Abstract
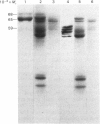
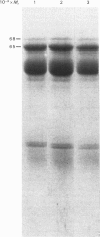
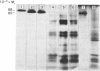
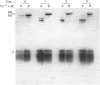
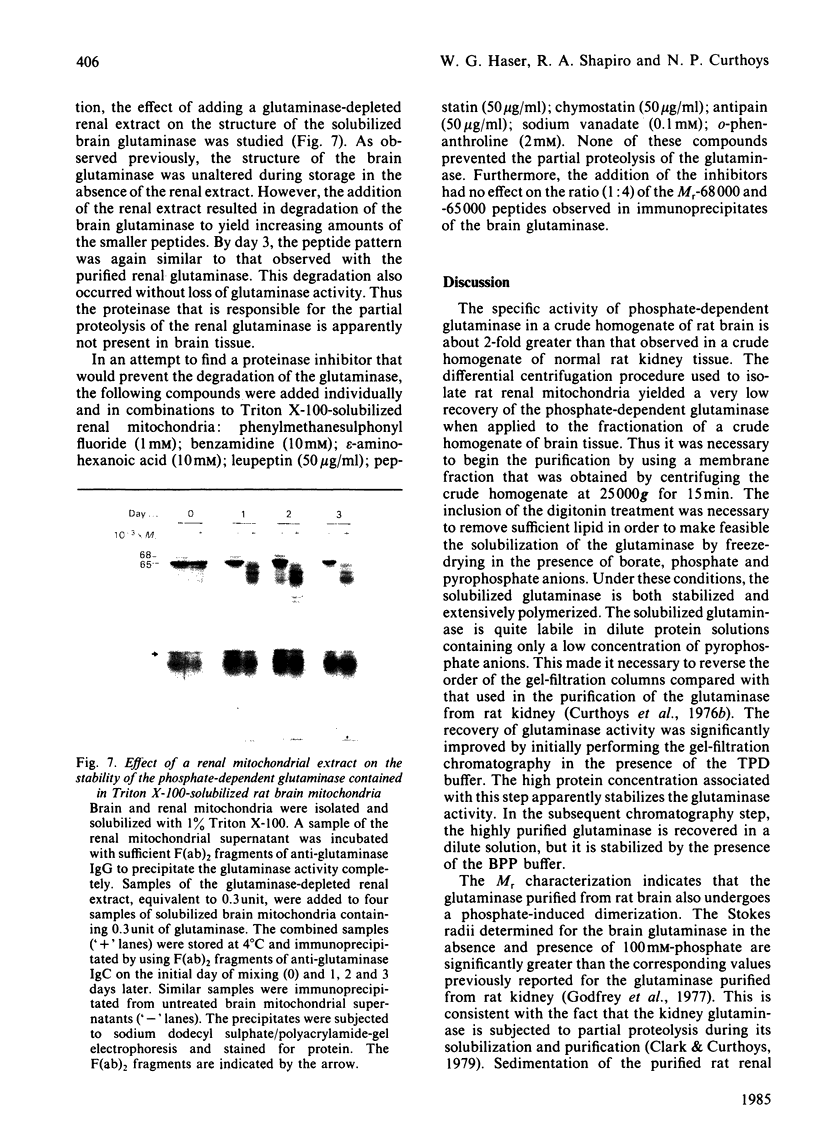
A phosphate-dependent glutaminase was purified 1200-fold from rat brain. In the absence of a polyvalent anion, the glutaminase exists as an inactive protomer which has an estimated Mr of 126000. The addition of 100mM-phosphate causes maximal activation and a dimerization (Mr 249000) of the glutaminase. The phosphate activation is sigmoidal, with a K0.5 of 25mM and a Hill coefficient (h) of 1.5 Glutamate inhibition is competitive with respect to glutamine and is decreased by increasing the concentration of phosphate. Phosphate also decreases the Km for glutamine. The purified glutaminase contains a predominant peptide (Mr 65000) and a minor peptide (Mr 68000) that are present in an approximate ratio of 4:1 respectively. The glutaminase immunoprecipitated from freshly solubilized brain tissue or from synaptosomal and non-synaptosomal brain mitochondria contains the same distribution of the two peptides. In contrast, the glutaminase purified from rat kidney contains five to seven peptides that range in Mr value from 59000 to 48000, and immunoprecipitates derived from freshly solubilized renal tissue contain only the Mr-65000 peptide. Partial proteolysis and size fractionation of the three immunoprecipitated peptides indicate that they are structurally related. The series of peptides characteristic of the purified renal glutaminase is generated on storage of the solubilized extract of kidney tissue. The glutaminase contained in the solubilized brain extract is not degraded unless a renal extract is added. Thus the difference in the pattern of peptides associated with the two purified enzymes is due to an endogenous renal proteinase that is not present in brain.
Full text
PDF



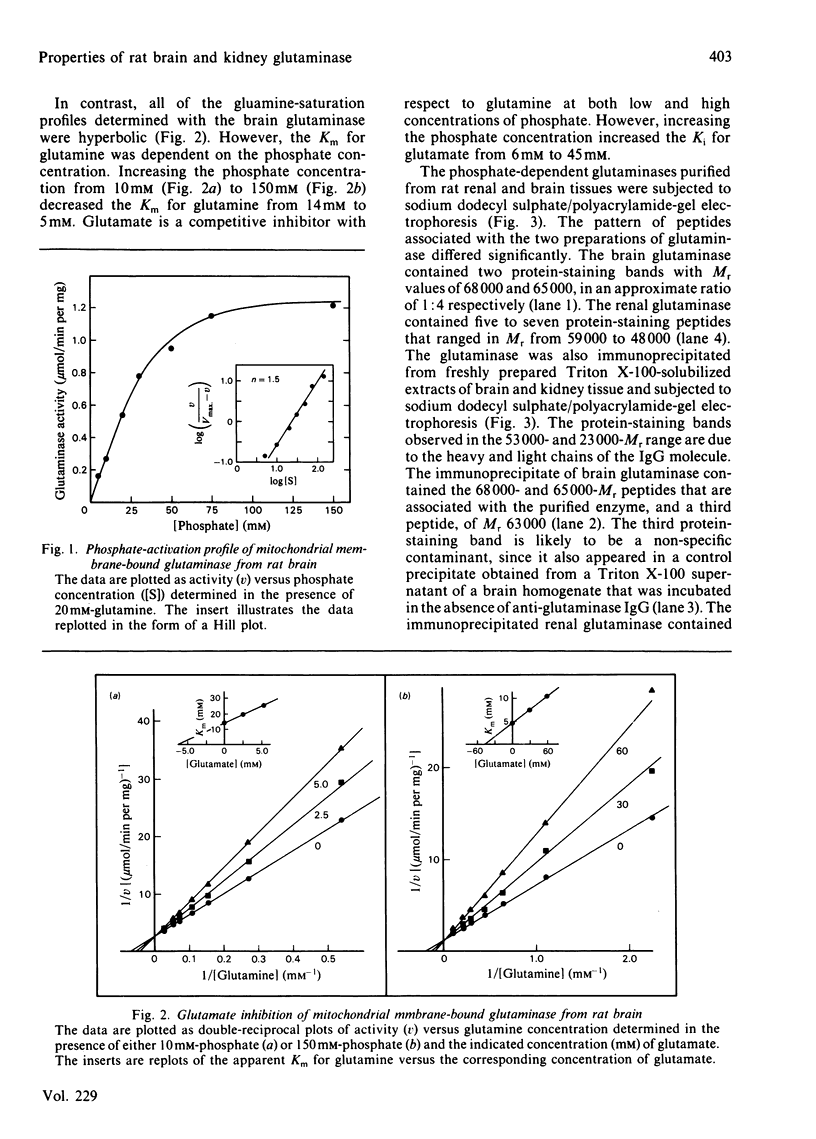
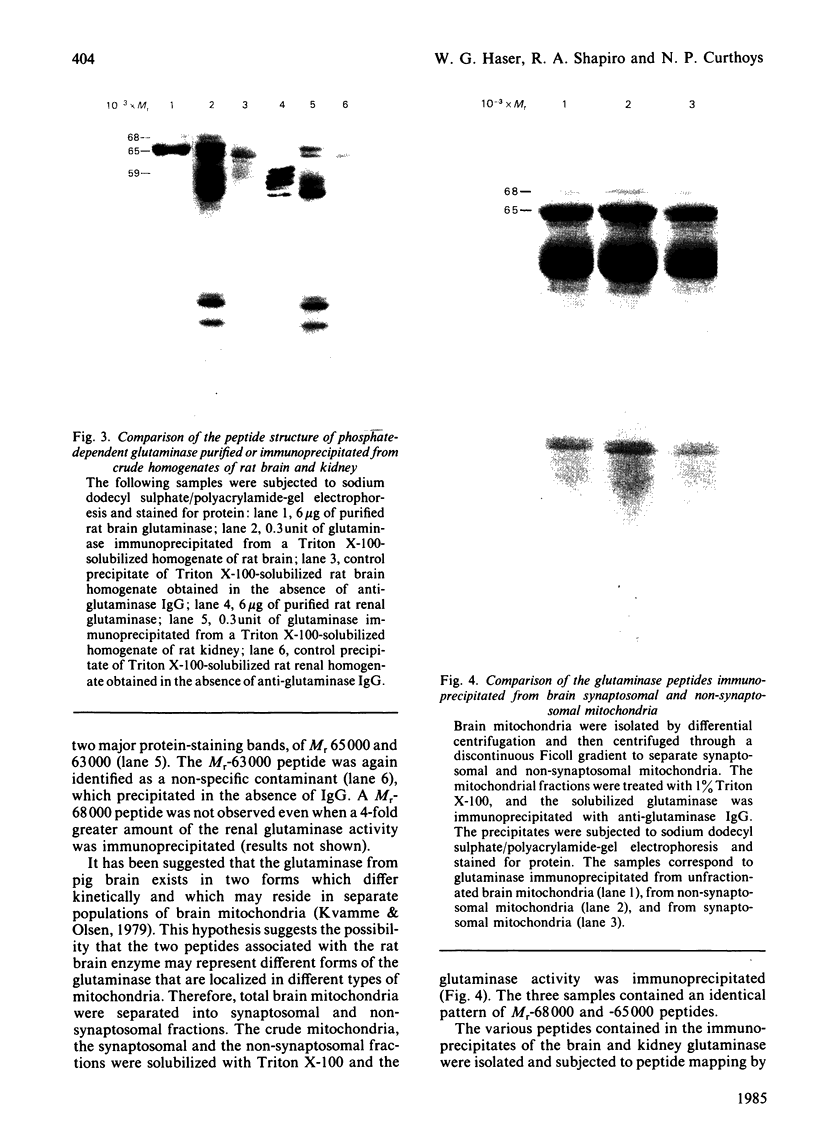
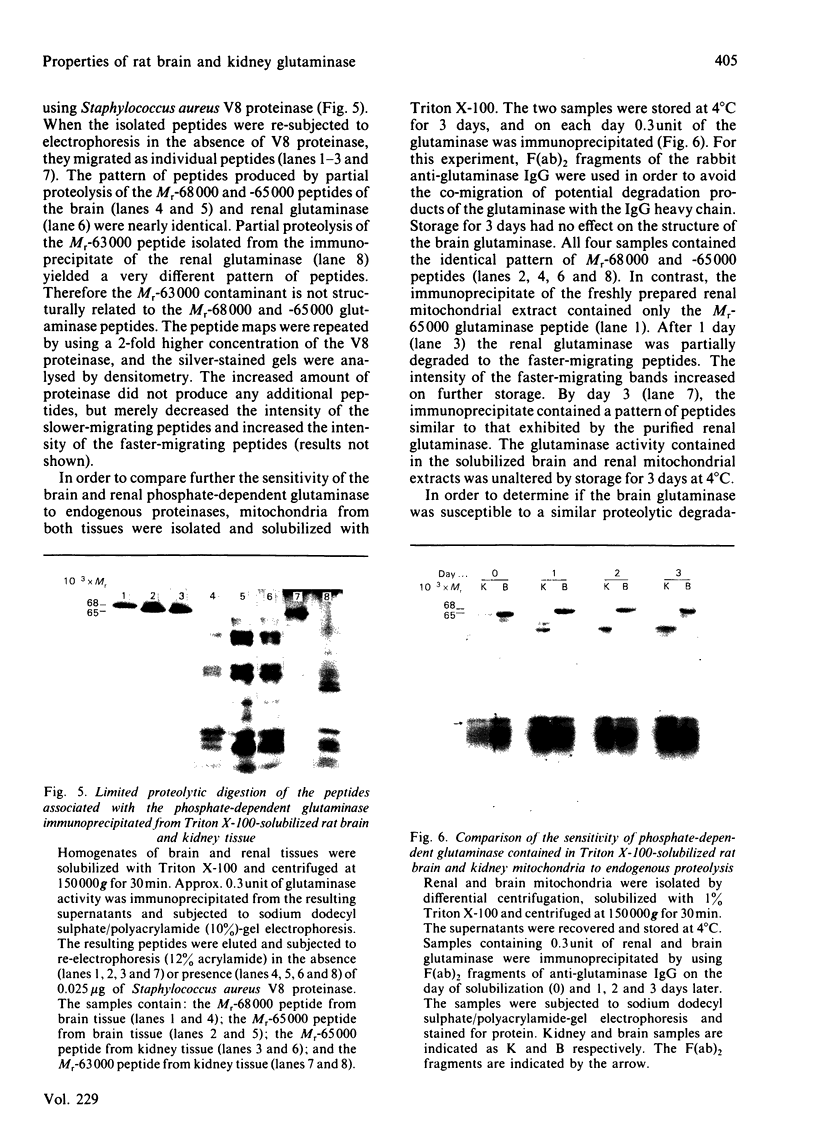


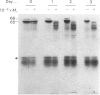
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Booth R. F., Clark J. B. A rapid method for the preparation of relatively pure metabolically competent synaptosomes from rat brain. Biochem J. 1978 Nov 15;176(2):365–370. doi: 10.1042/bj1760365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu J. F., Boeker E. A. Cow brain glutaminase: partial purification and mechanism of action. Arch Biochem Biophys. 1979 Sep;196(2):493–500. doi: 10.1016/0003-9861(79)90301-1. [DOI] [PubMed] [Google Scholar]
- Clark V. M., Curthoys N. P. Cause of subunit heterogeneity in purified rat renal phosphate-dependent glutaminase. J Biol Chem. 1979 Jun 25;254(12):4939–4941. [PubMed] [Google Scholar]
- Clark V. M., Shapiro R. A., Curthoys N. P. Comparison of the hydrolysis and the covalent binding of 6-diazo-5-oxo-L-[6-14C]norleucine by rat renal phosphate-dependent glutaminase. Arch Biochem Biophys. 1982 Jan;213(1):232–239. doi: 10.1016/0003-9861(82)90457-x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Curthoys N. P., Kuhlenschmidt T., Godfrey S. S., Weiss R. F. Phosphate-dependent glutaminase from rat kidney. Cause of increased activity in response to acidosis and identity with glutaminase from other tissues. Arch Biochem Biophys. 1976 Jan;172(1):162–167. doi: 10.1016/0003-9861(76)90062-x. [DOI] [PubMed] [Google Scholar]
- Curthoys N. P., Lowry O. H. The distribution of glutaminase isoenzymes in the various structures of the nephron in normal, acidotic, and alkalotic rat kidney. J Biol Chem. 1973 Jan 10;248(1):162–168. [PubMed] [Google Scholar]
- Curthoys N. P., Weiss R. F. Regulation of renal ammoniagenesis. Subcellular localization of rat kidney glutaminase isoenzymes. J Biol Chem. 1974 May 25;249(10):3261–3266. [PubMed] [Google Scholar]
- Godfrey S., Kuhlenschmidt T., Curthoys P. Correlation between activation and dimer formation of rat renal phosphate-dependent glutaminase. J Biol Chem. 1977 Mar 25;252(6):1927–1931. [PubMed] [Google Scholar]
- KARMEN A. A note on the spectrometric assay of glutamic-oxalacetic transaminase in human blood serum. J Clin Invest. 1955 Jan;34(1):131–133. [PubMed] [Google Scholar]
- Kovacevic Z., McGivan J. D. Mitochondrial metabolism of glutamine and glutamate and its physiological significance. Physiol Rev. 1983 Apr;63(2):547–605. doi: 10.1152/physrev.1983.63.2.547. [DOI] [PubMed] [Google Scholar]
- Kvamme E., Olsen B. E. Evidence for two species of mammalian phosphate-activated glutaminase having different regulatory properties. FEBS Lett. 1979 Nov 1;107(1):33–36. doi: 10.1016/0014-5793(79)80456-1. [DOI] [PubMed] [Google Scholar]
- Kvamme E., Tveit B., Svenneby G. Glutaminase from pig renal cortex. I. Purification and general properties. J Biol Chem. 1970 Apr 25;245(8):1871–1877. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Marchesi S. L., Steers E., Jr, Shifrin S. Purification and characterization of the multiple forms of beta-galactosidase of Escherichia coli. Biochim Biophys Acta. 1969 May;181(1):20–34. doi: 10.1016/0005-2795(69)90223-2. [DOI] [PubMed] [Google Scholar]
- Morehouse R. F., Curthoys N. P. Properties of rat renal phosphate-dependent glutaminase coupled to Sepharose. Evidence that dimerization is essential for activation. Biochem J. 1981 Mar 1;193(3):709–716. doi: 10.1042/bj1930709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISONOFF A., WISSLER F. C., LIPMAN L. N., WOERNLEY D. L. Separation of univalent fragments from the bivalent rabbit antibody molecule by reduction of disulfide bonds. Arch Biochem Biophys. 1960 Aug;89:230–244. doi: 10.1016/0003-9861(60)90049-7. [DOI] [PubMed] [Google Scholar]
- Nimmo G. A., Tipton K. F. Purification of soluble glutaminase from pig brain. Biochem Pharmacol. 1980 Feb;29(3):359–367. doi: 10.1016/0006-2952(80)90514-6. [DOI] [PubMed] [Google Scholar]
- Olsen B. R., Torgner I. A., Christensen T. B., Kvamme E. Ultrastructure of pig renal glutaminase. Evidence for conformational changes during polymer formation. J Mol Biol. 1973 Feb 25;74(2):239–251. doi: 10.1016/0022-2836(73)90109-5. [DOI] [PubMed] [Google Scholar]
- Patel M., McGivan J. D. Partial purification and properties of rat liver glutaminase. Biochem J. 1984 Jun 1;220(2):583–590. doi: 10.1042/bj2200583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regulation of renal ammoniagenesis. Purification and characterization of phosphate-dependent glutaminase from rat kidney. Arch Biochem Biophys. 1976 May;174(1):82–89. [PubMed] [Google Scholar]
- Schatz G., Butow R. A. How are proteins imported into mitochondria? Cell. 1983 Feb;32(2):316–318. doi: 10.1016/0092-8674(83)90450-6. [DOI] [PubMed] [Google Scholar]
- Shapiro R. A., Clark V. M., Curthoys N. P. Covalent interaction of L-2-amino-4-oxo-5-chloropentanoic acid with rat renal phosphate-dependent glutaminase. Evidence for a specific glutamate binding site and of subunit heterogeneity. J Biol Chem. 1978 Oct 10;253(19):7086–7090. [PubMed] [Google Scholar]
- Shapiro R. A., Morehouse R. F., Curthoys N. P. Inhibition by glutamate of phosphate-dependent glutaminase of rat kidney. Biochem J. 1982 Dec 1;207(3):561–566. doi: 10.1042/bj2070561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Svenneby G., Torgner I. A., Kvamme E. Purification of phosphate-dependent pig brain glutaminase. J Neurochem. 1973 Apr;20(4):1217–1224. doi: 10.1111/j.1471-4159.1973.tb00090.x. [DOI] [PubMed] [Google Scholar]
- Tveit B., Svenneby G., Kvamme E. Kinetic properties of glutaminase from pig renal cortex. Eur J Biochem. 1970 Jun;14(2):337–344. doi: 10.1111/j.1432-1033.1970.tb00294.x. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]