Abstract
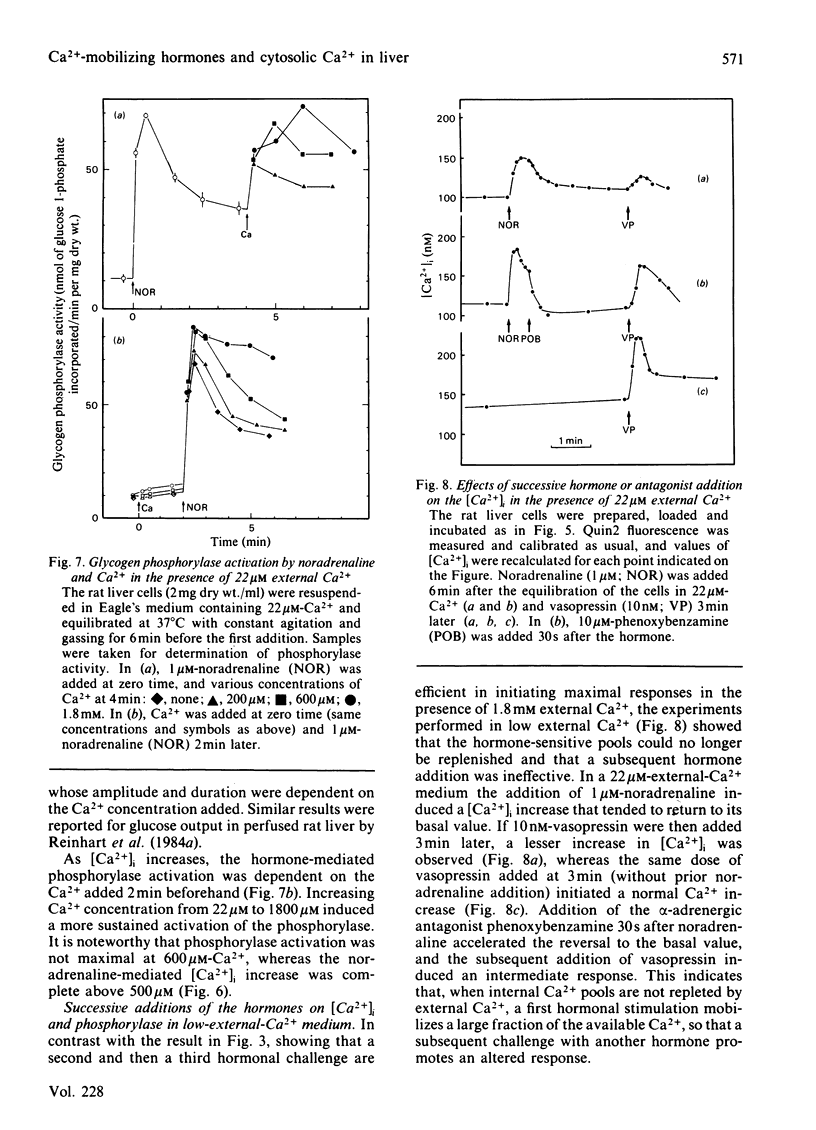
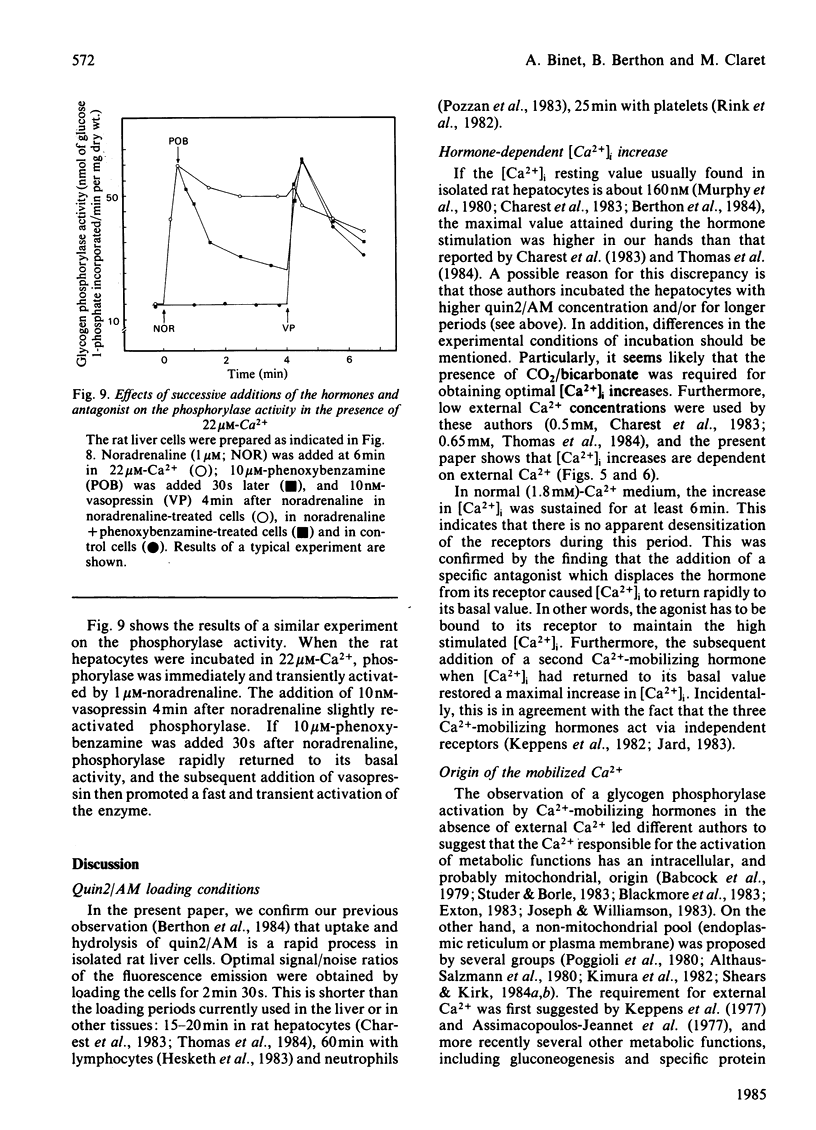
The action of alpha 1-adrenergic agonists (noradrenaline in the presence of propranolol), vasopressin and angiotensin on the intracellular free Ca2+ concentration, [Ca2+]i, was determined by using the fluorescent dye quin2 in isolated rat liver cells. In the presence of external Ca2+ (1.8 mM), 1 microM-noradrenaline induced an increase in [Ca2+]i up to about 800 nM without apparent delay, whereas 10 nM-vasopressin and 1 nM-angiotensin increased [Ca2+]i to values higher than 1500 nM with a lag period of about 6s. The successive addition of the hormones and of their specific antagonists indicated that the actions of the three Ca2+-mobilizing hormones occurred without apparent desensitization (over 6 min) and via independent receptors. The relative contributions of internal and external Ca2+ pools to the cell response were determined by studying the hormone-mediated [Ca2+]i increase and glycogen phosphorylase activation in low-Ca2+ media (22 microM). In this medium: (1) [Ca2+]i was lowered and the hormones initiated a transient instead of a sustained increase in [Ca2+]i; subsequent addition (2 min) of a second hormone promoted a lesser increase in [Ca2+]i; in contrast, the subsequent addition (2 min) of Ca2+ (1.8 mM) caused [Ca2+]i to increase to a value close to that initiated by the hormone in control conditions, the amplitude of the latter response being dependent on the concentration of Ca2+ added to the medium; (2) returning to normal Ca2+ (1.8 mM) restored the resting [Ca2+]i and allowed the hormone added 2 min later to promote a large increase in [Ca2+]i whose final amplitude was also dependent on the concentration of Ca2+ added beforehand. Similar results were found when the same protocol was applied to the glycogen phosphorylase activation. It is concluded that Ca2+ influx is required for a maximal and sustained response and to reload the hormone-sensitive stores.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Althaus-Salzmann M., Carafoli E., Jakob A. Ca2+, K+ redistributions and alpha-adrenergic activation of glycogenolysis in perfused rat livers. Eur J Biochem. 1980 May;106(1):241–248. doi: 10.1111/j.1432-1033.1980.tb06015.x. [DOI] [PubMed] [Google Scholar]
- Assimacopoulos-Jeannet F. D., Blackmore P. F., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. Studies on role of calcium in alpha-adrenergic activation of phosphorylase. J Biol Chem. 1977 Apr 25;252(8):2662–2669. [PubMed] [Google Scholar]
- Assimacopoulos-Jeannet F., McCormack J. G., Jeanrenaud B. Effect of phenylephrine on pyruvate dehydrogenase activity in rat hepatocytes and its interaction with insulin and glucagon. FEBS Lett. 1983 Aug 8;159(1-2):83–88. doi: 10.1016/0014-5793(83)80421-9. [DOI] [PubMed] [Google Scholar]
- Babcock D. F., Chen J. L., Yip B. P., Lardy H. A. Evidence for mitochondrial localization of the hormone-responsive pool of Ca2+ in isolated hepatocytes. J Biol Chem. 1979 Sep 10;254(17):8117–8120. [PubMed] [Google Scholar]
- Barritt G. J., Parker J. C., Wadsworth J. C. A kinetic analysis of the effects of adrenaline on calcium distribution in isolated rat liver parenchymal cells. J Physiol. 1981 Mar;312:29–55. doi: 10.1113/jphysiol.1981.sp013614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthon B., Binet A., Mauger J. P., Claret M. Cytosolic free Ca2+ in isolated rat hepatocytes as measured by quin2. Effects of noradrenaline and vasopressin. FEBS Lett. 1984 Feb 13;167(1):19–24. doi: 10.1016/0014-5793(84)80824-8. [DOI] [PubMed] [Google Scholar]
- Binet A., Claret M. alpha-adrenergic stimulation of respiration in isolated rat hepatocytes. Biochem J. 1983 Mar 15;210(3):867–873. doi: 10.1042/bj2100867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore P. F., Hughes B. P., Charest R., Shuman E. A., 4th, Exton J. H. Time course of alpha1-adrenergic and vasopressin actions on phosphorylase activation, calcium efflux, pyridine nucleotide reduction, and respiration in hepatocytes. J Biol Chem. 1983 Sep 10;258(17):10488–10494. [PubMed] [Google Scholar]
- Blackmore P. F., Hughes B. P., Shuman E. A., Exton J. H. alpha-Adrenergic activation of phosphorylase in liver cells involves mobilization of intracellular calcium without influx of extracellular calcium. J Biol Chem. 1982 Jan 10;257(1):190–197. [PubMed] [Google Scholar]
- Burgess G. M., Claret M., Jenkinson D. H. Effects of quinine and apamin on the calcium-dependent potassium permeability of mammalian hepatocytes and red cells. J Physiol. 1981 Aug;317:67–90. doi: 10.1113/jphysiol.1981.sp013814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess G. M., Godfrey P. P., McKinney J. S., Berridge M. J., Irvine R. F., Putney J. W., Jr The second messenger linking receptor activation to internal Ca release in liver. Nature. 1984 May 3;309(5963):63–66. doi: 10.1038/309063a0. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Fabiato A., Leslie B. A., Putney J. W., Jr Calcium pools in saponin-permeabilized guinea pig hepatocytes. J Biol Chem. 1983 Dec 25;258(24):15336–15345. [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Berthon B., Exton J. H. Changes in free cytosolic Ca2+ in hepatocytes following alpha 1-adrenergic stimulation. Studies on Quin-2-loaded hepatocytes. J Biol Chem. 1983 Jul 25;258(14):8769–8773. [PubMed] [Google Scholar]
- Chen J. L., Babcock D. F., Lardy H. A. Norepinephrine, vasopressin, glucagon, and A23187 induce efflux of calcium from an exchangeable pool in isolated rat hepatocytes. Proc Natl Acad Sci U S A. 1978 May;75(5):2234–2238. doi: 10.1073/pnas.75.5.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claret M., Binet A. Mécanismes d'action des hormones mobilisant le calcium dans les hépatocytes de Mammifères. J Physiol (Paris) 1984;79(2):120–128. [PubMed] [Google Scholar]
- Dawson A. P., Irvine R. F. Inositol (1,4,5)trisphosphate-promoted Ca2+ release from microsomal fractions of rat liver. Biochem Biophys Res Commun. 1984 May 16;120(3):858–864. doi: 10.1016/s0006-291x(84)80186-2. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Molecular mechanisms involved in alpha-adrenergic responses. Mol Cell Endocrinol. 1981 Sep;23(3):233–264. doi: 10.1016/0303-7207(81)90123-4. [DOI] [PubMed] [Google Scholar]
- Garrison J. C., Borland M. K., Florio V. A., Twible D. A. The role of calcium ion as a mediator of the effects of angiotensin II, catecholamines, and vasopressin on the phosphorylation and activity of enzymes in isolated hepatocytes. J Biol Chem. 1979 Aug 10;254(15):7147–7156. [PubMed] [Google Scholar]
- Hesketh T. R., Smith G. A., Moore J. P., Taylor M. V., Metcalfe J. C. Free cytoplasmic calcium concentration and the mitogenic stimulation of lymphocytes. J Biol Chem. 1983 Apr 25;258(8):4876–4882. [PubMed] [Google Scholar]
- Joseph S. K., Williamson J. R. The origin, quantitation, and kinetics of intracellular calcium mobilization by vasopressin and phenylephrine in hepatocytes. J Biol Chem. 1983 Sep 10;258(17):10425–10432. [PubMed] [Google Scholar]
- Keppens S., De Wulf H., Clauser P., Jard S., Morgat J. L. The liver angiotensin receptor involved in the activation of glycogen phosphorylase. Biochem J. 1982 Dec 15;208(3):809–817. doi: 10.1042/bj2080809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppens S., Vandenheede J. R., De Wulf H. On the role of calcium as second messenger in liver for the hormonally induced activation of glycogen phosphorylase. Biochim Biophys Acta. 1977 Feb 28;496(2):448–457. doi: 10.1016/0304-4165(77)90327-0. [DOI] [PubMed] [Google Scholar]
- Kimura S., Kugai N., Tada R., Kojima I., Abe K., Ogata E. Sources of calcium mobilized by alpha-adrenergic stimulation in perfused rat liver. Horm Metab Res. 1982 Mar;14(3):133–138. doi: 10.1055/s-2007-1018947. [DOI] [PubMed] [Google Scholar]
- Kneer N. M., Lardy H. A. Regulation of gluconeogenesis by norepinephrine, vasopressin, and angiotensin II: a comparative study in the absence and presence of extracellular Ca2+1. Arch Biochem Biophys. 1983 Aug;225(1):187–195. doi: 10.1016/0003-9861(83)90022-x. [DOI] [PubMed] [Google Scholar]
- Mauger J. P., Poggioli J., Guesdon F., Claret M. Noradrenaline, vasopressin and angiotensin increase Ca2+ influx by opening a common pool of Ca2+ channels in isolated rat liver cells. Biochem J. 1984 Jul 1;221(1):121–127. doi: 10.1042/bj2210121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. The activation of pyruvate dehydrogenase in the perfused rat heart by adrenaline and other inotropic agents. Biochem J. 1981 Feb 15;194(2):639–643. doi: 10.1042/bj1940639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E., Coll K., Rich T. L., Williamson J. R. Hormonal effects on calcium homeostasis in isolated hepatocytes. J Biol Chem. 1980 Jul 25;255(14):6600–6608. [PubMed] [Google Scholar]
- Poggioli J., Berthon B., Claret M. Calcium movements in in situ mitochondria following activation of alpha-adrenergic receptors in rat liver cells. FEBS Lett. 1980 Jun 30;115(2):243–246. doi: 10.1016/0014-5793(80)81178-1. [DOI] [PubMed] [Google Scholar]
- Poggioli J., Mauger J. P., Guesdon F., Claret M. A regulatory calcium-binding site for calcium channel in isolated rat hepatocytes. J Biol Chem. 1985 Mar 25;260(6):3289–3294. [PubMed] [Google Scholar]
- Pozzan T., Lew D. P., Wollheim C. B., Tsien R. Y. Is cytosolic ionized calcium regulating neutrophil activation? Science. 1983 Sep 30;221(4618):1413–1415. doi: 10.1126/science.6310757. [DOI] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. Calcium ion fluxes induced by the action of alpha-adrenergic agonists in perfused rat liver. Biochem J. 1982 Dec 15;208(3):619–630. doi: 10.1042/bj2080619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. The contribution of both extracellular and intracellular calcium to the action of alpha-adrenergic agonists in perfused rat liver. Biochem J. 1984 May 15;220(1):35–42. doi: 10.1042/bj2200035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. The role of calcium ions in the mechanism of action of alpha-adrenergic agonists in rat liver. Biochem J. 1984 Oct 1;223(1):1–13. doi: 10.1042/bj2230001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink T. J., Smith S. W., Tsien R. Y. Cytoplasmic free Ca2+ in human platelets: Ca2+ thresholds and Ca-independent activation for shape-change and secretion. FEBS Lett. 1982 Nov 1;148(1):21–26. doi: 10.1016/0014-5793(82)81234-9. [DOI] [PubMed] [Google Scholar]
- Shears S. B., Kirk C. J. Determination of mitochondrial calcium content in hepatocytes by a rapid cellular fractionation technique. Vasopressin stimulates mitochondrial Ca2+ uptake. Biochem J. 1984 Jun 1;220(2):417–421. doi: 10.1042/bj2200417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears S. B., Kirk C. J. Determination of mitochondrial calcium content in hepatocytes by a rapid cellular-fractionation technique. Alpha-adrenergic agonists do not mobilize mitochondrial Ca2+. Biochem J. 1984 Apr 15;219(2):383–389. doi: 10.1042/bj2190383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer R. K., Borle A. B. Differences between male and female rats in the regulation of hepatic glycogenolysis. The relative role of calcium and cAMP in phosphorylase activation by catecholamines. J Biol Chem. 1982 Jul 25;257(14):7987–7993. [PubMed] [Google Scholar]
- Studer R. K., Borle A. B. Sex difference in cellular calcium metabolism of rat hepatocytes and in alpha-adrenergic activation of glycogen phosphorylase. Biochim Biophys Acta. 1983 Apr 5;762(2):302–314. doi: 10.1016/0167-4889(83)90085-x. [DOI] [PubMed] [Google Scholar]
- Taylor W. M., Reinhart P. H., Bygrave F. L. Stimulation by alpha-adrenergic agonists of Ca2+ fluxes, mitochondrial oxidation and gluconeogenesis in perfused rat liver. Biochem J. 1983 Jun 15;212(3):555–565. doi: 10.1042/bj2120555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A. P., Alexander J., Williamson J. R. Relationship between inositol polyphosphate production and the increase of cytosolic free Ca2+ induced by vasopressin in isolated hepatocytes. J Biol Chem. 1984 May 10;259(9):5574–5584. [PubMed] [Google Scholar]
- Tsien R. Y. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature. 1981 Apr 9;290(5806):527–528. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Putney J. W., Jr Does calcium mediate the increase in potassium permeability due to phenylephrine or angiotensin II in the liver? J Pharmacol Exp Ther. 1978 Dec;207(3):669–676. [PubMed] [Google Scholar]


