Abstract
Purpose
Wheezing in early life is most frequently caused by viral lower respiratory tract illnesses, constituting a significant disease burden in children. This study aimed to investigate the association of wheezing in early life with autoimmune diseases throughout childhood.
Methods
A population-matched retrospective cohort study was conducted in Korea between 2002 and 2017. The cohort comprised 34,959 children admitted with viral wheezing before 2 years of age and an equal number of the matched unexposed children born in 2002 and 2003. Exposed infants were defined as those hospitalized for bronchiolitis or bronchial asthma before the age of 2. Unexposed controls were matched by sex and birth year at a 1:1 ratio, using incidence density sampling. A Cox proportional hazard model controlled for multiple risk factors was employed.
Results
The median age at hospitalization for wheeze was 9 months (interquartile range, 5–15 months), and 63% of the exposed infants were male. Over the mean 15-year follow-up period, the incidence rate of autoimmune diseases was 74.0 and 62.2 per 10,000 person-years in the exposed and matched unexposed cohorts, respectively. The adjusted hazard ratio for any autoimmune disease in the exposed cohort was 1.15 (95% confidence interval, 1.09–1.23) in comparison with the unexposed cohort. The exposed cohort revealed an augmented risk for specific autoimmune diseases, including juvenile idiopathic arthritis, Kawasaki disease, Henoch-Schönlein purpura, psoriasis, idiopathic thrombocytopenic purpura, and immunoglobulin A nephropathy. Risks were heightened for children with multiple wheezing episodes or a persistent wheezing episode after the age of 2 years.
Conclusions
This research identifies associations between early-life wheeze and the development of autoimmune diseases in childhood. Understanding these relationships can aid in recognizing the underlying pathophysiology of early-life wheeze and childhood autoimmune diseases, contributing to management strategies for these conditions.
Keywords: Asthma, autoimmune disease, bronchiolitis, children
INTRODUCTION
Lower respiratory tract illness in infants carries a significant disease burden due to its high incidence, morbidity, and impact on long-term health outcomes.1 Respiratory viruses are the most prevalent cause of lower respiratory tract illness in early life,2 and some infants with viral lower respiratory tract illness may present with wheeze, a symptom reflecting airway inflammation coupled with either airway obstruction or hyper-responsiveness.3,4,5 Particularly, more severe lower respiratory tract illnesses in infancy are often accompanied by wheezing and may lead to increased morbidity throughout childhood.6
Previous studies have explored the relationship between viral wheeze in early life and respiratory health in later stages, including its potential connection to childhood asthma.7 However, the research on how viral wheeze in early life influences life-long health outcomes is limited. Autoimmune diseases are generally characterized by Th1-promoted cellular immunity, while some phenotypes of viral wheeze are marked by Th2-mediated humoral immunity.8,9 Historically, these 2 disease categories were considered to possess mutually exclusive pathophysiology.10 Some research has suggested potential links between asthma in children or adults and autoimmune diseases.11,12,13 However, these findings are inconclusive, and the shared mechanisms between these disease categories remain unclear. In addition, no studies have specifically explored the relationship between viral wheeze in early life and autoimmune diseases during childhood.
In this study, we hypothesized that viral wheeze requiring hospitalization in early life could be linked to the subsequent development of autoimmune diseases. To test our hypothesis, we investigated the association between viral wheeze requiring hospitalization before 2 years of age and the development of autoimmune diseases during childhood, utilizing data from a national health insurance system database.
MATERIALS AND METHODS
Study population and data source
This study is a longitudinal observational and administrative cohort analysis. Utilizing a database procured from the National Health Insurance Service (NHIS) in conjunction with the mortality report of Statistics in Korea, a cohort comprising 997,154 children born between 2002 and 2003 in South Korea was followed from birth to December 2017. NHIS functions as a singular compulsory health insurance system in Korea, extending medical insurance benefits to virtually all residents. The NHIS database encompasses demographic data, such as gender, socioeconomic status, and residential area, as well as clinical information on medical usages. This includes International Classification of Diseases 10th revision (ICD-10) codes, prescription codes, and procedure codes relevant to hospitalizations, outpatient visits, and emergency room visits.
Exposed cohort
An exposed cohort was assembled, encompassing all children experiencing a wheezing episode. The definition of wheezing episode was specified as hospitalization for the principal diagnosis of acute bronchiolitis (ICD-10 code J21.X) or asthma (ICD-10 codes J45.X or J46.X) prior to 2 years of age, including those required intensive care unit treatment. The index date referred to the date of the first diagnosis of a wheezing episode. Moreover, children diagnosed with autoimmune diseases before the index date were excluded (Fig. 1), resulting in a total of 34,959 children being allocated to the exposed cohort. The diagnosis of a wheezing episode was found to consist of 44.5% asthma and 55.5% acute bronchiolitis (Supplementary Table S1).
Fig. 1. Study population.
The study included individuals born in South Korea in 2002 and 2003, using data from the National Health Insurance Service (NHIS) and supplementary mortality information provided by Statistics Korea. Participants who were hospitalized with a primary diagnosis of acute bronchiolitis (ICD-10 code J21.X) or asthma (ICD-10 code J45.X or J46.X) before the age of 2 were categorized into the exposed group. Those without such hospitalizations were allocated to the unexposed group. The index date was defined as the date of the first wheezing episode. From the exposed group, children previously diagnosed with autoimmune diseases prior to the index date (n = 458) were excluded. Using 1:1 incidence density sampling, 34,959 children were allocated to both the exposed and matched unexposed groups.
ICD-10, International Classification of Diseases 10th revision.
Matched unexposed cohort
Children who had never experienced a wheezing episode were assigned to an unexposed cohort. To reduce the significant numerical discrepancy between the exposed and unexposed groups, we implemented a 1:1 incidence density matching. Each exposed individual was paired with a randomly selected counterpart from the unexposed group, matched according to gender and birth year. The index date for participants in the unexposed group was randomly set to correspond with that of the matched individual in the exposed group. Subsequently, these matched individuals in the unexposed group were followed to determine whether they experienced wheezing episodes in the first 2 years of life; if so, they were reclassified into the exposed group. Follow-up for all participants extended from the index date to the first diagnosis of an autoimmune disease, death, or the end of the study period (December 31, 2017). To mitigate the risk of inverse causality or surveillance bias, occurrences of autoimmune diseases during the first year of follow-up were excluded from analysis.
Primary outcome
The outcome of interest was the diagnosis of pre-specified 41 autoimmune diseases, identified using ICD-10 codes (Supplementary Table S2). Any autoimmune diseases were defined as diagnosis of one or more preselected autoimmune diseases.
Covariates
Demographic covariates included gender, calendar year of birth, season of birth, residence area at the time of birth, and socioeconomic status. The residence areas at birth were classified into specific categories: Seoul, metropolitan regions, cities, and rural areas. Socioeconomic status was assessed using health insurance premiums and was divided into 2 categories based on the median value. Furthermore, information on perinatal diseases was obtained from the NHIS database. This encompassed disorders related to length of gestation and fetal growth (ICD-10 codes, P05.X to P08.X), birth trauma (ICD-10 codes, P10.X to P15.X), infections specific to the perinatal period (ICD-10 codes, P35.X to P39.X), hemorrhagic disorders of the fetus and newborn (ICD-10 codes, P50.X to T61.X), and congenital malformations and deformations (ICD-10 codes, Q00.X to Q89.X).
Statistical analysis
After assessing the proportional hazards assumption by conducting both the proportional test and the Schönfeld test, we evaluated the risk of developing autoimmune diseases in the exposed group using a Cox proportional hazards model. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated for the subsequent development of any autoimmune diseases in children who experienced viral wheeze before the age of 2 years. These calculations were performed using time after the index date as the underlying time scale. Additionally, we analyzed each association between atopic dermatitis and 41 specific autoimmune diseases. All analyses were controlled for variables, such as age and season at the index date, socioeconomic status, residential area at birth, and perinatal diseases.
Subgroup analyses were conducted according to gender, age group at index (≤ 6 months vs. 7–24 months), calendar year of birth (2002 vs. 2003), residence at birth (Seoul/metropolitans and cities vs. rural), socioeconomic status (< median vs. ≥ median), season at the index date (spring to early autumn vs. late autumn to winter), or perinatal status (yes vs. no). The differences in HRs were assessed by introducing an interaction term into the Cox model. We also investigated how the clinical characteristics of a wheezing episode before 2 years of age might influence the outcomes. The risk of development of autoimmune diseases was analyzed on the basis of various exposure characteristics, such as the diagnosis of the wheezing episode, the frequency of the wheezing episode, and the hospitalization duration of each wheezing episode.
To enhance the robustness of the results, we analyzed the associations of autoimmune diseases with acute bronchiolitis or asthma independently, using the same statistical methods as the main analysis. Additionally, to minimize the potential for reverse causality, we repeated the analyses, excluding the first 2, 3, and 5 years after study entry. Two-tailed p-values of less than 0.01 were considered statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Ethics statement
The utilization of de-identified individual data for research was sanctioned under the prevailing National Health Insurance Act. This research was conducted with ethical clearance under the current National Health Insurance Act. This retrospective study was performed in accordance with the relevant guidelines and regulations. The study protocol was reviewed and approved by the Institutional Review Board of the Korea National Institute for Bioethics Policy (P01-201603-21-005). Patient consents were not required as this study was based on de-identified and publicly available data. The need for informed consent was waived the Institutional Review Board of the Korea National Institute for Bioethics Policy.
RESULTS
Baseline characteristics of study population
In the population-matched cohort, a total of 34,959 individuals were allocated to both the exposed and matched unexposed groups. Table 1 presents the baseline characteristics of these groups. In each group, boys constituted 63.2% of the individuals. The median age at the index date was 9 months (interquartile range, 5 to 15 months), with 37.3% of children experiencing a wheezing episode before 6 months, and 62.7% of children experiencing it between 7 and 24 months. The mean duration of follow-up was 178.5 months (standard deviation [SD], 9.7 months) for the exposed group and 179.2 months (SD, 11.5 months) for the matched unexposed group. The socioeconomic status did not differ significantly between the exposed and matched unexposed groups. Moreover, higher proportions of children in the exposed group were hospitalized due to lower respiratory tract infections (such as wheezing episodes or pneumonia) in the year following the index date.
Table 1. Characteristics of the study cohorts.
| Characteristics | Population-matched cohort | ||
|---|---|---|---|
| Exposed group* (n = 34,959) | Matched unexposed group† (n = 34,959) | ||
| Follow-up time, mon | 178.54 ± 9.65 | 179.16 ± 11.47 | |
| Gender | |||
| Boy | 22,083 (63.17) | 22,083 (63.17) | |
| Girl | 12,876 (36.83) | 12,876 (36.83) | |
| Age at index date,‡ median (IQR), mon | 9.0 (5–15) | 9.0 (5–15) | |
| Age group at index date,‡ mon | |||
| ≤ 6 | 13,031 (37.28) | 13,339 (38.16) | |
| 7–24 | 21,928 (62.72) | 21,620 (61.84) | |
| Residence area at birth | |||
| Seoul/metropolitan | 18,304 (52.36) | 16,997 (48.62) | |
| City | 13,043 (37.31) | 14,246 (40.75) | |
| Rural | 3,597 (10.29) | 3,716 (10.63) | |
| Missing | 15 (0.04) | 0 (0.00) | |
| Socioeconomic status§ | |||
| < Median | 14,930 (42.71) | 14,941 (42.74) | |
| ≥ Median | 19,235 (55.02) | 18,800 (53.78) | |
| Missing | 794 (2.27) | 1,218 (3.48) | |
| Year of birth | |||
| 2002 | 16,731 (47.86) | 16,731 (47.86) | |
| 2003 | 18,228 (52.14) | 18,228 (52.14) | |
| Hospitalization due to lower respiratory infections for the first year after the index date∥ | |||
| None | 27,338 (78.20) | 33,188 (94.93) | |
| More than once to treat wheezing episode | 4,205 (12.03) | 569 (1.63) | |
| More than once to treat pneumonia | 4,301 (12.30) | 1,308 (3.74) | |
| Number of outpatient visits for the first year after the index date∥ | |||
| < 10/year¶ | 16,740 (47.90) | 16,052 (45.90) | |
| ≥ 10/year¶ | 18,219 (52.10) | 28,907 (54.10) | |
Values are presented as mean ± standard deviation or number (%).
IQR, interquartile range; ICD-10, International Classification of Diseases 10th revision.
*The exposed group comprises children hospitalized for bronchiolitis or asthma, identified by principal diagnosis codes of acute bronchiolitis (ICD-10 code J21.X) or asthma (ICD-10 codes J45.X or J46.X) before reaching 2 years of age.
†The matched unexposed group includes children without a wheezing episode and is created using incidence density matching at a 1:1 ratio with the exposed subjects, matching for gender.
‡The index date refers to the initial occurrence of a wheezing episode for exposed subjects; for unexposed subjects, the index date is randomly assigned to coincide with the index date of a corresponding exposed subject.
§Socioeconomic status was gauged using health insurance premiums as a proxy.
∥Data from the first month following the index date are excluded, as the exposed subjects utilized more healthcare services during this period.
¶Values are determined based on the mean value.
Clinical characteristics are detailed in Supplementary Table S1. A higher proportion of children diagnosed with perinatal diseases was observed in the exposed group, compared to the matched unexposed group (13,478 [38.6%] vs. 10,490 [30.0%]). The proportion of children with atopic dermatitis before 2 years of age was similar in both groups. The numbers of children diagnosed with wheezing episodes after 2 years of age were 3,867 (11.1%) in the exposed group and 1,200 (3.4%) in the matched unexposed group.
Associations between viral wheeze before age 2 and subsequent development of autoimmune diseases
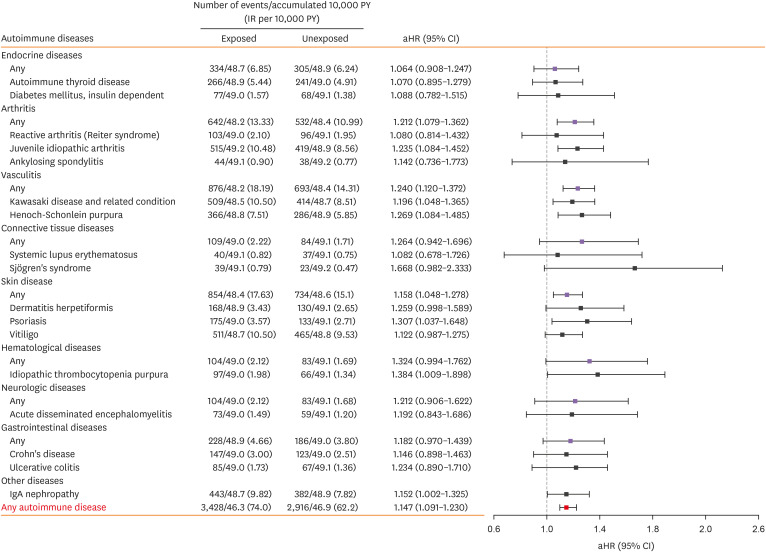
We investigated the association between early childhood wheezing episodes and the subsequent risk of developing autoimmune diseases (Fig. 2 and Supplementary Table S2). Among the exposed cohort, 3,428 children received diagnoses of autoimmune diseases, corresponding to an incidence rate of 74.0 cases per 10,000 person-years (PY). Conversely, in the unexposed cohort, 2,916 children were diagnosed, with an incidence rate of 62.2 cases per 10,000 PY. After adjusting for potential confounders, the exposed cohort demonstrated a 1.147-fold higher risk of developing autoimmune diseases (95% CI, 1.091–1.230) in comparison to the unexposed cohort.
Fig. 2. Risk of subsequent development of autoimmune diseases in children with bronchiolitis or asthma before 2 years of age.
Autoimmune diseases with fewer than 20 observed cases were pooled into their respective main categories rather than being analyzed individually. These contributed to the calculation of HRs. The Cox proportional hazards models were adjusted for multiple variables, including gender, age at index date, socioeconomic status, area of residence at birth, and history of perinatal diseases. Outcomes occurring during the first year following the index date were systematically excluded from all analyses.
PY, person year; IR, incidence rate; aHR, adjusted hazard ratio; CI, confidence interval; IgA, immunoglobulin A.
Specifically, the exposed cohort exhibited elevated risks for several individual autoimmune diseases. There was a statistically significant increase in the risk of juvenile idiopathic arthritis (adjusted hazard ratio [aHR], 1.235; 95% CI, 1.084–1.452) and other arthritis-related diseases (aHR, 1.212; 95% CI, 1.079–1.362). Moreover, this cohort also presented higher risks for Kawasaki disease (aHR, 1.196; 95% CI, 1.048–1.365) and Henoch-Schönlein purpura (aHR, 1.269; 95% CI, 1.084–1.485), as well as diseases with any types of vasculitis included in the present study (aHR, 1.240; 95% CI, 1.120–1.372). Notably, there was a significant association between wheezing episodes occurring within the first 2 years of life and conditions classified as skin diseases (aHR, 1.158; 95% CI, 1.048–1.278). Specifically, these early-life wheezing episodes were linked to a significantly higher risk of developing psoriasis (aHR, 1.307; 95% CI, 1.037–1.648). Regarding hematologic conditions, the exposed cohort displayed an increased risk of idiopathic thrombocytopenic purpura (aHR, 1.384; 95% CI, 1.009–1.898). Further, an elevated risk was noted for immunoglobulin A (IgA) nephropathy (aHR, 1.152; 95% CI, 1.002–1.325).
Associations between wheezing episode before 2 years of age and autoimmune diseases according to characteristics of individuals
We examined the association between episodes of wheezing before 2 years of age and the subsequent development of autoimmune diseases, stratified by individual baseline characteristics, to identify populations with high-risk groups (Table 2). With regard to gender, both males and females in the exposed group demonstrated an increased risk for developing autoimmune diseases, with no statistically significant gender-dependent difference in risk (P for interaction = 0.795). However, the risk was different depending on age at the index date (P for interaction = 0.035), with a higher risk observed in cases between 7–24 months, compared to those before 6 months. Moreover, the risk of developing autoimmune diseases within the exposed group was influenced by socioeconomic status (P for interaction = 0.046). Children from higher socioeconomic backgrounds faced a greater risk, compared to those from lower socioeconomic backgrounds (aHR, 1.202; 95% CI, 1.123–1.286; aHR, 1.084; 95% CI, 1.005–1.168; P for interaction = 0.046). However, there were no significant variations based on calendar year of birth, geographic area of residence at birth, season at index date, or perinatal status, in autoimmune disease risks (all P for interaction > 0.05).
Table 2. Risk of any autoimmune disease among individuals exposed to acute bronchiolitis or asthma before 2 years of age* compared with matched-unexposed individuals.
| Characteristics | Total number | No. of autoimmune disease cases/No. of accumulated person-years × 10,000 (incidence rate/10,000 person-years) | Absolute rate difference/10,000 person-years (95% CI) | Adjusted HR (95% CI)† | P for interaction‡ | ||
|---|---|---|---|---|---|---|---|
| Exposed group | Matched unexposed group | ||||||
| All | 34,959 | 3,428/46.3 (74.0) | 2,916/46.9 (62.2) | 11.764 (8.413–15.116) | 1.147 (1.091–1.230) | ||
| Gender | 0.795 | ||||||
| Boy | 22,083 | 2,104/29.4 (71.7) | 1,777/29.7 (59.9) | 11.819 (7.676–15.962) | 1.150 (1.078–1.226) | ||
| Girl | 12,876 | 1,324/17.0 (77.8) | 1,139/17.2 (66.1) | 11.674 (5.998–17.359) | 1.141 (1.053–1.238) | ||
| Age group at the index date,§ mon | 0.035 | ||||||
| ≤ 6 | 12,723 | 1,295/17.9 (72.4) | 1,156/17.6 (65.7) | 6.661 (1.192–12.130) | 1.066 (0.983–1.156) | ||
| 7–24 | 22,236 | 2,133/28.5 (75.0) | 1,760/29.3 (60.1) | 14.873 (10.630–19.116) | 1.197 (1.122–1.276) | ||
| Year of birth | 0.940 | ||||||
| 2002 | 16,731 | 1,713/22.2 (77.0) | 1,459/22.5 (64.9) | 12.169 (7.231–17.107) | 1.150 (1.071–1.235) | ||
| 2003 | 18,228 | 1,715/24.1 (71.2) | 1,457/24.4 (59.8) | 11.391 (6.835–15.947) | 1.144 (1.066–1.229) | ||
| Residence area at birth | 0.874 | ||||||
| Seoul/metropolitan | 18,309 | 1,779/22.5 (79.1) | 1,612/24.5 (65.9) | 13.275 (8.390–18.160) | 1.140 (1.048–1.240) | ||
| City | 13,043 | 1,296/18.9 (68.5) | 1,020/17.6 (58.1) | 10.342 (5.184–15.501) | 1.154 (1.077–1.235) | ||
| Rural | 3,597 | 353/4.9 (71.6) | 284/4.8 (58.7) | 12.879 (2.755–23.002) | 1.134 (0.936–1.334) | ||
| Socioeconomic status∥ | 0.046 | ||||||
| < Median | 14,941 | 1,452/19.8 (73.3) | 1,313/20.0 (65.8) | 7.560 (2.376–12.744) | 1.084 (1.005–1.168) | ||
| ≥ Median | 18,800 | 1,904/25.5 (74.7) | 1,518/25.3 (60.1) | 14.628 (10.110–19.146) | 1.202 (1.123–1.286) | ||
| Season at the index date | 0.981 | ||||||
| Spring to early autumn | 23,311 | 1,909/25.3 (75.5) | 1,951/31.3 (62.4) | 13.100 (8.725–17.475) | 1.126 (1.037–1.223) | ||
| Late autumn to winter | 11,648 | 1,519/21.1 (72.1) | 965/15.6 (61.8) | 10.319 (4.992–15.645) | 1.160 (1.088–1.237) | ||
| Any perinatal status¶ | 0.221 | ||||||
| Yes | 10,490 | 1,616/17.6 (91.6) | 1,152/13.9 (83.0) | 8.610 (2.057–15.163) | 1.103 (1.021–1.191) | ||
| No | 24,469 | 1,812/28.7 (63.1) | 1,764/33.0 (53.5) | 9.666 (5.836–13.497) | 1.173 (1.097–1.253) | ||
CI, confidence interval; HR, hazard ratio; ICD-10, International Classification of Diseases 10th revision.
*A wheezing episode is defined as hospitalization with a principal diagnosis of acute bronchiolitis (ICD-10 code J21.X) or asthma (ICD-10 code J45.X or J46.X) occurring before the age of 2.
†In stratified analyses, Cox models were calculated, adjusting for gender, age at the index date, economic status, place of residence at birth, and perinatal diseases—excluding the variable used for stratification. The first year of follow-up was excluded from all analyses.
‡The P value was obtained through an interaction test by incorporating an interaction term into the Cox model.
§The index date signifies the initial occurrence of a wheezing episode for exposed subjects; for unexposed subjects, the index date is randomly aligned with the index date of a matching exposed subject.
∥Economic status was assessed using health insurance premiums as an indicator.
¶Perinatal conditions include disorders related to gestation length and fetal growth, birth injuries, infections specific to the perinatal period, hemorrhagic disorders in the fetus and newborn, and congenital malformations.
Associations between wheezing episodes before age 2 and autoimmune diseases according to clinical characteristics of wheezing episodes
The risk of developing autoimmune diseases was associated with both the frequency of wheezing episodes experienced before 2 years of age and the duration of hospital stays (Table 3). Specifically, children who experienced 3 or more wheezing episodes demonstrated a higher risk for subsequent development of autoimmune diseases, compared to those who had 2 or fewer episodes (aHR, 1.427; 95% CI, 1.270–1.604 vs. aHR, 1.124; 95% CI, 1.068–1.183; P for interaction < 0.001). When compared to the unexposed group, children hospitalized for equal to or longer than the median stay of 6 days had a 1.22-fold increased risk of developing autoimmune diseases, whereas those hospitalized for less than 6 days showed a 1.08-fold elevated risk (P for interaction < 0.001). However, there was no difference in the risk of autoimmune diseases based on the primary diagnosis of a wheezing episode (P for interaction = 0.66).
Table 3. Risk of autoimmune disease associated with wheezing episodes* in early childhood according to clinical conditions.
| Group | No. of autoimmune disease cases/No. of Accumulated person-years × 10,000 (incidence rate/10,000 person-years) | Adjusted HR (95% CI)† | P value for interaction | ||
|---|---|---|---|---|---|
| Matched unexposed group (n = 34,969) | 2,916/46.9 (62.2) | Ref | |||
| Exposed group | |||||
| Diagnostic codes for wheezing | 0.662 | ||||
| Acute bronchiolitis (n = 19,389) | 1,902/25.9 (73.4) | 1.127 (1.062–1.195) | |||
| Asthma (n = 15,570) | 1,526/20.4 (74.7) | 1.171 (1.009–1.248) | |||
| Number of wheezing episodes | < 0.001 | ||||
| Two or fewer (n = 32,285) | 3,100/42.9 (72.3) | 1.124 (1.068–1.183) | |||
| Three times or more (n = 2,674) | 328/3.4 (95.3) | 1.427 (1.270–1.604) | |||
| Duration of admissions for wheezing episode | < 0.001 | ||||
| < Median (6 days) (n = 18,310) | 1,688/24.4 (69.3) | 1.082 (1.018–1.150) | |||
| ≥ Median (6 days) (n = 16,648) | 1,740/22.0 (79.2) | 1.217 (1.146–1.293) | |||
HR, hazard ratio; CI, confidence interval; ICD-10, International Classification of Diseases 10th revision.
*Wheezing episodes were identified through hospitalization records with a primary diagnosis of either acute bronchiolitis (ICD-10 code J21.X) or asthma (ICD-10 code J45.X or J46.X) occurring before the age of 2 years.
†All Cox models were adjusted for the following covariates: gender, age at the index date, economic status, area of residence at birth, and perinatal diseases. The first year of follow-up was omitted from all analyses.
Sensitivity analysis
We performed additional analyses on the association between autoimmune diseases and each specific diagnosis—acute bronchiolitis or asthma—related to wheezing episodes (Supplementary Table S3). Initially, using a 1:1 incidence density matching approach, we identified 16,871 children who had been diagnosed with asthma before reaching 2 years of age, as well as an equal number who had not been diagnosed. The IR for autoimmune diseases was 74.6 among children with asthma and 63.2 among those without. This corresponded to a 1.16-fold (95% CI, 1.08–1.25) increase in the risk of developing autoimmune diseases in children diagnosed with asthma, compared to those without asthma. Similarly, after performing matching, we found 19,735 children with and without a diagnosis of acute bronchiolitis prior to the age of 2 years. In this cohort, the IR for autoimmune diseases was 73.6 for children with acute bronchiolitis and 63.7 for those without acute bronchiolitis, signifying a heightened risk for autoimmune diseases in children with acute bronchiolitis (HR, 1.14; 95% CI, 1.07–1.22).
Furthermore, we executed sensitivity analyses using varied lag times of 2, 3, and 5 years following the index date (Supplementary Table S4). Despite extending the lag-time intervals to these durations, we observed consistent outcomes linking viral wheeze before the age of 2 years with the subsequent development of autoimmune diseases during childhood.
DISCUSSION
In the present study, we demonstrated a significant association between viral wheeze before the age of 2 and increased risks of developing autoimmune diseases in later childhood in a large population-based cohort analysis. Notably, children who had viral wheeze before the age of 2 showed an increased risk for specific autoimmune diseases, the highest risk being for idiopathic thrombocytopenic purpura, followed by psoriasis, Henoch-Schönlein purpura, juvenile idiopathic arthritis, Kawasaki disease, and IgA nephropathy. Additionally, the risk was further amplified in children with an index age between 7 and 24 months, those from higher socioeconomic backgrounds, and those with frequent and prolonged hospitalizations due to viral wheeze prior to 2 years of age. These findings provide significant implications for the long-term management of early-life viral wheeze and underscore the necessity for its consideration in the management strategies.
Several studies have explored the relationship between childhood and adult asthma and the subsequent development of autoimmune diseases.14,15 One nationwide registry study revealed that children diagnosed with asthma between the ages of 5 and 7 showed increased risks of developing type 1 diabetes and inflammatory bowel disease beyond the age of 8.16 Another cross-sectional study found that patients with asthma at 8–29 years exhibited heightened risks for a range of autoimmune diseases, defined as any one of the arthritis-related conditions, systemic connective tissue disorders, ankylosing spondylitis, non-infective enteritis and colitis, and psoriasis.17
It is important to differentiate asthma in older children and adults from that in infants,18 as the epithelial reticular basement membrane in infants with reversible airflow obstruction is different in infants, even when associated with atopy.19 The pathophysiology of viral wheeze before the age of 2 is associated with small airway involvement, which is triggered by respiratory viral infections early in life and results in airway inflammation, airflow obstruction, and hyperreactivity.20 The occurrence of viral wheeze in early life is shaped by complex interactions among host genetics, environmental factors, and the characteristics of respiratory pathogens.20,21,22 Therefore, conclusions about the link between viral wheeze prior to the age of 2 and childhood autoimmune diseases cannot be drawn from data pertaining to childhood asthma and adult asthma. To date, no studies have examined the association between early-life viral wheeze and the later development of autoimmune diseases, although research is required to investigate this relationship specifically among children who experience viral wheeze before the age of 2. To our knowledge, the present study is the first and most extensive to investigate the association between viral wheeze in infancy and the subsequent development of autoimmune diseases during childhood.
The potential mechanisms that underlie the association between viral wheeze in infancy and subsequent development of autoimmune diseases are multifaceted and likely involve a combination of shared genetic susceptibility, environmental influences, and cytokine milieu.13,20,23,24 Rather than acting in isolation, these factors likely interact in a complex manner, influenced by both genetic and environmental backgrounds, to contribute to the observed association between viral wheeze during infancy and autoimmune diseases. Although genes, such as TLR-2, GSDMB, and HLA-DRB1, have been identified as conferring a predisposition to both asthma and autoimmune diseases,24,25,26 genetic factors alone are insufficient to explain the development of asthma. Environmental factors, such as diet, infection, and pollution, also play a significant role in the onset and progression of bronchiolitis, asthma, and autoimmune diseases.20,27,28 For instance, a higher intake of advanced glycation end products has been associated with both childhood wheeze29 and autoimmune diseases.23 Chronic inflammation, characterized by elevated levels of interleukin (IL)-17 and tumor necrosis factor-α, may partially explain the link between early-life viral wheeze and autoimmune disorders, as these cytokines are also elevated in autoimmune diseases and asthma.30,31 In addition, regulatory T cells (Treg), which play a key role in the regulation of immune homeostasis and tolerance, are commonly involved in the pathogenesis in the autoimmune disease and allergic diseases by suppressing autoreactive immune response and Th2 response, respectively.32,33 Therefore, research on the role of Treg on the association of autoimmune diseases with wheeze in early life is required. The epithelial barrier hypothesis has recently been proposed to account for the rising prevalence of allergic and autoimmune diseases, particularly in the context of modern societal shifts such as industrialization and urbanization. 34 Furthermore, alterations in the gut microbiome during the vulnerable period of early-life immune system development may provide a partial explanation for the association between early-life viral wheeze and autoimmune diseases.35 Dysregulated immune responses, coupled with airway inflammation, emerge as key features in both autoimmune diseases and respiratory conditions like asthma and bronchiolitis, particularly among genetically predisposed individuals.35,36
In the present study, we found that wheezing episode before the age of 2 significantly increased the risk of various pediatric autoimmune diseases, such as juvenile idiopathic arthritis, Kawasaki disease, Henoch-Schönlein purpura, idiopathic thrombocytopenic purpura, and IgA nephropathy. However, no significant associations were observed with endocrine or connective tissue diseases. The notable link between early-life wheezing episode and specific types of vasculitis, such as Kawasaki disease and Henoch-Schönlein purpura, may be partly attributed to certain subtypes of respiratory involvement in systemic vasculitis in genetically predisposed individuals.37 Alternatively, these associations might be the result of sequential manifestations stemming from pre-existing immune complex, a condition detectable in both asthma and vasculitis.38 Previous research has indicated that respiratory viral infections, specifically respiratory syncytial virus, can act as a trigger for Kawasaki disease within a relatively short interval of 2 months.39 In contrast, our study demonstrated a lag time of 1–5 years between early-life viral wheeze and the initial diagnosis of Kawasaki disease. This suggests that respiratory viral infection alone is insufficient to fully account for the observed associations between viral wheeze in infancy and the later development of Kawasaki disease. A more plausible explanation may lie in the intricate interplay of multiple factors, such as dysregulated immune responses in susceptible populations, contributing to these associations.20,40 The absence of a significant correlation between early-life wheezing episode and endocrine autoimmune diseases may be due to the lack of overlapping pathophysiological mechanisms, which originate from diverse factors.
Atopic dermatitis and psoriasis are commonly T-cell mediated chronic inflammatory skin diseases.41 Although atopic dermatitis and psoriasis are considered as distinct diseases in the aspects of clinical, pathologic, and molecular background, some subtypes of atopic dermatitis and psoriasis have areas of overlaps in the pathologic mechanisms, including IL-17 component.41 Furthermore, psoriasis shared 35% genes with atopic dermatitis.42 In addition, the association between psoriasis and respiratory comorbidities has been infrequently explored in existing literature.43,44 The presence of systemic inflammatory markers, such as elevated IL-17, which are commonly found in both early-life viral wheeze and psoriasis, could offer a partial explanation for these observed associations.45 Accumulating evidence suggested that atopic dermatitis is associated with increased risks of multiple autoimmune diseases.42,46,47 As one of the major allergic comorbidities, atopic dermatitis can share the common pathophysiology with autoimmune diseases in the aspect of genetic susceptibility and predominant cytokines,48 which might partially explain the link between atopic dermatitis and autoimmune diseases. While one investigation has demonstrated that RSV bronchiolitis and juvenile idiopathic arthritis exhibit inverse distributions in IL-8 polymorphisms,49 another study has indicated that children with allergic diseases, including asthma, are at a heightened risk for developing juvenile idiopathic arthritis.50 These results imply that genetic predispositions alone cannot fully account for the association between viral wheeze and juvenile idiopathic arthritis, when considering that RSV bronchiolitis may also act as a risk factor for subsequent asthma development.20 Further research is warranted to elucidate the underlying mechanisms driving these associations.
We defined the exposure of interest as early life wheezing episodes requiring hospitalization, rather than all wheezing episodes, including those treated as outpatients. This was due to a lack of validation studies on wheezing episodes in the administrative cohorts and the nature of our data, which necessitated a more precise selection of wheezing episodes. When considering previous studies that found children with severe symptoms, specifically those with acute bronchiolitis requiring hospitalization at a young age, had a higher risk of asthma, compared to those treated as outpatients,6,51 it's important to acknowledge that our study cannot generalize the association between all wheezing episodes and autoimmune diseases. Furthermore, our study revealed that an elevated risk for childhood autoimmune diseases has been observed in children with more extended hospital stays and frequent hospitalizations due to early-life viral wheeze. This suggests that the severity of early-life viral wheeze may contribute to an increased likelihood of developing autoimmune diseases during childhood. These findings suggest that factors correlating with the severity of viral wheeze in early life could partially overlap with the etiological mechanisms of childhood autoimmune diseases.
Several limitations warrant mention in the present study. Although asthma and bronchiolitis can be distinct entities in children older than 2 years, we defined the concept of viral wheeze in infancy using ICD-10 codes for either asthma or bronchiolitis occurring before the age of 2, coupled with a bronchodilator prescription in this study. This categorization can be problematic given that bronchodilators are frequently prescribed for bronchiolitis in infancy when asthmatic features are present, rendering asthma and bronchiolitis virtually indistinguishable in children under 2 years of age. As this study included only hospitalized for bronchiolitis or bronchial asthma before 2 years of age, the results of the present study might suggest that infants with higher risk of asthma or more severe bronchiolitis might have the associations with the development of autoimmune diseases in childhood.52 In addition, since our study targeted hospitalizations for wheezing episodes, the matched control group likely included cases that visited outpatient services for wheezing episodes. It is important to consider this aspect when interpreting the results of the present study. Additionally, while various factors, such as genetic susceptibility can influence the onset of autoimmune diseases,53 these were not accounted for as potential confounders in our analysis because of lack of information in genetic susceptibility in the dataset used in the present study. Despite these limitations, our study holds significance for elucidating the association between early-life viral wheeze and the subsequent development of autoimmune diseases in childhood. Utilizing diverse lag-time analyses enhanced the robustness of our findings.
In summary, early-life viral wheeze is significantly associated with an increased risk of developing autoimmune diseases in later childhood. These findings underscore the need for heightened clinical vigilance regarding this risk in children who experience viral wheeze early in life, aiming to improve disease outcomes. Enhanced understanding of these associations may facilitate the elucidation of underlying pathophysiological mechanisms, thereby aiding in the formulation of targeted management strategies for both viral wheeze and autoimmune diseases in children.
ACKNOWLEDGMENTS
This work was supported by Korea Health Technology R&D Through the Korea (HR22C1605030022), the 2022 SAMA PHARM CO grant from the Korean Academy of Pediatric Allergy and Respiratory Disease, and a grant (BCRI-24082) of Chonnam National University Hospital Biomedical Research Institute. The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
Data Sharing Statement: This study was based on the National Health Claims Database (NHIS-2019-1-560) established by the NHIS of the Republic of Korea. Applications for using NHIS data are be reviewed by the Inquiry Committee of Research Support; if the application is approved, raw data is provided to the applicant for a fee. We cannot provide access to the data, analytic methods, and research materials to other researchers because of the intellectual property rights of this database that is owned by the National Health Insurance Corporation. However, investigators who wish to reproduce our results or replicate the procedure can be used in the database, which is open for research purposes (https://nhiss.nhis.or.kr/).
SUPPLEMENTARY MATERIALS
Clinical characteristics of study participants
Risk of subsequent development of autoimmune diseases in children with wheezing episodes*
Association between acute bronchiolitis or asthma and subsequent autoimmune diseases, analyzed independently
Association between wheezing episodes* and subsequent autoimmune diseases across varying lag-times
References
- 1.Safiri S, Mahmoodpoor A, Kolahi AA, Nejadghaderi SA, Sullman MJ, Mansournia MA, et al. Global burden of lower respiratory infections during the last three decades. Front Public Health. 2023;10:1028525. doi: 10.3389/fpubh.2022.1028525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalziel SR, Haskell L, O’Brien S, Borland ML, Plint AC, Babl FE, et al. Bronchiolitis. Lancet. 2022;400:392–406. doi: 10.1016/S0140-6736(22)01016-9. [DOI] [PubMed] [Google Scholar]
- 3.Douros K, Everard ML. Time to say goodbye to bronchiolitis, viral wheeze, reactive airways disease, wheeze bronchitis and all that. Front Pediatr. 2020;8:218. doi: 10.3389/fped.2020.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruotsalainen M, Heikkilä P, Backman K, Korppi M. An increased asthma risk continued until young adulthood after early-childhood hospitalisation for wheezing. Acta Paediatr. 2022;111:157–162. doi: 10.1111/apa.16099. [DOI] [PubMed] [Google Scholar]
- 5.Chung HL. Diagnosis and management of asthma in infants and preschoolers. Clin Exp Pediatr. 2022;65:574–584. doi: 10.3345/cep.2021.01746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dumas O, Erkkola R, Bergroth E, Hasegawa K, Mansbach JM, Piedra PA, et al. Severe bronchiolitis profiles and risk of asthma development in Finnish children. J Allergy Clin Immunol. 2022;149:1281–1285.e1. doi: 10.1016/j.jaci.2021.08.035. [DOI] [PubMed] [Google Scholar]
- 7.Muñoz-Quiles C, López-Lacort M, Díez-Domingo J, Orrico-Sánchez A. Bronchiolitis, regardless of its aetiology and severity, is associated with an increased risk of asthma: a population-based study. J Infect Dis. 2023;228:840–850. doi: 10.1093/infdis/jiad093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skapenko A, Leipe J, Lipsky PE, Schulze-Koops H. The role of the T cell in autoimmune inflammation. Arthritis Res Ther. 2005;7(Suppl 2):S4–14. doi: 10.1186/ar1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YD. Systemic autoinflammatory disorders: autoinflammatory and autoimmune disorders. Clin Exp Pediatr. 2023;66:439–440. doi: 10.3345/cep.2023.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tedeschi A, Asero R. Asthma and autoimmunity: a complex but intriguing relation. Expert Rev Clin Immunol. 2008;4:767–776. doi: 10.1586/1744666X.4.6.767. [DOI] [PubMed] [Google Scholar]
- 11.Smew AI, Lundholm C, Sävendahl L, Lichtenstein P, Almqvist C. Familial coaggregation of asthma and type 1 diabetes in children. JAMA Netw Open. 2020;3:e200834. doi: 10.1001/jamanetworkopen.2020.0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukherjee M, Nair P. Autoimmune responses in severe asthma. Allergy Asthma Immunol Res. 2018;10:428–447. doi: 10.4168/aair.2018.10.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy RC, Morrell ED, Hallstrand TS. The intersection between autoimmunity, macrophage dysfunction, endotype, and exacerbations in severe asthma. Am J Respir Crit Care Med. 2023;207:383–385. doi: 10.1164/rccm.202211-2074ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng R, Wang Z, Zhang J, Liang Z, Xu C, Wang J, et al. Type 1 diabetes and asthma: a systematic review and meta-analysis of observational studies. Endocrine. 2022;75:709–717. doi: 10.1007/s12020-021-02973-x. [DOI] [PubMed] [Google Scholar]
- 15.Yun HD, Knoebel E, Fenta Y, Gabriel SE, Leibson CL, Loftus EV, Jr, et al. Asthma and proinflammatory conditions: a population-based retrospective matched cohort study. Mayo Clin Proc. 2012;87:953–960. doi: 10.1016/j.mayocp.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liljendahl MS, Sevelsted A, Chawes BL, Stokholm J, Bønnelykke K, Andersen ZJ, et al. Childhood asthma is associated with development of type 1 diabetes and inflammatory bowel diseases: a Danish nationwide registry study. Sci Rep. 2022;12:21728. doi: 10.1038/s41598-022-26067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlstad Ø, Nafstad P, Tverdal A, Skurtveit S, Furu K. Comorbidities in an asthma population 8–29 years old: a study from the Norwegian Prescription Database. Pharmacoepidemiol Drug Saf. 2012;21:1045–1052. doi: 10.1002/pds.2233. [DOI] [PubMed] [Google Scholar]
- 18.Lee E, Rhee EH, Kim K, Kim HS, Kim WK, Song DJ, et al. Frequency of exacerbation and degree of required asthma medication can characterize childhood longitudinal asthma trajectories. Ann Allergy Asthma Immunol. 2023;131:444–450. doi: 10.1016/j.anai.2023.05.035. [DOI] [PubMed] [Google Scholar]
- 19.Saglani S, Malmström K, Pelkonen AS, Malmberg LP, Lindahl H, Kajosaari M, et al. Airway remodeling and inflammation in symptomatic infants with reversible airflow obstruction. Am J Respir Crit Care Med. 2005;171:722–727. doi: 10.1164/rccm.200410-1404OC. [DOI] [PubMed] [Google Scholar]
- 20.Jartti T, Smits HH, Bønnelykke K, Bircan O, Elenius V, Konradsen JR, et al. Bronchiolitis needs a revisit: distinguishing between virus entities and their treatments. Allergy. 2019;74:40–52. doi: 10.1111/all.13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manti S, Licari A, Leonardi S, Marseglia GL, Baraldi E. Reply to correspondence: “Bronchiolitis needs a revisit: Distinguishing between virus entities and their treatments”. Allergy. 2020;75:1531–1532. doi: 10.1111/all.14154. [DOI] [PubMed] [Google Scholar]
- 22.Tahamtan A, Askari FS, Bont L, Salimi V. Disease severity in respiratory syncytial virus infection: role of host genetic variation. Rev Med Virol. 2019;29:e2026. doi: 10.1002/rmv.2026. [DOI] [PubMed] [Google Scholar]
- 23.Virtanen SM. Dietary factors in the development of type 1 diabetes. Pediatr Diabetes. 2016;17(Suppl 22):49–55. doi: 10.1111/pedi.12341. [DOI] [PubMed] [Google Scholar]
- 24.Bjørnvold M, Munthe-Kaas MC, Egeland T, Joner G, Dahl-Jørgensen K, Njølstad PR, et al. A TLR2 polymorphism is associated with type 1 diabetes and allergic asthma. Genes Immun. 2009;10:181–187. doi: 10.1038/gene.2008.100. [DOI] [PubMed] [Google Scholar]
- 25.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41:703–707. doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adami G, Pontalti M, Cattani G, Rossini M, Viapiana O, Orsolini G, et al. Association between long-term exposure to air pollution and immune-mediated diseases: a population-based cohort study. RMD Open. 2022;8:e002055. doi: 10.1136/rmdopen-2021-002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bacharier LB, Boner A, Carlsen KH, Eigenmann PA, Frischer T, Götz M, et al. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy. 2008;63:5–34. doi: 10.1111/j.1398-9995.2007.01586.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang JG, Liu B, Kroll F, Hanson C, Vicencio A, Coca S, et al. Increased advanced glycation end product and meat consumption is associated with childhood wheeze: analysis of the National Health and Nutrition Examination Survey. Thorax. 2021;76:292–294. doi: 10.1136/thoraxjnl-2020-216109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Liu Y, Chu CQ. Th17 cells in type 1 diabetes: role in the pathogenesis and regulation by gut microbiome. Mediators Inflamm. 2015;2015:638470. doi: 10.1155/2015/638470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kainonen E, Rautava S, Korkeamäki M, Isolauri E. Unique cytokine secretion profile in children with both type I diabetes and asthma distinct from that of solely diabetic or asthmatic children. Cytokine. 2006;34:198–205. doi: 10.1016/j.cyto.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Goswami TK, Singh M, Dhawan M, Mitra S, Emran TB, Rabaan AA, et al. Regulatory T cells (Tregs) and their therapeutic potential against autoimmune disorders - Advances and challenges. Hum Vaccin Immunother. 2022;18:2035117. doi: 10.1080/21645515.2022.2035117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson DS, Larché M, Durham SR. Tregs and allergic disease. J Clin Invest. 2004;114:1389–1397. doi: 10.1172/JCI23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akdis CA. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat Rev Immunol. 2021;21:739–751. doi: 10.1038/s41577-021-00538-7. [DOI] [PubMed] [Google Scholar]
- 35.Miyauchi E, Shimokawa C, Steimle A, Desai MS, Ohno H. The impact of the gut microbiome on extra-intestinal autoimmune diseases. Nat Rev Immunol. 2023;23:9–23. doi: 10.1038/s41577-022-00727-y. [DOI] [PubMed] [Google Scholar]
- 36.Wark PA. Asthma and the dysregulated immune response to rhinovirus. Am J Respir Crit Care Med. 2020;202:157–159. doi: 10.1164/rccm.202003-0634ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nasser M, Cottin V. The respiratory system in autoimmune vascular diseases. Respiration. 2018;96:12–28. doi: 10.1159/000486899. [DOI] [PubMed] [Google Scholar]
- 38.Guest IC, Sell S. Bronchial lesions of mouse model of asthma are preceded by immune complex vasculitis and induced bronchial associated lymphoid tissue (iBALT) Lab Invest. 2015;95:886–902. doi: 10.1038/labinvest.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang JM, Jung J, Kim YE, Huh K, Hong J, Kim DW, et al. Temporal correlation between Kawasaki disease and infectious diseases in South Korea. JAMA Netw Open. 2022;5:e2147363. doi: 10.1001/jamanetworkopen.2021.47363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noval Rivas M, Arditi M. Kawasaki disease: pathophysiology and insights from mouse models. Nat Rev Rheumatol. 2020;16:391–405. doi: 10.1038/s41584-020-0426-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:68–73. doi: 10.1016/j.coi.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Ahn J, Shin S, Lee GC, Han BE, Lee E, Ha EK, et al. Unraveling the link between atopic dermatitis and autoimmune diseases in children: Insights from a large-scale cohort study with 15-year follow-up and shared gene ontology analysis. Allergol Int. 2024;73:243–254. doi: 10.1016/j.alit.2023.12.005. [DOI] [PubMed] [Google Scholar]
- 43.Fang HY, Liao WC, Lin CL, Chen CH, Kao CH. Association between psoriasis and asthma: a population-based retrospective cohort analysis. Br J Dermatol. 2015;172:1066–1071. doi: 10.1111/bjd.13518. [DOI] [PubMed] [Google Scholar]
- 44.Kao LT, Lee CZ, Liu SP, Tsai MC, Lin HC. Psoriasis and the risk of pneumonia: a population-based study. PLoS One. 2014;9:e116077. doi: 10.1371/journal.pone.0116077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santus P, Rizzi M, Radovanovic D, Airoldi A, Cristiano A, Conic R, et al. Psoriasis and respiratory comorbidities: the added value of fraction of exhaled nitric oxide as a new method to detect, evaluate, and monitor psoriatic systemic involvement and therapeutic efficacy. BioMed Res Int. 2018;2018:3140682. doi: 10.1155/2018/3140682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Lusignan S, Alexander H, Broderick C, Dennis J, McGovern A, Feeney C, et al. Atopic dermatitis and risk of autoimmune conditions: population-based cohort study. J Allergy Clin Immunol. 2022;150:709–713. doi: 10.1016/j.jaci.2022.03.030. [DOI] [PubMed] [Google Scholar]
- 47.Kim JH, Lee E, Ha EK, Shin J, Lee GC, Rha YH, et al. Cascade of atopic dermatitis comorbidities in children after birth for 15 years. Allergy. 2024;79:153–163. doi: 10.1111/all.15917. [DOI] [PubMed] [Google Scholar]
- 48.Ellinghaus D, Baurecht H, Esparza-Gordillo J, Rodríguez E, Matanovic A, Marenholz I, et al. High-density genotyping study identifies four new susceptibility loci for atopic dermatitis. Nat Genet. 2013;45:808–812. doi: 10.1038/ng.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heinzmann A, Ahlert I, Kurz T, Berner R, Deichmann KA. Association study suggests opposite effects of polymorphisms within IL8 on bronchial asthma and respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2004;114:671–676. doi: 10.1016/j.jaci.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 50.Lin CH, Lin CL, Shen TC, Wei CC. Epidemiology and risk of juvenile idiopathic arthritis among children with allergic diseases: a nationwide population-based study. Pediatr Rheumatol Online J. 2016;14:15. doi: 10.1186/s12969-016-0074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewis KM, De Stavola BL, Cunningham S, Hardelid P. Socioeconomic position, bronchiolitis and asthma in children: counterfactual disparity measures from a national birth cohort study. Int J Epidemiol. 2023;52:476–488. doi: 10.1093/ije/dyac193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65:1045–1052. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 53.Pisetsky DS. Pathogenesis of autoimmune disease. Nat Rev Nephrol. 2023;19:509–524. doi: 10.1038/s41581-023-00720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical characteristics of study participants
Risk of subsequent development of autoimmune diseases in children with wheezing episodes*
Association between acute bronchiolitis or asthma and subsequent autoimmune diseases, analyzed independently
Association between wheezing episodes* and subsequent autoimmune diseases across varying lag-times