Abstract
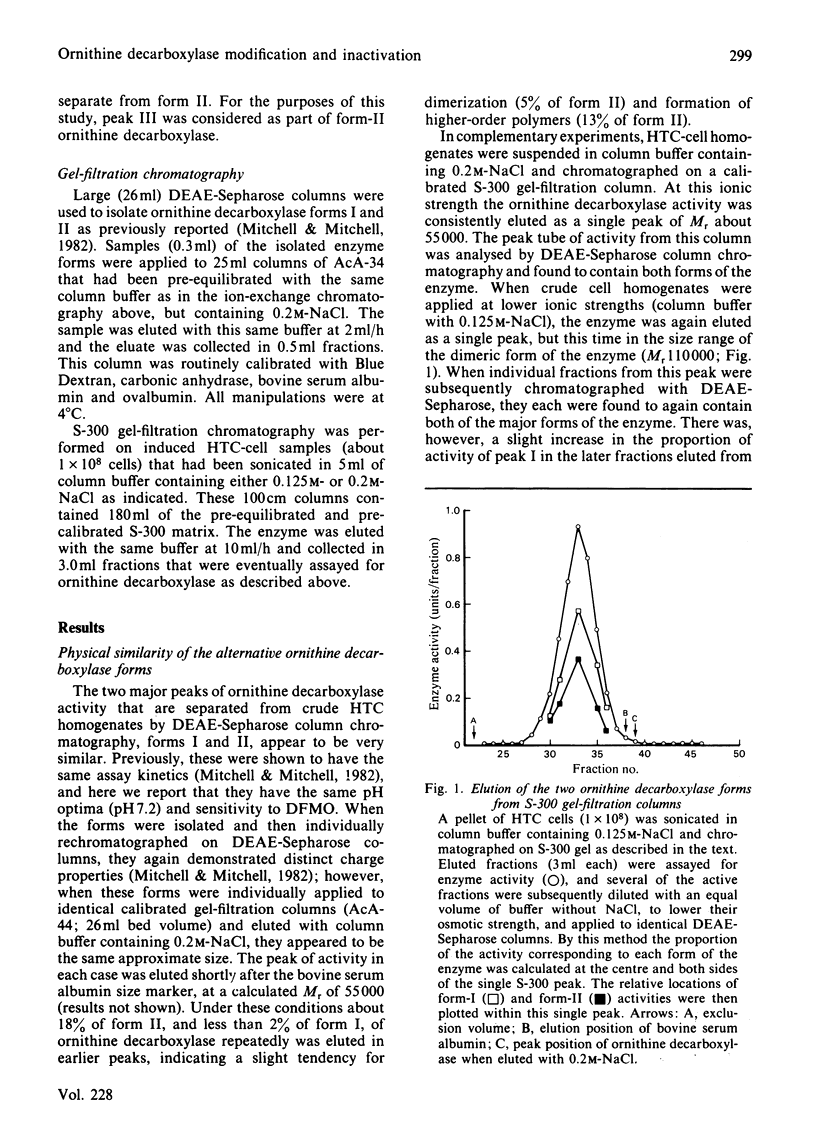
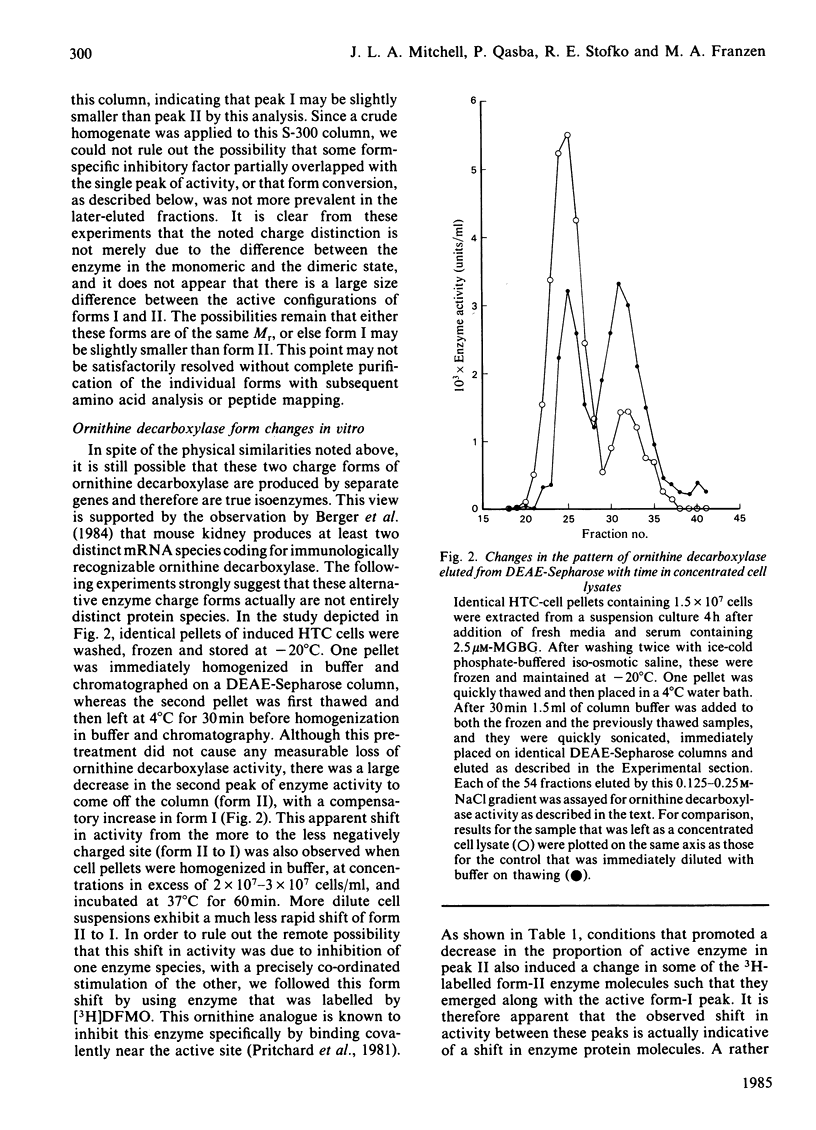
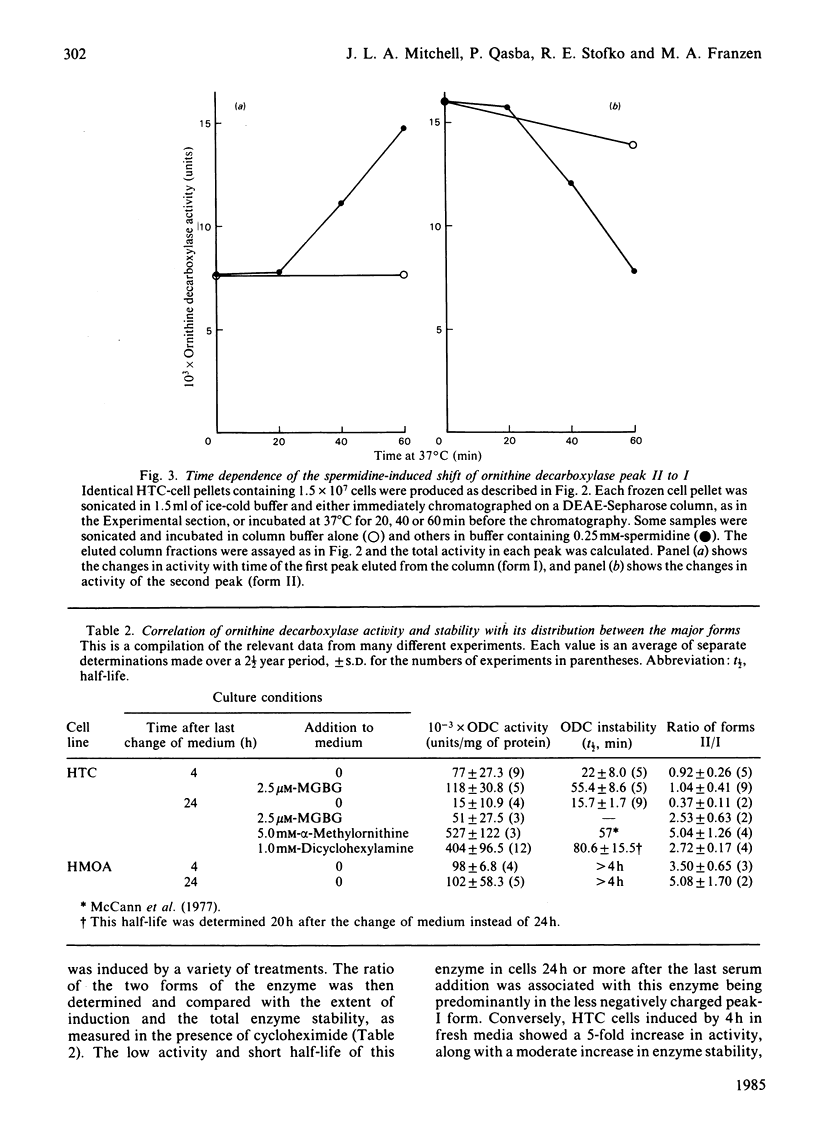
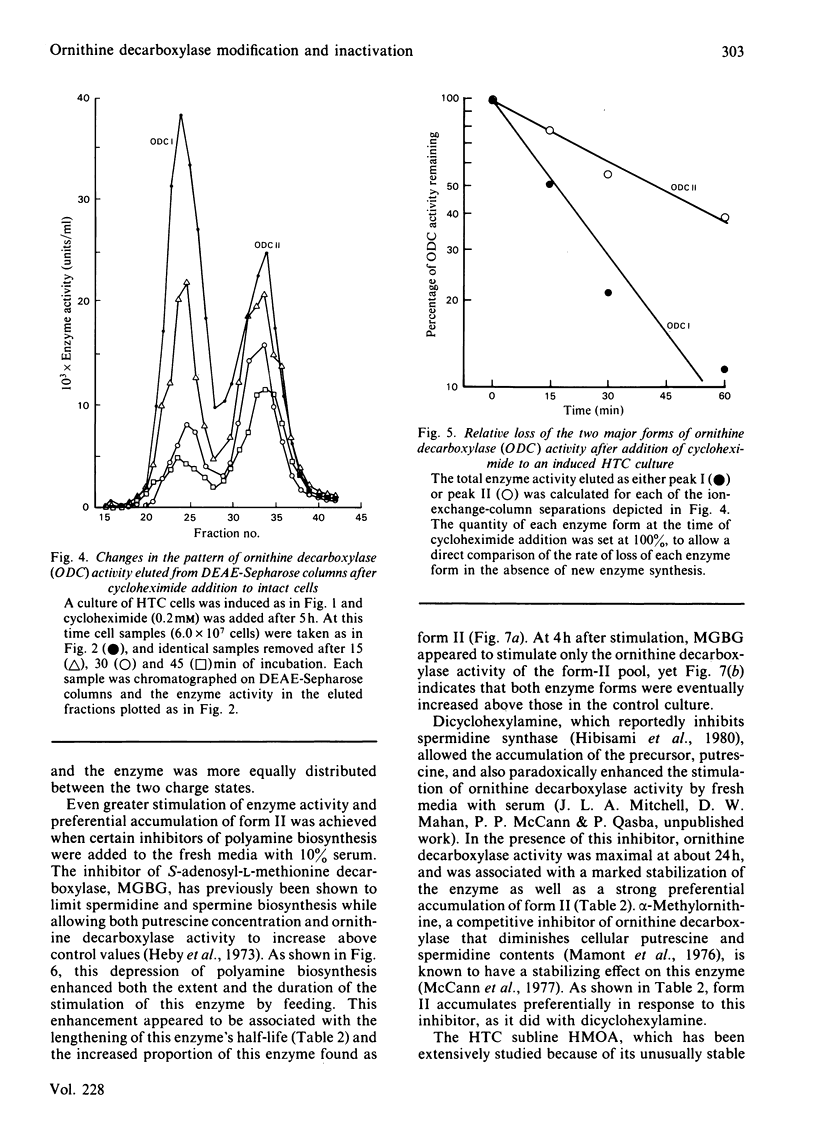
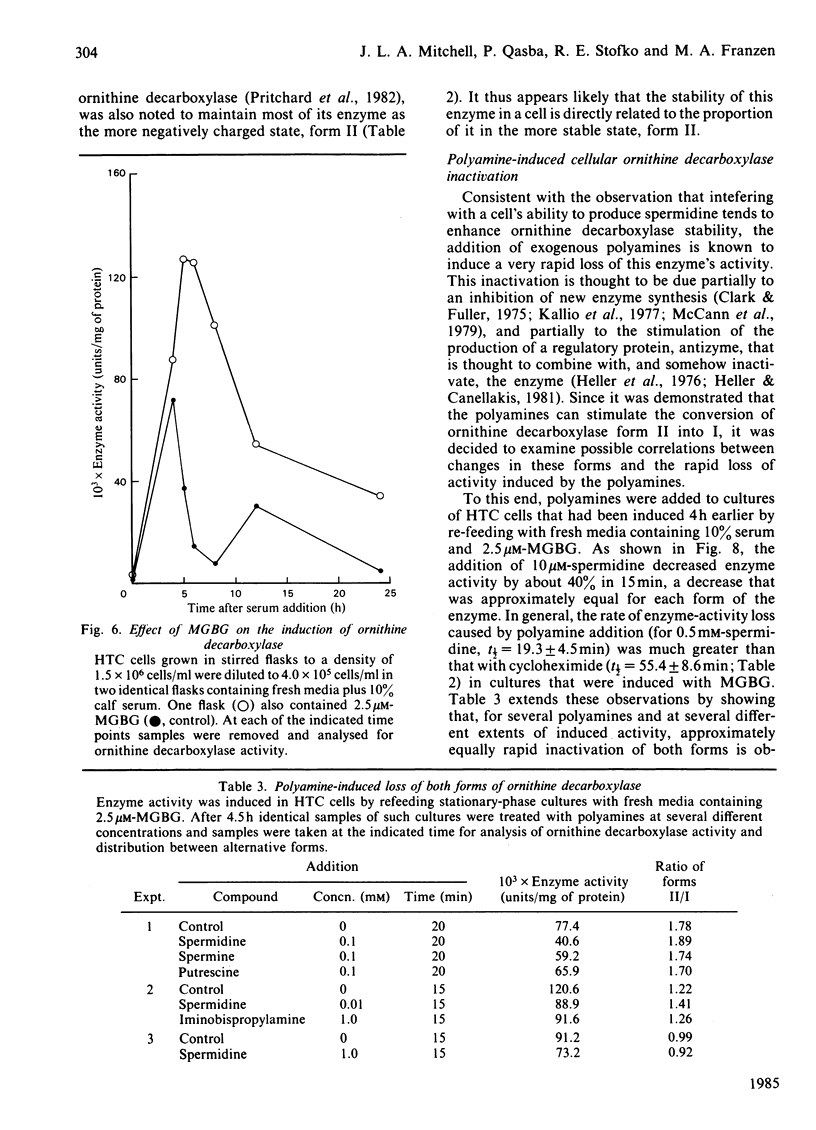
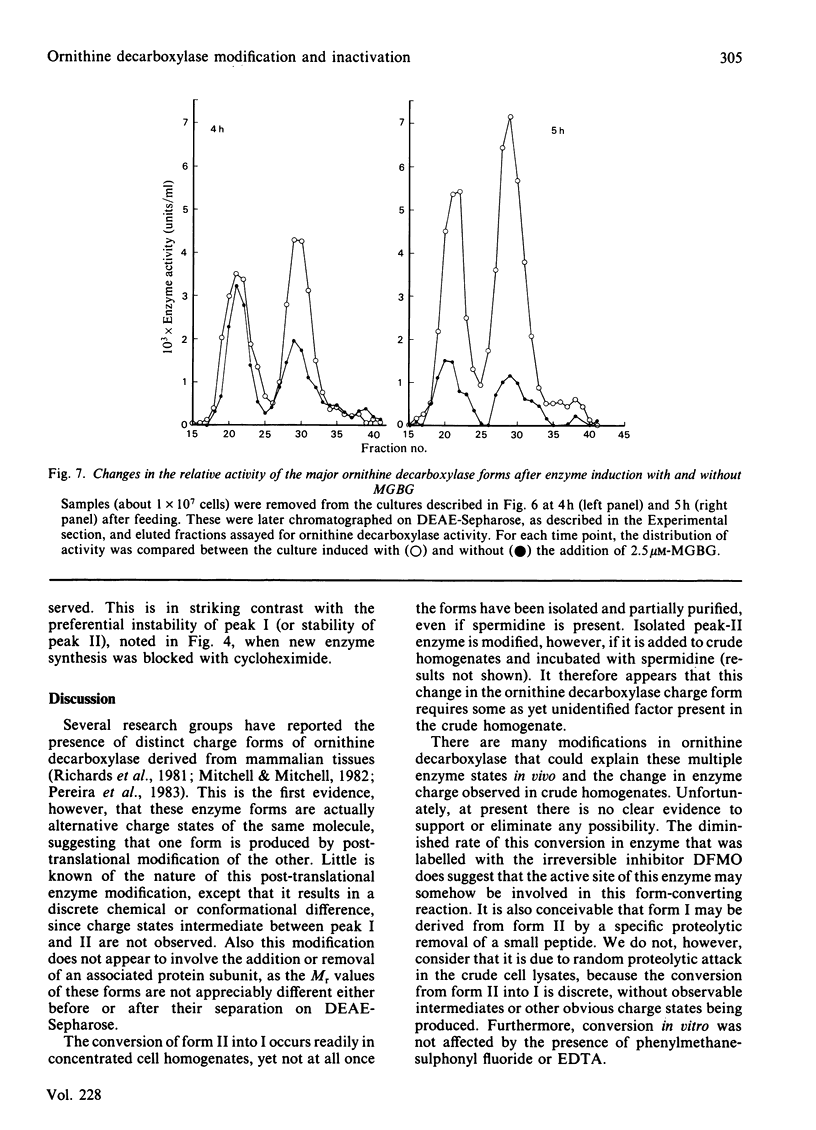
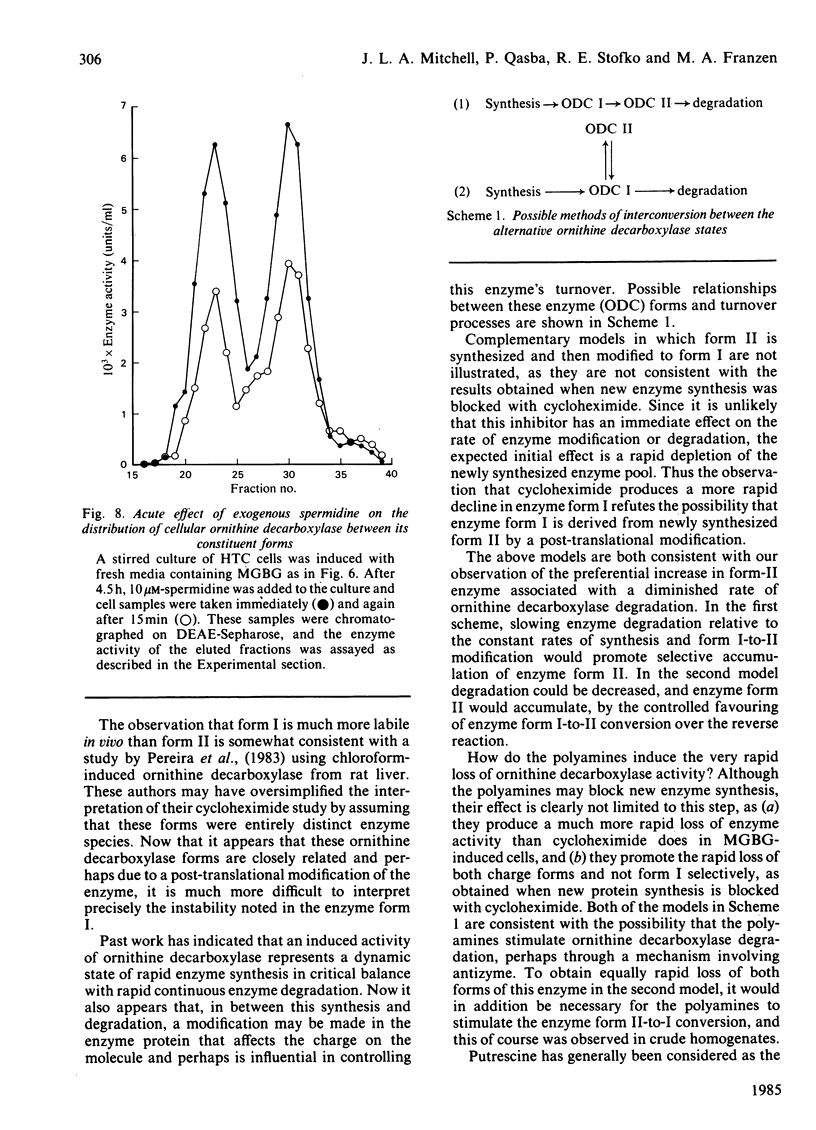
Ornithine decarboxylase isolated from HTC cells was separated into two distinct charged states by salt-gradient elution from DEAE-Sepharose columns. This charge difference between the enzyme forms was maintained in partially purified preparations, but enzyme form II was observed to change to form I in a time-dependent polyamine-stimulated fashion in crude cell homogenates. The enzyme modification that produces this charge diversity between the alternative enzyme states was further investigated for its role in enzyme activity induction, protein stability and rapid turnover. Inhibition of new protein synthesis by cycloheximide resulted in a much more rapid loss of form I enzyme than of form II, suggesting that during normal enzyme turnover the latter enzyme state may be derived from the former. Culture conditions that favour the stabilization of this usually labile enzyme generally induced an increased proportion of the enzyme in the form II charge state. In particular, inhibitors of synthesis of spermidine and spermine induced the stabilization of cellular ornithine decarboxylase and promoted a marked accumulation in form II. Conversely, polyamines added to the cells in culture induced a very rapid loss in both forms of the enzyme, an effect that could not be attributed merely to an inhibition of new enzyme synthesis. It appears that the polyamines, but not putrescine, may be an essential part of the rapid ornithine decarboxylase inactivation process and that they may function in part by stimulating the conversion of the more stable enzyme form II into the less stable enzyme state, form I.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atmar V. J., Kuehn G. D. Phosphorylation of ornithine decarboxylase by a polyamine-dependent protein kinase. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5518–5522. doi: 10.1073/pnas.78.9.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger F. G., Szymanski P., Read E., Watson G. Androgen-regulated ornithine decarboxylase mRNAs of mouse kidney. J Biol Chem. 1984 Jun 25;259(12):7941–7946. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Clark J. L., Fuller J. L. Regulation of ornithine decarboxylase in 3T3 cells by putrescine and spermidine: indirect evidence for translational control. Biochemistry. 1975 Oct 7;14(20):4403–4409. doi: 10.1021/bi00691a010. [DOI] [PubMed] [Google Scholar]
- Erwin B. G., Seely J. E., Pegg A. E. Mechanism of stimulation of ornithine decarboxylase activity in transformed mouse fibroblasts. Biochemistry. 1983 Jun 7;22(12):3027–3032. doi: 10.1021/bi00281a037. [DOI] [PubMed] [Google Scholar]
- Heby O., Sauter S., Russell D. H. Stimulation of ornithine decarboxylase activity and inhibition of S-adenosyl-L-methionine decarboxylase activity in leukaemic mice by methylglyoxal bis(guanylhydrazone). Biochem J. 1973 Dec;136(4):1121–1124. doi: 10.1042/bj1361121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller J. S., Canellakis E. S. Cellular control of ornithine decarboxylase activity by its antizyme. J Cell Physiol. 1981 May;107(2):209–217. doi: 10.1002/jcp.1041070206. [DOI] [PubMed] [Google Scholar]
- Heller J. S., Fong W. F., Canellakis E. S. Induction of a protein inhibitor to ornithine decarboxylase by the end products of its reaction. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1858–1862. doi: 10.1073/pnas.73.6.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibasami H., Tanaka M., Nagai J., Ikeda T. Dicyclohexylamine, a potent inhibitor of spermidine synthase in mammalian cells. FEBS Lett. 1980 Jul 11;116(1):99–101. doi: 10.1016/0014-5793(80)80537-0. [DOI] [PubMed] [Google Scholar]
- Hogan B. L., McIlhinney A., Murden S. Effect of growth conditions on the activity of ornithine decarboxylase in cultured hepatoma cells. II. Effect of serum and insulin. J Cell Physiol. 1974 Jun;83(3):353–357. doi: 10.1002/jcp.1040830305. [DOI] [PubMed] [Google Scholar]
- Kallio A., Löfman M., Pösö H., Jänne J. Inhibition of ornithine decarboxylase by diamines in regenerating rat liver. Evidence for direct action on the accumulation of the enzyme protein. FEBS Lett. 1977 Jul 1;79(1):195–199. [PubMed] [Google Scholar]
- Mamont P. S., Böhlen P., McCann P. P., Bey P., Schuber F., Tardif C. Alpha-methyl ornithine, a potent competitive inhibitor of ornithine decarboxylase, blocks proliferation of rat hepatoma cells in culture. Proc Natl Acad Sci U S A. 1976 May;73(5):1626–1630. doi: 10.1073/pnas.73.5.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann P. P., Tardif C., Duchesne M. C., Mamont P. S. Effect of alpha-methyl ornithine on ornithine decarboxylase activity of rat hepatoma cells in culture. Biochem Biophys Res Commun. 1977 Jun 6;76(3):893–899. doi: 10.1016/0006-291x(77)91585-6. [DOI] [PubMed] [Google Scholar]
- McCann P. P., Tardif C., Hornsperger J. M., Böhlen P. Two distinct mechanisms for ornithine decarboxylase regulation by polyamines in rat hepatoma cells. J Cell Physiol. 1979 May;99(2):183–190. doi: 10.1002/jcp.1040990204. [DOI] [PubMed] [Google Scholar]
- McConlogue L., Coffino P. Ornithine decarboxylase in difluoromethylornithine-resistant mouse lymphoma cells. Two-dimensional gel analysis of synthesis and turnover. J Biol Chem. 1983 Jul 10;258(13):8384–8388. [PubMed] [Google Scholar]
- Mitchell J. L., Mitchell G. K., Carter D. D. Amine-specificity of the inactivating ornithine decarboxylase modification in Physarum polycephalum. Biochem J. 1982 Sep 1;205(3):551–557. doi: 10.1042/bj2050551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. L., Mitchell G. K. Ornithine decarboxylase protein diversity and activity modulation in HTC cells. Biochem Biophys Res Commun. 1982 Apr 14;105(3):1189–1197. doi: 10.1016/0006-291x(82)91095-6. [DOI] [PubMed] [Google Scholar]
- Pereira M. A., Savage R. E., Jr, Guion C. Induction by chloroform of two forms of ornithine decarboxylase in rat liver. Half-life of isozymes. Biochem Pharmacol. 1983 Sep 1;32(17):2511–2514. doi: 10.1016/0006-2952(83)90011-4. [DOI] [PubMed] [Google Scholar]
- Pritchard M. L., Pegg A. E., Jefferson L. S. Ornithine decarboxylase from hepatoma cells and a variant cell line in which the enzyme is more stable. J Biol Chem. 1982 May 25;257(10):5892–5899. [PubMed] [Google Scholar]
- Pritchard M. L., Seely J. E., Pösö H., Jefferson L. S., Pegg A. E. Binding of radioactive alpha-difluoromethylornithine to rat liver ornithine decarboxylase. Biochem Biophys Res Commun. 1981 Jun;100(4):1597–1603. doi: 10.1016/0006-291x(81)90701-4. [DOI] [PubMed] [Google Scholar]
- Prouty W. F. Ornithine decarboxylase inactivation in HeLa cells. J Cell Physiol. 1976 Sep;89(1):65–76. doi: 10.1002/jcp.1040890107. [DOI] [PubMed] [Google Scholar]
- Richards J. F., Lit K., Fuca R., Bourgeault C. Multiple species of ornithine decarboxylase in rat tissues: effects of dexamethasone. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1461–1467. doi: 10.1016/0006-291x(81)90783-x. [DOI] [PubMed] [Google Scholar]
- Russell D. H. Posttranslational modification of ornithine decarboxylase by its product putrescine. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1167–1172. doi: 10.1016/0006-291x(81)90741-5. [DOI] [PubMed] [Google Scholar]
- Russell D., Snyder S. H. Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1420–1427. doi: 10.1073/pnas.60.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely J. E., Pegg A. E. Changes in mouse kidney ornithine decarboxylase activity are brought about by changes in the amount of enzyme protein as measured by radioimmunoassay. J Biol Chem. 1983 Feb 25;258(4):2496–2500. [PubMed] [Google Scholar]
- Seely J. E., Pösö H., Pegg A. E. Effect of androgens on turnover of ornithine decarboxylase in mouse kidney. Studies using labeling of the enzyme by reaction with [14C] alpha-difluoromethylornithine. J Biol Chem. 1982 Jul 10;257(13):7549–7553. [PubMed] [Google Scholar]


