Abstract
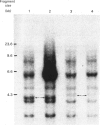
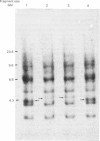
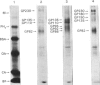
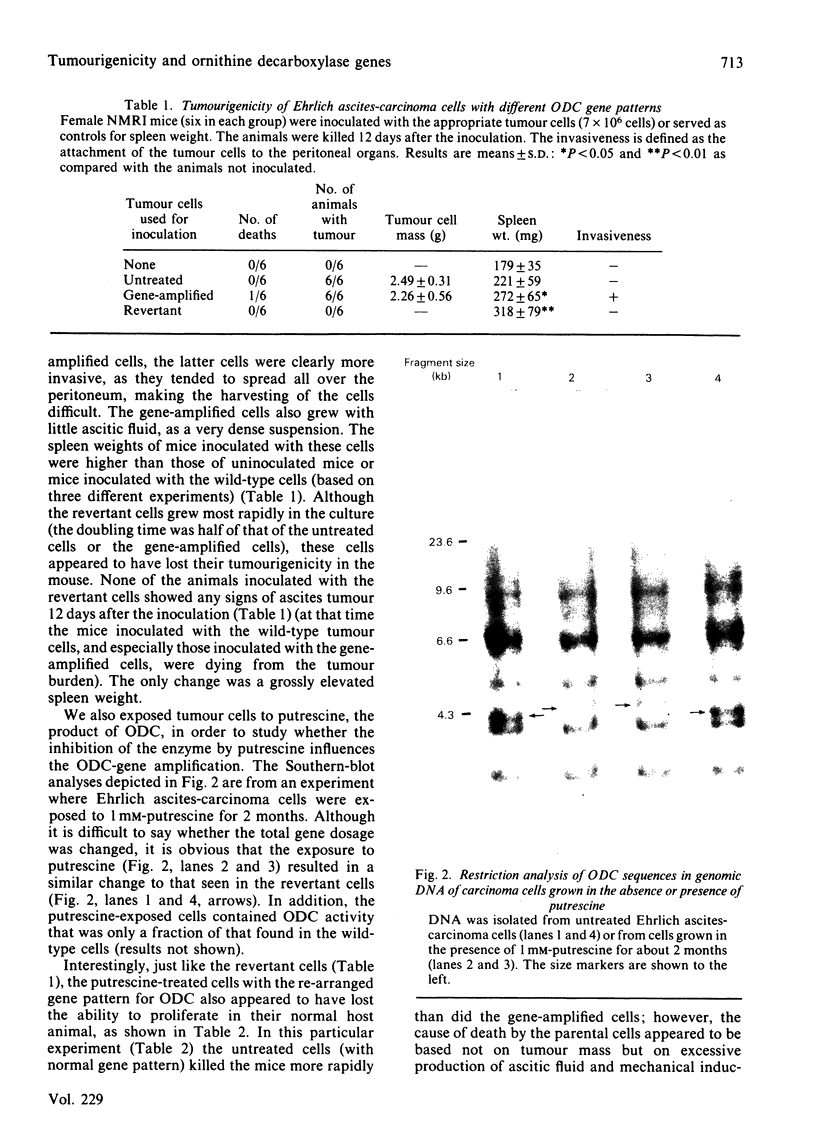
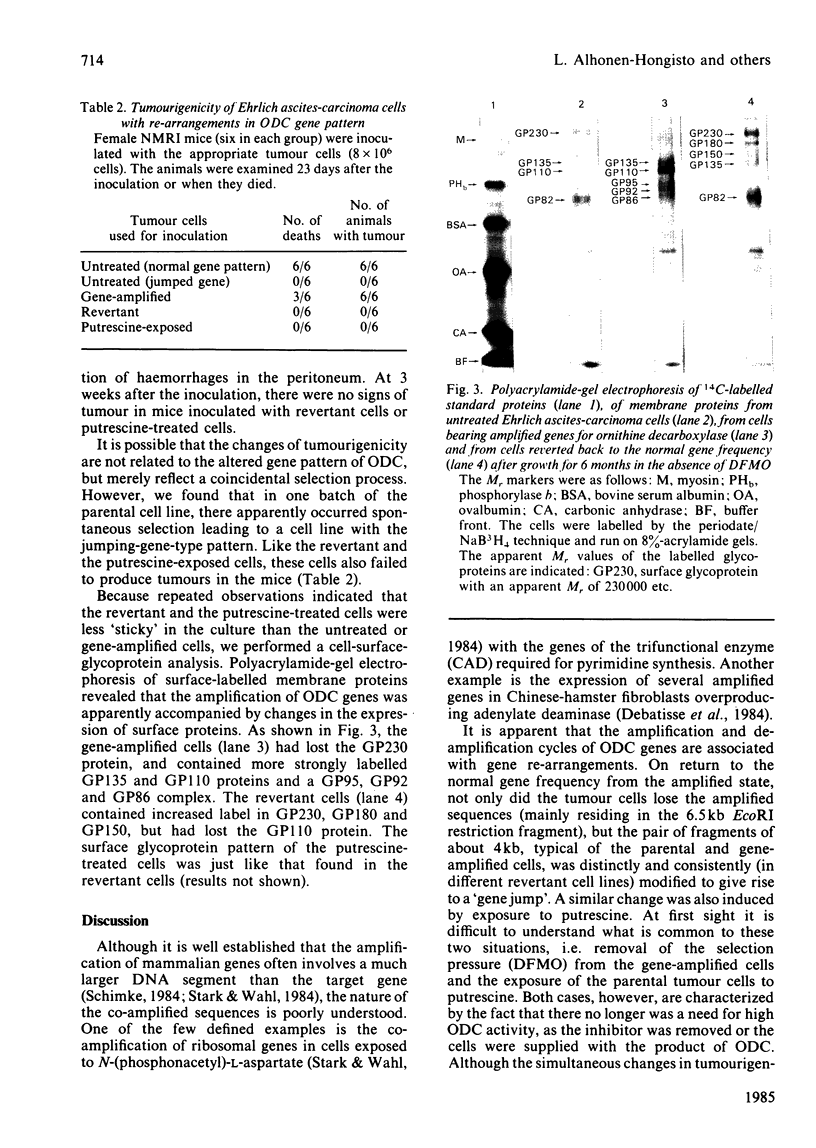
We selected a 2-difluoromethylornithine-resistant Ehrlich ascites-carcinoma cell line that grows in the presence of 20 mM-difluoromethylornithine. These cells contain 10-20 times the normal amount of hybridizable sequences for ornithine decarboxylase (EC 4.1.1.17) in their genomic DNA. We used these gene-amplified cells, their revertant counterparts (grown in the absence of the drug after an established gene amplification) and tumour cells grown in the presence of putrescine to investigate the changes of ornithine decarboxylase gene pattern and simultaneously occurring phenotypic changes, such as tumourigenicity and the expression of cell-surface glycoproteins. In the tumour cells reverted back to the normal gene frequency, not only did the amplified sequences disappear, but there were also signs of gene re-arrangements seen as a "gene jump', when a signal evidently moved to a heavier restriction fragment. Similar gene re-arrangement likewise occurred in cells exposed to putrescine. Although the wild-type tumour cells and the gene-amplified cells readily grew in the peritoneal cavity of mice, the revertant cells and the putrescine-treated cells had lost their tumourigenicity in mice. Gene-amplified tumour cells and the revertant cells showed distinct changes in their surface glycoprotein pattern in comparison with the parental cell line. These findings indicate that alterations of ornithine decarboxylase gene pattern/dosage may be associated with phenotypic changes possibly related to the tumourigenicity of these carcinoma cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alhonen-Hongisto L., Kallio A., Sinervirta R., Seppänen P., Kontula K. K., Jänne O. A., Jänne J. Difluoromethylornithine-induced amplification of ornithine decarboxylase genes in Ehrlich ascites carcinoma cells. Biochem Biophys Res Commun. 1985 Jan 31;126(2):734–740. doi: 10.1016/0006-291x(85)90246-3. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Collard J. G., van Beek W. P., Janssen J. W., Schijven J. F. Transfection by human oncogenes: concomitant induction of tumorigenicity and tumor-associated membrane alterations. Int J Cancer. 1985 Feb 15;35(2):207–213. doi: 10.1002/ijc.2910350211. [DOI] [PubMed] [Google Scholar]
- Debatisse M., de Saint Vincent B. R., Buttin G. Expression of several amplified genes in an adenylate-deaminase overproducing variant of Chinese hamster fibroblasts. EMBO J. 1984 Dec 20;3(13):3123–3127. doi: 10.1002/j.1460-2075.1984.tb02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg C. G., Andersson L. C. Selective radioactive labeling of cell surface sialoglycoproteins by periodate-tritiated borohydride. J Biol Chem. 1977 Aug 25;252(16):5888–5894. [PubMed] [Google Scholar]
- Jänne O. A., Kontula K. K., Isomaa V. V., Bardin C. W. Ornithine decarboxylase mRNA in mouse kidney: a low abundancy gene product regulated by androgens with rapid kinetics. Ann N Y Acad Sci. 1984;438:72–84. doi: 10.1111/j.1749-6632.1984.tb38277.x. [DOI] [PubMed] [Google Scholar]
- Kahana C., Nathans D. Isolation of cloned cDNA encoding mammalian ornithine decarboxylase. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3645–3649. doi: 10.1073/pnas.81.12.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontula K. K., Torkkeli T. K., Bardin C. W., Jänne O. A. Androgen induction of ornithine decarboxylase mRNA in mouse kidney as studied by complementary DNA. Proc Natl Acad Sci U S A. 1984 Feb;81(3):731–735. doi: 10.1073/pnas.81.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McConlogue L., Gupta M., Wu L., Coffino P. Molecular cloning and expression of the mouse ornithine decarboxylase gene. Proc Natl Acad Sci U S A. 1984 Jan;81(2):540–544. doi: 10.1073/pnas.81.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured animal cells. Cell. 1984 Jul;37(3):705–713. doi: 10.1016/0092-8674(84)90406-9. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Wahl G. M. Gene amplification. Annu Rev Biochem. 1984;53:447–491. doi: 10.1146/annurev.bi.53.070184.002311. [DOI] [PubMed] [Google Scholar]