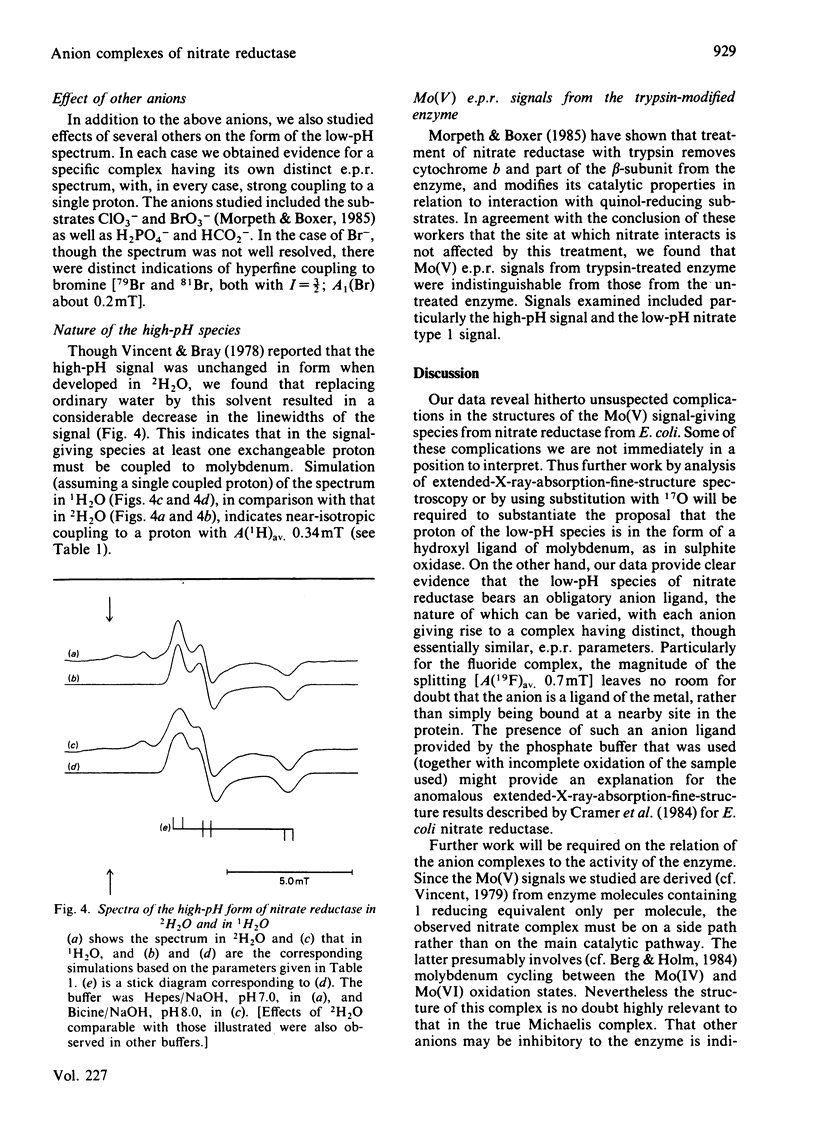
The interconversion of nitrate reductase from Escherichia coli between low-pH and high-pH Mo(V) e.p.r. signal-giving species was re-investigated [cf. Vincent & Bray (1978) Biochem. J. 171, 639-647]. The process cannot be described by a single pK value, since the apparent pK for interconversion is raised by the presence of various anions. The low-pH form of the enzyme exists as a series of complexes with different anion ligands of molybdenum. Each complex has specific and slightly different e.p.r. parameters, but all show strong coupling of Mo(V) to a single proton, exchangeable with the solvent, having A(1H)av. 1.0 to 1.3 mT. Complexes with Cl-, F- [A(19F)av. 0.7 mT], NO3- and NO2- give particularly well-defined spectra. The high-pH form of the enzyme is now shown to bear a coupled proton. Like that in the low-pH species, this proton is exchangeable with the solvent, but the coupling is much weaker, with A(1H)av. 0.3 mT. Thus, contrary to earlier assumptions, the proton detectable by e.p.r. is probably not identical with the proton whose dissociation controls interconversion between the two species; the latter proton could be located in the protein rather than on a ligand of molybdenum. Treatment of the enzyme with trypsin [Morpeth & Boxer (1985) Biochemistry 24, 40-46] did not affect its Mo(V) e.p.r. signals.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bray R. C., Barber M. J., Lowe D. J. Electron-paramagnetic-resonance spectroscopy of complexes of xanthine oxidase with xanthine and uric acid. Biochem J. 1978 Jun 1;171(3):653–658. doi: 10.1042/bj1710653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray R. C., Gutteridge S., Lamy M. T., Wilkinson T. Equilibria amongst different molybdenum (V)-containing species from sulphite oxidase. Evidence for a halide ligand of molybdenum in the low-pH species. Biochem J. 1983 Apr 1;211(1):227–236. doi: 10.1042/bj2110227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray R. C., Gutteridge S. Numbers and exchangeability with water of oxygen-17 atoms coupled to molybdenum (V) in different reduced forms of xanthine oxidase. Biochemistry. 1982 Nov 9;21(23):5992–5999. doi: 10.1021/bi00266a041. [DOI] [PubMed] [Google Scholar]
- Bray R. C., Lamy M. T., Gutteridge S., Wilkinson T. Evidence from electron-paramagnetic-resonance spectroscopy for a complex of sulphite ions with the molybdenum centre of sulphite oxidase. Biochem J. 1982 Jan 1;201(1):241–243. doi: 10.1042/bj2010241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray R. C. The reactions and the structures of molybdenum centers in enzymes. Adv Enzymol Relat Areas Mol Biol. 1980;51:107–165. doi: 10.1002/9780470122969.ch3. [DOI] [PubMed] [Google Scholar]
- Cohen H. J., Fridovich I., Rajagopalan K. V. Hepatic sulfite oxidase. A functional role for molybdenum. J Biol Chem. 1971 Jan 25;246(2):374–382. [PubMed] [Google Scholar]
- Godfrey C., Greenwood C., Thomson A. J., Bray R. C., George G. N. Electron-paramagnetic-resonance spectroscopy studies on the dissimilatory nitrate reductase from Pseudomonas aeruginosa. Biochem J. 1984 Dec 1;224(2):601–608. doi: 10.1042/bj2240601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge S., Bray R. C., Notton B. A., Fido R. J., Hewitt E. J. Studies by electron-paramagnetic-resonance spectroscopy of the molybdenum centre of spinach (Spinacia oleracea) nitrate reductase. Biochem J. 1983 Jul 1;213(1):137–142. doi: 10.1042/bj2130137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamy M. T., Gutteridge S., Bary R. C. Electron-paramagnetic-resonance parameters of molybdenum(V) in sulphite oxidase from chicken liver. Biochem J. 1980 Feb 1;185(2):397–403. doi: 10.1042/bj1850397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morpeth F. F., Boxer D. H. Kinetic analysis of respiratory nitrate reductase from Escherichia coli K12. Biochemistry. 1985 Jan 1;24(1):40–46. doi: 10.1021/bi00322a007. [DOI] [PubMed] [Google Scholar]
- Solomonson L. P., Barber M. J., Howard W. D., Johnson J. L., Rajagopalan K. V. Electron paramagnetic resonance studies on the molybdenum center of assimilatory NADH:nitrate reductase from Chlorella vulgaris. J Biol Chem. 1984 Jan 25;259(2):849–853. [PubMed] [Google Scholar]
- Vincent S. P., Bray R. C. Electron-paramagnetic-resonance studies on nitrate reductase from Escherichia coli K12. Biochem J. 1978 Jun 1;171(3):639–647. doi: 10.1042/bj1710639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent S. P. Oxidation--reduction potentials of molybdenum and iron--sulphur centres in nitrate reductase from Escherichia coli. Biochem J. 1979 Feb 1;177(2):757–759. doi: 10.1042/bj1770757. [DOI] [PMC free article] [PubMed] [Google Scholar]


