ABSTRACT
Background
Despite achieving endoscopic remission, over 20% of inflammatory bowel disease (IBD) patients experience chronic abdominal pain. Visceral pain and the microbiome exhibit sex-dependent interactions, while visceral pain in IBD shows a sex bias. Our aim was to evaluate whether post-inflammatory microbial perturbations contribute to visceral hypersensitivity in a sex-dependent manner.
Methods
Males, cycling females, ovariectomized, and sham-operated females were given dextran sodium sulfate to induce colitis and allowed to recover. Germ-free recipients received sex-appropriate and cross-sex fecal microbial transplants (FMT) from post-inflammatory donor mice. Visceral sensitivity was assessed by recording visceromotor responses to colorectal distention. The composition of the microbiota was evaluated via 16S rRNA gene V4 amplicon sequencing, while the metabolome was assessed using targeted (short chain fatty acids – SCFA) and semi-targeted mass spectrometry.
Results
Post-inflammatory cycling females developed visceral hyperalgesia when compared to males. This effect was reversed by ovariectomy. Both post-inflammatory males and females exhibited increased SCFA-producing species, but only males had elevated fecal SCFA content. FMT from post-inflammatory females transferred visceral hyperalgesia to both males and females, while FMT from post-inflammatory males could only transfer visceral hyperalgesia to males.
Conclusions
Female sex, hormonal status as well as the gut microbiota play a role in pain modulation. Our data highlight the importance of considering biological sex in the evaluation of visceral pain.
KEYWORDS: Microbiome, fecal microbiota transplant, sex-differences, visceral pain, colorectal distention, DSS colitis, short-chain fatty acids
Introduction
Inflammatory bowel diseases (IBDs), including Crohn’s disease and ulcerative colitis, are relapsing remitting inflammatory diseases of the intestine. These carry a significant economic impact and are currently increasing in prevalence, projected to affect 1% of the Canadian population by 2030.1
Pain, defined as an unpleasant sensory or emotional experience associated with potential tissue damage,2 is a hallmark symptom of IBD. Up to 70% of patients with acute flares of disease experience abdominal pain, which is one of the main reasons that patients seek medical attention. However, 20–60% of IBD patients in endoscopic remission also experience chronic abdominal pain.3–6 Chronic visceral pain is a devastating outcome for patients, leading to high levels of anxiety, depression, and associated poor quality of life.5 The pathophysiological mechanisms underlying chronic pain in the absence of inflammation are not known; however, altered sensory neural processing, resulting in both peripheral and central sensitization, plays a key role in this process.7 Unfortunately, effective treatments for chronic visceral pain are limited, such that 10–20% of IBD outpatients are on chronic narcotic therapy, which is not only ineffective but also leads to increased morbidity and mortality.8,9
The gut microbiome affects a wide variety of gastrointestinal processes, including motility, secretion, barrier, and immune function.10 Dysbiosis, or a change in microbial composition and metabolism, is known to be associated with the pathogenesis of IBD, although it is unclear whether these changes are a cause or a consequence of intestinal inflammation.10 Interestingly, there is evidence that some IBD patients in endoscopic remission continue to exhibit dysbiosis.11–15 The gut microbiome has been recently demonstrated to play a role in pain signalling.16,17 Microbial products (e.g. short chain fatty acids [SCFA18–20] lipopolysaccharides,21,22 pore-forming toxins,23 and N-formyl peptides23) can directly activate nociceptors through their corresponding receptors; indirectly, microbial metabolites can activate both endocrine (enterochromaffin cells24) and immune cells,25 which in turn can modulate pain neurotransmission and the expression of molecular targets in pain signaling, such as the transient receptor potential vanilloid-1 receptor (TRPV1). The enterochromaffin/mucosal afferent circuit was recently shown to be both necessary and sufficient in mediating visceral pain in rodent models; enterochromaffin cells were stimulated by microbial metabolites including the SCFA isobutyrate and isovalerate in a sex-dependent manner.24,26 Our laboratory has previously demonstrated that fecal microbial transplant from a mouse model of post-inflammatory colitis increased visceral hypersensitivity in antibiotic-treated mice.18 Post-inflammatory mice exhibited both increased SCFA-producing species as well as fecal SCFA content; SCFAs, including butyrate and propionate, were able to increase capsaicin-evoked calcium responses in isolated nociceptors, suggesting that microbial-derived metabolites contribute to the pathogenesis of post-inflammatory visceral pain.18
It is well established that women have a higher prevalence of chronic pain conditions compared to men.27 Irritable bowel syndrome (IBS), a disorder characterized by changes in bowel habits and abdominal pain in the absence of overt inflammation, has a well-described female predominance.28 This sex-bias has also been demonstrated for chronic pain in IBD, where female sex has been repeatedly shown to be a predictive factor for the development of chronic abdominal pain in remission.29,30 Unfortunately, current preclinical research demonstrates a sex bias, with most studies using only male animals, leading to a lack of understanding of biological mechanisms specific to visceral pain in females.31
Female rodents have been shown to have increased visceral sensitivity to colorectal distention compared to males.32,33 Interestingly, sex also has a known effect on shaping gut microbiota composition, in both humans34,35 and animals.36 In adults, males and females present different taxa abundance in their gut microbiota and changes in the metabolome across their lifespan.35,37,38 Sex hormones play a key role in shaping the microbiome, as a study conducted on castrated male mice showed similar microbial composition to female mice.37 More recently, the sex-dependent interaction of pain and the microbiome has been studied, where visceral pain was seen to vary across the estrous cycle in specific pathogen-free (SPF) mice but not in germ-free mice.39 Furthermore, ovariectomy-induced visceral hypersensitivity was observed only in SPF mice, but not germ-free mice, further emphasizing an interaction between sex and the microbiome in pain modulation.39 These data suggest that the role of sex and the microbiome in pain modulation is complex.
Our aim was to evaluate whether the dysbiotic microbiota in the post-inflammatory state contributed to visceral hypersensitivity in a sex-dependent manner. We used a well-established post-inflammatory DSS mouse model of colitis, where animals display increased visceral hypersensitivity to colorectal distention, 5 weeks after DSS administration.18,40 We hypothesized that the gut microbiota plays a key role in modulating post-inflammatory chronic visceral pain in female mice.
Methods
Animals
Six-week-old C57BL/6 male and female Elite mice from Charles River Laboratories (Montreal, Quebec, Canada) were housed separately in groups of three in individually ventilated cages (Green Line GM500, SealSafe Plus, Techniplast) with unrestricted access to chow (5062 Pico-Vac Mouse Diet 20 (9% fat; LabDiet, Richmond IN, USA) and water. Cages were housed in controlled conditions with a 12 h:12 h light:dark cycle. All mice were allowed to acclimate for 1 week at the Animal Resource Centre at the University of Calgary prior to the initiation of experiments. Germ-free C57BL/6J were bred and maintained at the International Microbiome Centre (IMC), University of Calgary, Canada, with unrestricted access to chow (LabDiet JL Rat and Mouse/Auto 6F 5K52). Germ-free status was routinely monitored by culture-dependent and -independent methods. ARRIVE tables for the SPF and germ-free facilities can be found in supplementary tables 1 and 2. All protocols were approved by the University of Calgary Animal Care Committee and are in accordance with the guidelines for the ethical use of animals in research of the Canadian Council on Animal Care (AC20–0149).
Dss-induced colitis and damage scoring
Male and female mice were given either 2.5% (wt./vol) DSS (40,000 MW, Alfa Aesar, Waltham, MA, USA) in drinking water, or water alone for 5 days. After 5 days of DSS, all mice were given water alone until day 42 post-DSS. Bodyweight changes were recorded daily for 15 days post-DSS and every 3 days thereafter until day 42. To evaluate the severity of DSS colitis, a previously validated disease activity index (DAI,41) consisting of weight loss, stool consistency, and bleeding, was used for the first 15 days post-DSS administration. All animals were observed and evaluated individually. Control animals did not exhibit any disease activity. Colon length and thickness were assessed at the time of death.18,40 Colon length was assessed by measuring the length of the full colon; the maximum inner thickness of the colon was measured at the distal most region of the colon using a digital calliper42 (Mitutoyo Canada, Mississauga, ON). Microscopic damage scores were assessed using histology. A straight midsection of the colon (1 cm) was dissected and fixed in 10% formalin for 4 days and later embedded in paraffin. Mid-sagittal cuts (7 mm) were collected on coated super-frost slides and dried overnight. For microscopic damage scoring, tissue slides were stained with Alcian blue, hematoxylin and eosin for morphologic analysis. The microscopic damage score was calculated from a 6-point score43 which was developed and validated using chemically induced models of colitis.
Myeloperoxidase enzyme activity assay (MPO)
MPO was measured as previously described.44 Briefly, proximal colon samples (2 cm) from post-inflammatory or control mice were collected immediately after sacrifice, snap-frozen in microcentrifuge tubes, and analyzed 3 days after collection. All solutions were freshly prepared for each experiment. Colonic tissue was thawed and homogenized in 1 mL HTAB buffer (Sigma-Aldrich, Oakville, ON, Canada) for each 75 mg of tissue. The lysate was centrifuged at 13,200 rpm for 30 min at 4°C. Supernatants were transferred into a new microcentrifuge tube. 7 µl of the sample was loaded in triplicate on a 96-well plate. For a complete plate, 20 ml of o-34 Dianisidine (Santa Cruz Biotechnology, Santa Cruz, CA, USA) buffer with 10 µl of 1.2% H2O2 were combined. Samples were read immediately for 30 s, 12 times at 405 nm, using a microplate reader (SpectraMax Plus 384, Molecular Devices, VWR, Edmonton, AB, Canada). Values are presented as MPO units/milligram of tissue/minute.
Estrous cycle evaluation and synchronization of female mice
To control for the effect of sex hormones, all in vivo pain measurements were performed during diestrus in cycling female mice. Vasectomized male C57BL/6 Elite mice (6–7 weeks old) were allowed to rest for 10 days after surgery. Vasectomy effectiveness was determined by the inability of male mice to father a pregnancy when co-housed with female mice after recovery. Five days prior to somatic pain assessment, two cycling female mice were introduced into a vasectomized male mouse’s cage. Pseudopregnancy was confirmed 24–48 h after introducing the females by the presence of a copulatory vaginal plug after mating.45 48 hours thereafter, all females were in diestrus and remained in this stage for at least 7 days.46 In cases where pseudopregnancy was delayed, pain assessment was postponed until 24–48 h after pseudopregnancy was confirmed. Diestrus was confirmed in cycling females on the day of in vivo visceral pain evaluation only using vaginal smear cytology.47 Vaginal smears were not performed 24-48 hours after the presence of a copulatory vaginal plug, to prevent stress to the animals.48,49
Ovariectomy
Ovariectomy was performed as previously described.50 Briefly, five-week-old C57BL/6 female Elite mice were anesthetized with intraperitoneal ketamine and xylazine (100 mg/kg and 10 mg/kg, respectively). A single midline dorsal incision along the lumbar vertebrae was made, and the muscle was perforated to access the ovaries. The ovaries were resected at the tip of the uterine horn, leaving the oviduct and uterus intact. Sham surgery consisted of accessing the ovaries by performing the same incision, but no tissue was resected. Meloxicam (5 mg/kg) was administered subcutaneously once every 24 h for 2 days after the surgery as post-operative care for pain relief. Chow pellets were placed in the bottom of the cage during the post-operative period. Mice were housed in pairs and were allowed to recover for a week before DSS treatment.
Fecal microbiota transplant (FMT)
Fecal samples from donor-controlled or post-inflammatory DSS male and female mice were collected immediately upon defecation on day 42; a total of 200 mg of fecal sample per mouse was collected. Fecal samples were weighted and pooled into their respective group in a 15 mL falcon tube, snap-frozen, and stored at −80°C until use.
On the day of FMT, donor fecal samples were thawed on ice and moved into an anaerobic chamber (Ruskin, Sony Technology Centre, Pencoed, UK). Only a single group of donor fecal samples was processed at a time to control for possible contamination. Inside the anaerobic chamber, pre-reduced PBS was added in a 1:7.5 dilution to the samples and homogenized using an 18 G needle and syringe. Each tube’s supernatant was collected and transferred as 250 µL aliquots into sterile screw-top tubes. Separate aliquots were used for each orogastric gavage. An additional 1 mL aliquot was collected for donor microbiota profiling. Aliquots containing donor slurry for the first orogastric gavage were used immediately. For the second orogastric gavage, Brain Heart Infusion (BHI) broth with 30% glycerol was added in a 1:1 ratio to samples. Samples were subsequently homogenized to cryopreserve the bacteria and stored at −80°C until use.51
Germ-free male and female six-week-old C57BL/6 recipient mice were housed in sterile isolators (NKP-Isotec, Coalville, United Kingdom) at the International Microbiome Centre at the University of Calgary in groups of the same sex, with up to three (male) or five (female) mice per cage. Male and female germ-free mice received either sex-appropriate or cross-sex FMT, from control or post-inflammatory DSS groups. Each recipient mouse received two orogastric gavages of 200 µL of fecal supernatant, the first on day 1 and the second on day 4 to allow for optimal microbiota engraftment.51,52 The inoculated germ-free mice were given a three-week engraftment period before any behavioral testing was performed. Electrode implantation was performed inside the International Microbiome Centre, while visceral pain evaluation was performed outside the facility. Upon transfer from the facility, stool samples from the recipient mice were immediately collected, snap-frozen, and stored at −80°C until evaluation of microbial composition.
Measurement of somatic and visceral pain
For the evaluation of somatic sensitivity, we assessed both mechanical and thermal sensitivity.18 Mechanical and thermal sensitivity in both SPF and FMT-recipient mice were evaluated on the same day. A week later, visceral pain was assessed in all mice using the visceromotor response to colorectal distention. Testing was performed by a blinded investigator.
Mechanical pain sensitivity was evaluated using the automated aesthesiometer for the Von Frey hair test53 (Ugo Basile, Gemonio, Italy). Before the test, all mice were placed in plastic compartments with a wire base for 1 h on three consecutive days to allow for room and equipment acclimation. On the day of the experiment, mice were allowed to habituate in individual opaque compartments until exploratory behavior was no longer observed. The hind paw withdrawal was measured by applying constant pressure with an automated filament to the center of the plantar surface until withdrawal. Each mouse was tested five times, alternating right and left paws with a 10-min resting period. In each animal, the left or right hind paw was chosen at random, and the withdrawal threshold was measured in all animals in a row, followed by evaluation of the opposite hind paw. The automated force exerted by the von Frey filament and latency of paw withdrawal were recorded for each mouse.
Thermal sensitivity was measured using the hot plate test53 (Bioseb, Pinellas Park, FL, USA), which was video recorded for later review. Immediately after the Von Frey test, mice were allowed to rest for 30 min. Mice were placed on a metal hot plate set to 52°C. The latency from placement of the mouse on the heated surface until the first behavioral sign of nociception, such as licking a hind paw, lifting, or jumping, was recorded and the animal was immediately removed. Mice were placed on the hot plate for a maximum of 20 s to prevent tissue damage.
The visceromotor response (VMR) evoked by colorectal distention was performed as outlined below.18,40 48 hours prior to the procedure, mice were anesthetized with ketamine and xylazine. Two electrodes were implanted into the external oblique abdominal muscle and exteriorized through the back of their neck and protected by a plastic tube attached to the skin. On the test day, mice were restrained in a 50 mL falcon tube, and a 10.5 mm diameter balloon catheter was gently inserted 0.5 cm deep into the mouse rectum. Animals were allowed to acclimatize for 1 h. Electrodes were connected to a Bio Amplifier that signals into an electromyogram acquisition system to record and display the response (both from ADInstruments, Colorado Springs, CO, USA). The rectal balloon was stepwise inflated at 15, 30, 45, and 60 mmHg pressures, each for 10 s; mice were allowed a 5-min resting interval between each distention. The electromyographic activity from the abdominal contractions was recorded, and visceromotor responses were calculated using LabChart 7 software (ADInstruments, Colorado Springs, CO, USA).
Microbiota sequencing
Bacterial composition was evaluated using 16S rRNA gene amplicon sequencing as previously described.18 Fecal pellets were freshly collected from mice at different time points during colitis recovery. The collection timepoints were baseline (day 0), 1 week after DSS was last administered (day 13), prior to behavioral tests (day 35) and upon sacrifice (day 42). Upon defecation, 2–3 pellets were immediately collected into sterile microcentrifuge tubes and placed on ice. Samples were stored at −80°C until use. DNA extraction and purification from feces were performed using a QIAamp Fast DNA Stool Mini Kit (Qiagen, Toronto, ON, Canada). DNA quality and quantity were determined using the NanoDrop 2000c spectrophotometer (ThermoFisher, Waltham, MA, USA). DNA was stored at −80°C until 16S rRNA gene amplicon sequencing. The V4 hypervariable region of the bacterial 16S rRNA gene was amplified by using barcoded primers: (16SV4Fwd: AATGATACGGCGACCACCGABARCODETATGGTAATTGTGTGCCAGCMGCCGCGGTAA and 16SV4Rev: CAAGCAGAAGACGGCATACGAGATBARCODEAGTCAGTCAGCCGGACTACHVGGGTWTCTAAT). KAPA HiFi polymerase (Roche, Mannheim, Germany) was used under the following cycling conditions: initial denaturation at 98°C for 2 min, 25 cycles of 98°C for 30s, annealing at 55°C for 30s, extension at 72°C for 20s and final elongation at 72°C for 7 min. Individual PCR libraries were pooled, and then the concentration was measured on a Qubit fluorometer (ThermoFisher). The 16S rRNA v4 gene amplicon sequencing was performed by using a V2–500 cycle cartridge (Illumina) on the MiSeq platform (Illumina, San Diego, CA, USA). Raw fastq files were processed using the DADA2 pipeline 1.16 (R package, version 1.38.0). After removing the V4 primers and reads shorter than 50 base pairs, quality filtering was done with the ’Filter and Trim’ function removing reads with an expected error higher than 2 for forward and reverse reads. An amplicon sequence variants (ASV) table was generated, and taxonomic classification was assigned to each ASV using the Ribosomal Data Project (RDP) classifier training set as the reference database.54 A phyloseq object was constructed from the DADA2 outputs using the phyloseq package (Version 1.30.0) to further compare different ordination methods. Low-occurrence and poorly represented ASVs were filtered to remove noise variables achieve robustness of the ASVs findings.
Evaluation of engraftment
The effectiveness of the FMT was determined by performing 16S rRNA sequencing of the donor and the inoculated recipient fecal samples and identifying the percent of ASVs shared between groups.55 Donor fecal pellets used for FMT were sequenced to determine the total taxa present. The number of taxa identified were used as a reference point to further assess the engraftment percentage. The ASV engraftment rate was defined as the number of engrafted ASVs in the post-FMT recipient mice stool divided by the total number of ASV in the donor stool.56 Microbial diversity was determined by comparing the relative abundance, alpha diversity, and beta diversity between donor and recipient FMT groups.
Liquid chromatography-mass spectrometry (LC-MS)
Metabolic profiling was performed by LC-MS as previously described.18,57 Briefly, 3–4 fecal pellets were freshly collected in microcentrifuge tubes and snap-frozen on dry ice at baseline and at the time of sacrifice. Samples were stored at −80°C until use. For targeted metabolomics, SCFAs were extracted (1:2 ratio wet sample weight [mg] to extraction solvent [mL]) from fecal samples with ice-cold extraction solvent (50% water/acetonitrile, v/v) spiked with stable isotope-labeled SCFA standards (acetic acid -13C2, 5 mm final concentration; propionic acid-13C3, 1.5 mm final concentration; butyric acid-13C2, 500 µM final concentration; isobutyric-d7 acid, 100 µM final concentration; valeric-d9 acid, 50 µM final concentration; Isovaleric-d9 acid, 100 µM final concentration), derivatized with N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) and aniline, and then submitted to liquid chromatography-mass spectrometry analysis. The platform consists of a VanquishTM ultrahigh-performance liquid chromatography system coupled to a TSQ FortisTM triple quadrupole Mass Spectrometer (UHPLC-MS/MS; Thermo Scientific) equipped with an electrospray ionization (HESI-II) probe. SCFAs were separated on a Hypersil GOLDTM C18 column (200 × 2.1 mm, 1.9 mm; Thermo Scientific) using a binary solvent system composed of LC-MS grade water and methanol both containing 0.1% (%v/v) formic acid and monitored with a mass spectrometer operating in positive ionization mode and selected reaction monitoring (SRM) mode.
The semi-targeted metabolomics methods used in this study have been described in detail elsewhere.58 Briefly, analysis was performed on a Q Exactive™ Hybrid Quadrupole-Orbitrap™ Mass Spectrometer (Thermo-Fisher) coupled to a Vanquish™ UHPLC System (Thermo-Fisher). Chromatographical separation of metabolites was performed on a Syncronis HILIC UHPLC column (2.1 mm x 100 mm x 1.7 µm, Thermo-Fisher) at a flow rate of 600 µL/min using a binary solvent system: solvent A, 20 mm ammonium formate pH 3.0 in mass spectrometry grade H20 and solvent B, mass spectrometry grade acetonitrile with 0.1% formic acid (%v/v). Sample injection volume was 2 µL. Data were acquired in negative full scan ionization mode at a resolution of 140,000 scanning from m/z 70–1000.
Data analysis was performed using the open-source software Metabolomic Analysis and Visualization Engine (MAVEN,59,60) and the absolute quantification of native SCFA concentration was calculated based on the12C:13C signal intensity ratio and the respective13C-internal standard concentration. For semi-targeted metabolomics, metabolite peak assignments were determined by matching the previously established m/z and retention time (RT) of authentic standards with observed metabolite signals. To generate the heat map, signal intensities were normalized using z-scores: mean-centered, variance-stabilized signal intensities. For row clustering, a hierarchical clustering algorithm that merged or split rows based on their similarity, forming a tree-like structure known as a dendrogram was used. Semi-targeted metabolomics data was further analyzed using MetaboAnalyst 5.0. Data was normalized by log transformation base 10 to remove heteroscedasticity.
Statistical analysis
All data are expressed as the mean ± standard error of the mean (SEM), and the total number of mice as n, unless otherwise stated. Colonic length, thickness, MPO, hot plate, and SCFA data are presented as mean ± standard deviation (SD), given that only a single measurement was made per mouse presented. Statistical analyses were performed using Prism 9.0 software (GraphPad, La Jolla, CA, USA). The Shapiro–Wilk normality test was used, given that the sample size is n < 50, to determine the distribution of the data. If data followed a normal distribution, the mean was compared using parametric tests; otherwise, nonparametric methods were used. If a Gaussian distribution was confirmed, one-way or two-way analysis of variance (ANOVA) with Bonferroni or Tukey post-hoc test was applied for comparison of multiple groups with one or two variables; otherwise, the Kruskal–Wallis nonparametric test was used with a Dunn’s post-hoc test. A p value < 0.05 was considered to be significant.
The 16s rRNA sequencing analysis was carried out by using the R package phyloseq61 (version 1.38.0). Alpha diversity was calculated using the Shannon diversity index. Beta diversity was represented using a Bray-Curtis dissimilarity index with non-metric multidimensional scaling (NMDS), graphically plotting samples based on taxonomic composition dissimilarities between samples with an 80% confidence interval. Differential abundance was determined using the DESeq2 function, representing which taxa significantly differ in relative abundance between groups. Data were normalized by applying size factor normalization to ensure that the counts for each ASV was normalized to the same sequencing depth. Differential abundance analysis was carried out by using the R package DESeq2 to identify ASVs that were differentially abundant across treatments.62 To build the results table, independent filtering was done by including an alpha argument cutoff set to p < 0.01 to decrease the false discovery rate (FDR). Significant changes in ASVs abundance were represented on a logarithmic scale base 2-fold change, treating the increase and decrease ratio in a similar fashion. Finally, each function performed the following significance tests: two-way analysis of variance test with Tukey’s post-hoc test (alpha diversity), PERMANOVA (beta diversity), and Wald test (differential abundance). Supplementary data containing summary statistics at different steps of the analysis pipeline can be found in Supplementary data 1. Raw sequencing data are available at http://www.ncbi.nlm.nih.gov/bioproject/1132548, as well as the mapping file to Supplementary data 2. For semi-targeted metabolomic analysis, a one-way ANOVA with a p-value (FDR) of 0.05 was performed to evaluate whether any of the 83 measured metabolites had significant differences across groups. Raw metabolomic data can be found in Supplementary data 3.
Results
Biological sex modulates post-inflammatory visceral hypersensitivity
We first evaluated whether inflammation-induced microbial perturbation had a sex-specific effect on visceral hypersensitivity. Male and cycling female mice were given 2.5% DSS for 5 days and allowed to recover for 5 weeks (Figure 1a). Both male and female mice exposed to DSS demonstrated marked weight loss when compared to control counterparts (Figure 1(b)) as well as increased macroscopic disease activity (Figure 1(c)). Male mice treated with DSS experienced a significantly greater degree of weight loss compared to cycling females, as well as a significant increase in the disease activity index, suggesting that males exhibited increased colitis severity, as previously reported.63 By 5 weeks after DSS administration, DSS-treated mice recovered their body weight to the same level as the untreated mice (Figure 1(b)). A significant decrease in colonic length was seen in male mice treated with DSS (Figure 1(d)); however, no other evidence of macroscopic or microscopic inflammation was seen (Figures 1(f-g)). At this time point, sex differences were no longer detected.
Figure 1.
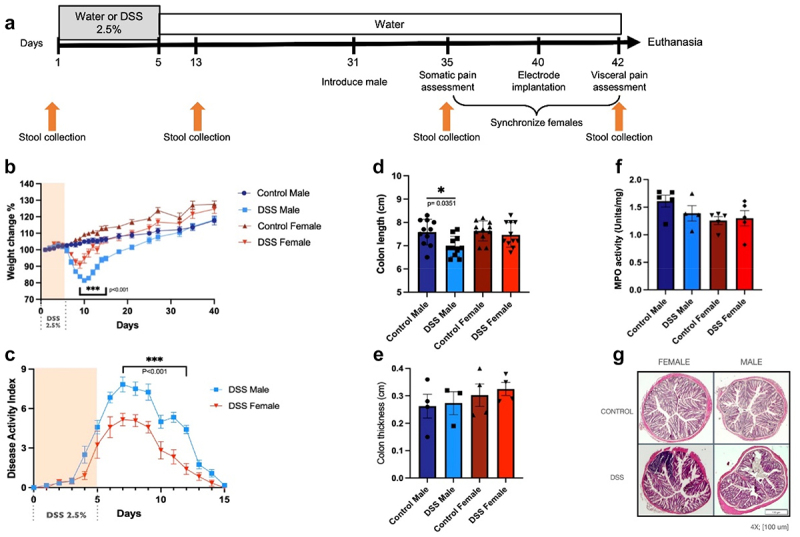
Assessment of DSS colitis severity in males and females. (a). Experimental timeline. Male and female mice were given 5 days of DSS or water and allowed to recover for 5 weeks. At day 31, a vasectomized male was introduced to synchronize females. Somatic pain assessment was performed at day 35. Electrodes were implanted at day 40 and visceral pain assessment was performed at day 42 followed by euthanasia of animals. Stool collection time points are indicated by arrows. (b). Weight changes in post-inflammatory mice. Two-way ANOVA, Tukey post hoc test, ***p≤0.001, comparing male DSS vs female DSS (from day 9–15), male control vs male DSS (from days 7–20), female control vs female DSS group (from day 8–13), n=12/group. (c). Disease activity index in DSS mice. Two-way ANOVA, Tukey post hoc test ***p≤0.001 from day 7–12, n=12/group. (d-f). Macroscopic and microscopic inflammation in DSS male and cycling females. (d). Colonic length changes in post-inflammatory mice. One-way ANOVA, Bonferroni post hoc test, *p=0.035, comparing male DSS vs male control (n=11-13/group). No significant differences were seen between male DSS vs female DSS (p=0.14) or between female control and female DSS (p>0.99). No changes in thickness (e; n=4/group), or tissue myeloperoxidase levels (f; n=4-5/group) were seen. (g). Representative photomicrographs of colonic cross sections. No microscopic evidence of inflammation was seen. Scale bar, 100 µm.
Visceral and somatic pain measurements were performed in both males and diestrus cycling females, as pain is known to vary across the estrus cycle.39 Mechanical sensitivity was assessed by using the automated Von Frey test in the hind paw, and thermal sensitivity was evaluated by using the hot plate test. Both post-inflammatory DSS males and cycling females exhibited mechanical allodynia (Figure 2(a)) with no sex-differences observed between groups. Only post-inflammatory female mice developed thermal hypersensitivity (Figure 2(b)), although a similar trend was seen in post-inflammatory males.
Figure 2.
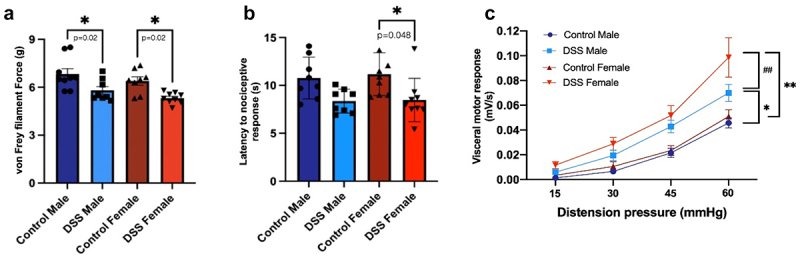
Somatic and visceral sensitivity in post-inflammatory males and females. (a). Mechanical sensitivity assessed using the automated Von Frey test. One-way ANOVA, Bonferroni post-hoc test *p=0.02 control vs DSS males and control vs DSS females. N=8-9/group. (b). Thermal sensitivity was evaluated using the hot plate test. One-way ANOVA, Bonferroni post-hoc test *p=0.048 control vs. DSS females. No differences were seen when comparing control vs DSS males, p=0.18. N=8-9/group. (c). Visceral sensitivity was assessed using the visceromotor response to colorectal distention. 45mmHg distention: control males vs DSS males, *p≤0.05; control females vs DSS females, ** p≤0.01. 60mmHg distention: control males vs DSS males, *p≤0.05; control females vs DSS females: **p≤0.01, DSS males vs DSS females, ##p=0.003. Two-way ANOVA, Tukey post-hoc test, n=11-12/group.
The visceromotor response to colorectal distention was increased in both male and female post-inflammatory mice when compared to their control counterparts (Figure 2(c)), similar to previous studies.18,40 However, post-inflammatory females demonstrated significantly increased visceral hypersensitivity compared to males (Figure 2(c)), suggesting that biological sex plays a role in visceral hypersensitivity.
Sex hormones modulate post-inflammatory visceral hypersensitivity
Given the increase in visceral hypersensitivity in post-inflammatory females, we next explored whether sex hormones were involved. Ovariectomy (OVX) or sham surgery was performed 1 week prior to DSS administration (Figure 3(a)). OVX or sham-control female mice were given DSS and allowed to recover as above. Evaluation of visceral and somatic pain was performed in diestrus in sham females; ovariectomized mice were introduced to vasectomized males without synchronization. Sham-treated females experienced a significantly increased severity of weight loss after DSS administration compared to OVX mice (Figure 3(b)), as well as increased disease activity (Figure 3(c)), as previously reported.63,64 Five weeks after DSS administration, DSS-treated mice recovered their body weight to the same levels as that of untreated mice and did not display evidence of microscopic inflammation (Figures 3(e-f)). Control mice that had an ovariectomy had a decrease in colonic length and increased thickness compared to sham surgery, indicating a possible effect on intestinal smooth muscle by sex hormones.65 A significant decrease in colonic length was also seen in sham operated DSS mice (Figure 3(d)), however the colonic length was similar in females treated with DSS (Figure 1(d)) and sham operated mice given DSS (Figure 3(d)). No differences in MPO levels were seen.
Figure 3.
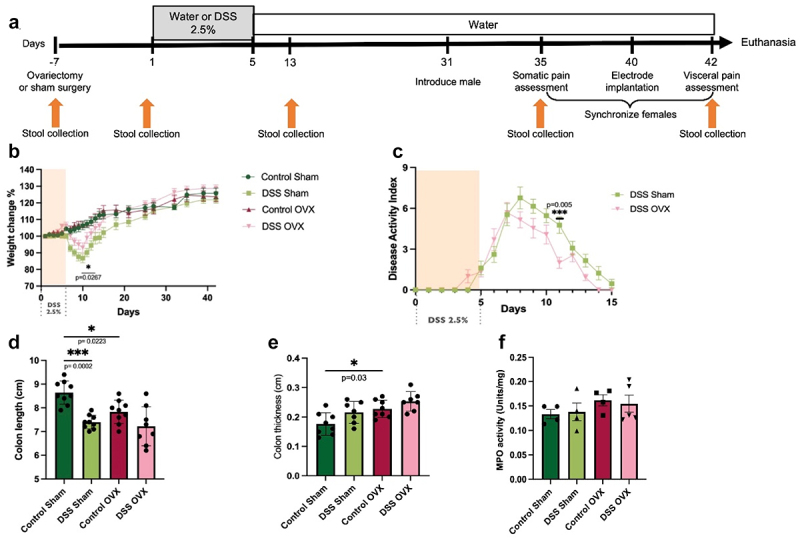
Assessment of DSS colitis severity in sham-operated and OVX-females. (a). Experimental timeline. Ovariectomy or sham surgery was performed one week prior to DSS administration. Mice were then given 2.5% DSS and allowed to recover as outlined above. Stool collection time points are indicated by arrows. (b). Weight changes in sham and OVX females. Two-way ANOVA, Tukey post hoc test, *p≤0.05, comparing sham DSS vs OVX DSS on day 11, ***p≤0.001 comparing sham control vs sham DSS (from day 8–12), and ***p≤0.001 OVX control vs OVX DSS (from day 9–11), n=13/group. (c). Disease severity in sham and OVX females. Two-way ANOVA, Tukey post hoc test, **p=0.005 at day 11, n=13/group. (d). Colonic length. One-way ANOVA, Bonferroni post-hoc test, *p=0.02, sham control vs OVX control, ***p=0.0002, sham control vs DSS control, n=11-13/group. No significant differences were seen between DSS sham vs DSS OVX (p>0.99). (e). Colonic thickness. One-way ANOVA, Bonferroni post-hoc test, *p≤0.05, sham control vs OVX control, n=7-8/group. (f). MPO activity, n=4-5/group.
No differences in mechanical (Figure 4(a)) or thermal sensitivity (Figure 4(b)) were seen when comparing female sham vs. OVX mice. It is possible that the difference noted between sham-operated females and cycling females (Figure 2(b)) is secondary to the effect of anesthesia, analgesia, surgery-induced stress or indeed, decreased severity of intestinal inflammation in OVX mice. OVX females given DSS displayed a significant decrease in visceral hypersensitivity when compared to DSS sham females (Figure 4(c)), suggesting that sex hormones play a role in visceral but not somatic hypersensitivity.
Figure 4.
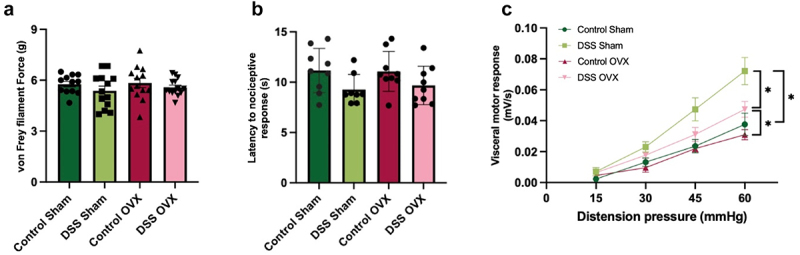
Somatic and visceral sensitivity in post-inflammatory sham-operated and OVX females (a). Mechanical sensitivity in sham vs OVX females. N=11-12/group. One-way ANOVA, Bonferroni post-hoc test. (b). Thermal sensitivity in sham vs OVX females N=8-9/group. One-way ANOVA, Bonferroni post-hoc test. (c). Visceral sensitivity in sham vs OVX mice. OVX DSS females displayed a significant decrease in visceral hypersensitivity compared to sham DSS females. 45mmHg distension: control sham vs DSS sham group, *p≤0.05. 60mmHg distension: control sham vs DSS sham, *p≤0.05; control OVX vs DSS OVX *p≤0.05, DSS sham vs DSS OVX p≤0.05. Two-way ANOVA, Tukey post hoc test. n=12-13/group.
Gut microbiota and fecal short-chain fatty acid content differ between control and post-inflammatory males and females
As our previous study demonstrated that microbial manipulation modulates the development of visceral hypersensitivity,18 and as microbes and bacterial metabolites, such as SCFA can sensitize afferent neurons,18,19,66 we next evaluated the gut microbiota composition and metabolic profile (metabolome) of post-inflammatory cycling and OVX females compared to males. Stool was collected from mice at baseline [day 0], 1 week after DSS treatment [day 13], prior to somatic pain assessment [i.e., after 4 weeks of recover at day 35], and at day 42, prior to visceral pain assessment (Figure 1(a)). To control for microbiota changes due to sham or OVX surgery, we collected additional samples prior to surgery [day −7; Figure 3(a)). For metabolomic profiling, stool was collected at baseline [day 0] and immediately prior to visceral pain testing [day 42; Figure 1(a)).
Amplicon sequencing of the V4 region of the bacterial 16S rRNA gene was used to compare the gut microbiota profile of post-inflammatory males, females and controls at the timepoints outlined above. Evaluation of the alpha diversity [Shannon; Figure 5(a)) demonstrated a significant decrease in diversity in male mice 1 week after DSS treatment compared to baseline, suggesting a decrease in the overall species richness and evenness in the microbial community. By day 35, microbial diversity in post-inflammatory males had recovered to baseline levels. In contrast, post-inflammatory female mice displayed a significant increase in their microbial diversity during DSS recovery. By day 42, the diversity in the post-inflammatory female microbiota had returned to baseline levels. No change was seen in alpha diversity between control male or female mice at any point of the experiment. Changes in the Shannon diversity index could indicate a decrease in the abundance of some microbial species or an increase in the dominance of one or a few species, affecting the evenness of distribution with the sample. Altogether, we saw a decrease in the Shannon diversity index in both post-inflammatory males and females at different time points during the experiment; however, both returned to baseline, suggesting that there was a recovery in the overall species richness over the course of the experiment.
Figure 5.
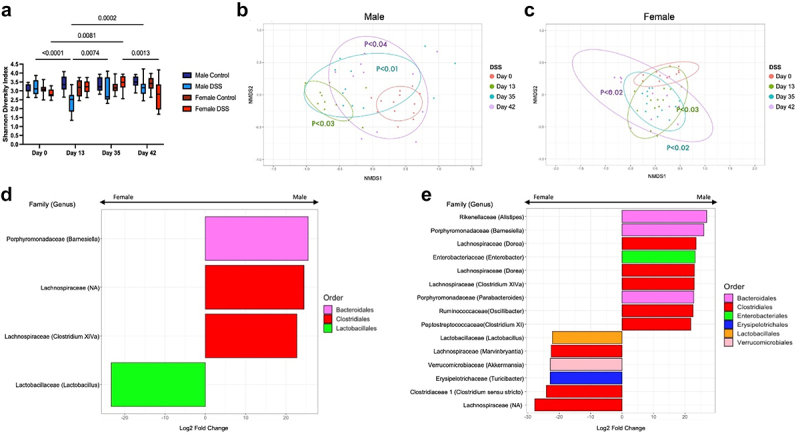
Changes in gut microbiota composition in post-inflammatory males and females. (a). Shannon diversity index. There was a significant decrease in the alpha diversity [p<0.0001, two-way ANOVA, Tukey post-hoc test] in male mice 1 week after DSS treatment compared to baseline, which recovered to baseline levels by day 35. Female post-inflammatory mice displayed a significant increase in their microbial diversity compared to baseline [p=0.008, two-way ANOVA, Tukey post-hoc test] at day 35, which recovered to baseline levels by day 42 [p=0.001, two-way ANOVA, Tukey post-hoc test compared to day 35]. No change was seen in alpha diversity between control male or female mice at any point of the experiment. (b-c). Beta diversity (Bray-Curtis index, NMDS) in male (b) and female (c) post-inflammatory mice. Circles indicate 80% confidence interval. Males and females treated with DSS showed a significant shift in diversity after DSS administration, which persisted until day 42. All timepoints compared to control with PERMANOVA. (d). Log2 fold change of the ASVs differentially increased in males (right) and females (left) at day 0. ASVs with an adjusted p-value below p<0.01 were considered differentially abundant. DESeq2, Wald test. (e). Log2 fold change of the ASVs differentially increased in males (right) and females (left) at day 42. ASVs with an adjusted p-value below p<0.01 were considered differentially abundant. DESeq2, Wald test.
Beta diversity was analyzed using the Bray-Curtis index. One week after DSS administration, both male (Figure 5(b)) and female (Figure 5(c)) post-inflammatory mice displayed a significant shift from their baseline diversity. As male mice recovered from DSS, the distance between the groups narrowed, but remained significantly different from baseline at day 42 (Figure 5(b))]. In contrast, the degree of dissimilarity increased as the post-inflammatory females recovered from colitis and remained increased at day 42 (Figure 5(c)). Neither male nor female control mice showed any change in beta-diversity over the course of the experiment (Figure S1(a-b)). These data suggest that the microbial community was significantly different in both male and female mice in the post-inflammatory state compared to baseline.
We next evaluated the differential abundance of the gut microbiota at baseline and day 42 between post-inflammatory males and females. Few changes were seen between sexes at day 0 (Figure 5d). At baseline, the Barnesiella variant from the Bacteroides phylum and Lachnospiracea were found to be significantly increased in males when compared to females, whereas females demonstrated significant enrichment in Lactobacillus, as has been previously demonstrated.67 An increase in the abundance of different genera was seen in the post-inflammatory DSS state, when comparing sexes at day 42 (Figure 5(e)). Enterobacter and Oscillobacter variants from the Proteobacteria and Firmicutes phylum were found in increased abundance in post-inflammatory males compared to post-inflammatory females. In contrast, an increased abundance of variants from the Firmicutes phylum as well as Akkermansia were found in post-inflammatory females compared to males in the post-inflammatory state. Increased abundance of SCFA-producing Firmicutes, such as Lachnospiracea and Ruminococaceae, were seen in both groups, similar to our previous study in males.18 Together, these data suggest that significant sex differences in the gut microbiome exist in the post-inflammatory state. In addition, both males and females exhibit increases in some SCFA-producing families in the post-inflammatory state.
To further understand the sex-specific differences in microbiota composition, we evaluated the metabolic profile of these microbial communities through both targeted and semi-targeted metabolomic analysis of fecal samples. We first evaluated SCFA content, since our previous data showed that these metabolites were significantly increased in the post-inflammatory state in male mice and were able to directly sensitize visceral afferent neurons.18 Fecal SCFA levels, including acetate, butyrate, and isobutyrate were significantly increased in male post-inflammatory mice when compared to controls (Figure 6(a-f)). In contrast, post-inflammatory females exhibited a decrease in fecal content of acetate, butyrate, isobutyrate and isovalerate when compared to males, despite the increase in SCFA-producing families. These data suggest that biological sex plays a role in bacterial metabolism.
Figure 6.
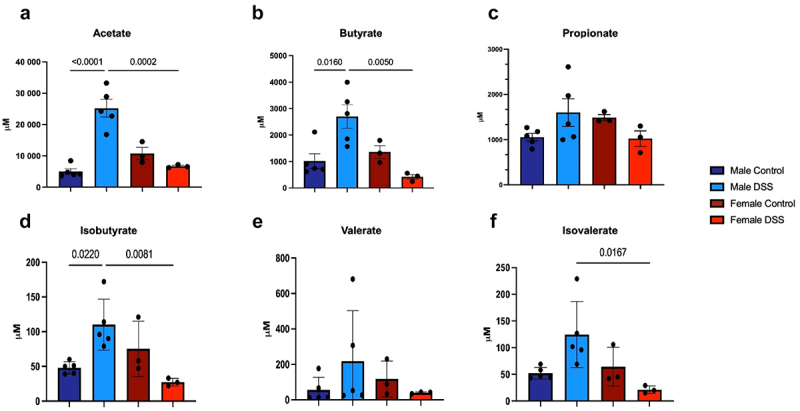
Changes in fecal short chain fatty acids in post-inflammatory male vs female mice. (a). Fecal acetate levels; p<0.001 DSS males vs male controls and p=0.0002 DSS males vs DSS females. (b). Fecal butyrate levels; p=0.0123 DSS males vs male controls, p=0.0040 DSS males vs DSS females. (c). Fecal propionate levels. (d). Fecal isobutyrate levels; p= 0.0167 DSS males vs control males, p=0.0064 DSS males vs DSS females. (e). Fecal valerate levels. (f). Fecal isovalerate levels. p=0.0167 DSS males vs DSS females. One-way ANOVA, Bonferroni post-hoc test, n=3-5/group.
Semi-targeted metabolomic analysis was performed for control and post-inflammatory male and female mice. With this approach, we identified 83 metabolites (Figure S2; a heatmap of the signal intensity of these at day 42 is shown in Figure S3), with 32 of these metabolites displaying significant differences between groups (Figure S4). As we were interested in metabolites that may modulate visceral pain, we evaluated metabolites that were similar at baseline when comparing males and females, given that baseline visceral pain was comparable between the sexes. Of these 14 metabolites, two are known to play a role in visceral hypersensitivity: Adenine (Figure 7(a)) and Tryptophan (Figure 7(b)). Data for the other metabolites can be found in the supplementary data (Figure S5).
Figure 7.
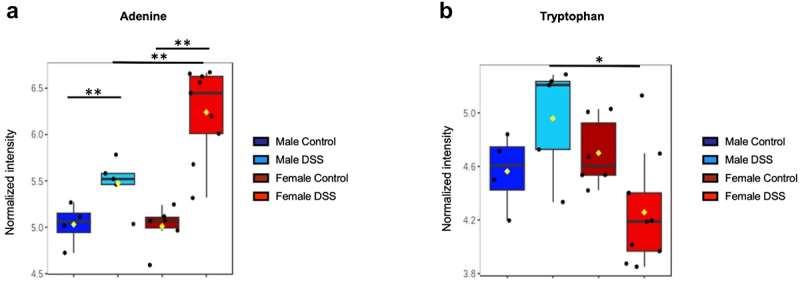
Fecal metabolites known to participate in visceral hyperalgesia. (a). Adenine signal intensity across groups. (b). Tryptophan signal intensity across groups. The box plot represents the median value ± the interquartile range. One-way ANOVA, Tukey’s post-hoc test; FDR <0.05. **p<0.01, *p<0.05. n=4-9 samples per group.
Few changes in the differential abundance are seen after ovariectomy
As sex hormones modulated visceral hypersensitivity, we next evaluated whether they affected gut microbiota composition. The alpha diversity was evaluated between sham and OVX control mice. No significant changes were observed at any point during the experiment (Figure 8(a)) DSS Sham females showed a significant increase in diversity at day 42 compared to baseline, while DSS OVX females displayed a significant increase in diversity at days 35 and 42 when compared to baseline. No differences between the DSS sham and DSS OVX group were noted. Altogether, we saw a change in the Shannon diversity index in both sham-operated and OVX females at different timepoints from DSS recovery, suggesting that there was an overall increase in species richness over the course of the experiment.
Figure 8.
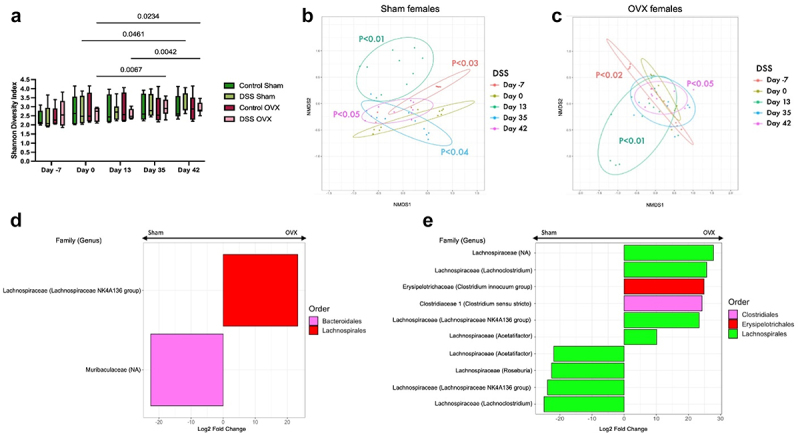
Changes in gut microbiota composition in post-inflammatory sham-operated and OVX females (a). Shannon diversity index. Post-inflammatory sham females showed a significant increase in alpha diversity at day 42 compared to day 0 (p=0.0461), while post-inflammatory OVX females displayed a significant increase in diversity at days 35 (p=0.0067) and 42 (p=0.0234) when compared to baseline. No differences were seen between OVX or sham control mice. Two-way ANOVA, Tukey post-hoc test. (b-c). Beta diversity (Bray-Curtis index, NMDS) in sham-operated (b) and OVX (c) post-inflammatory mice. Circles indicate 80% confidence interval. Sham [p<0.03, PERMANOVA] and OVX [p<0.02, PERMANOVA] surgery resulted in a significant shift in the gut microbiota. Sham and OVX females treated with DSS showed a significant shift in diversity after DSS administration [p<0.01 PERMANOVA] which persisted until day 42. (d). Log2 fold change of the ASVs differentially increased in OVX (right) and sham-operated females (left) at day 0. ASVs with an adjusted p-value below p<0.01 were considered differentially abundant. DESeq2, Wald test. (e). Log2 fold change of the ASVs differentially increased in OVX (right) and sham-operated females (left) at day 42. ASVs with an adjusted p-value below p<0.01 were considered differentially abundant. DESeq2, Wald test.
Beta diversity was analyzed using the Bray-Curtis index. Sham and OVX surgery did cause a significant shift in the gut microbiota in the post-inflammatory mice, likely secondary to the use of analgesia, anesthesia and surgery-induced stress (Figures 8(b-c)), with a similar trend seen in the control sham-operated and OVX mice (Figures S6A and B]. Sham and OVX females treated with DSS showed a similar trend to cycling females, with a shift in diversity after DSS administration (Figures 8(b-c)) which did not recover to baseline. These data suggest that sex hormones also contribute to the differences in the microbial community in the post-inflammatory state.
We next evaluated the differential abundance of the gut microbiota at baseline and day 42 between sham-operated and OVX post-inflammatory females. Few changes were seen at baseline, with only one ASV increased in each group: Lachnospiraceae NK4A136 in OVX females and Muribaculaceae in sham females (Figure 8(d)). In the post-inflammatory state, both sham and OVX female mice showed an increase in differential abundance of ASVs from the Lachnospiraceae family (Figure 8(e)). Additionally, the post-inflammatory OVX female group exhibited an increase in Clostridium genera. These findings suggest that minor differences between sham and OVX females were observed before and after recovery from DSS treatment, when compared to the differences seen between males and cycling females.
FMT from post-inflammatory females transfers visceral hypersensitivity to both males and females, while FMT from post-inflammatory males only transfers visceral hypersensitivity phenotype to male recipients
To evaluate whether the post-inflammatory microbiota had sex-specific effects on visceral hypersensitivity, we performed FMT experiments. Germ-free male and female recipients received stool from both male and female post-inflammatory mice (Figure 9(a)). Three weeks post-FMT,51,52 visceral sensitivity was evaluated as described above.
Figure 9.
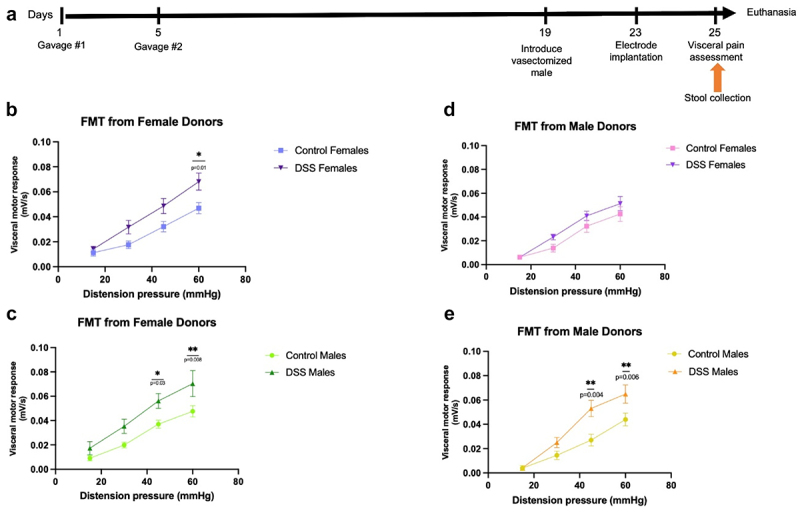
Evaluation of visceral sensitivity following sex-appropriate or cross-sex FMT. (a). Experimental timeline. Germ-free male and female recipients received sex-appropriate or cross-sex FMT at days 1 and 5. At day 19, a vasectomized male mouse was introduced to female recipients to synchronize the estrous cycle. Electrodes were implanted at day 23 and visceral sensitivity assessment was performed at day 25, 3 weeks post-FMT. Stool collection time point is indicated. (b). Visceromotor response to colorectal distention in female recipients who received stool from female donors. 60mmHg distention: p=0.01. Two-way ANOVA, Tukey post-hoc test, n=10. (c). Visceromotor response to colorectal distention in male recipients who received stool from female donors. 45mmHg distention: p=0.03 and 60mmHg distention: p=0.008. Two-way ANOVA, Tukey post-hoc test, n=8-11. (d). Visceromotor response to colorectal distention in female recipients who received stool from male donors, n=8-9. (e). Visceromotor response to colorectal distention in male recipients who received stool from male donors. 45mmHg distention: p=0.004 and 60mmHg distention: p=0.006. Two-way ANOVA, Tukey post-hoc test, n=9.
Both female (Figure 9(b)) and male (Figure 9(c)) germ-free recipients demonstrated a significant increase in visceral hypersensitivity when given stool from female post-inflammatory donors compared to female control donors. However, only male germ-free recipients (Figure 9(e)) demonstrated a significant increase in visceral hypersensitivity when given stool from post-inflammatory donors compared to male control donors. In contrast, female germ-free recipient mice did not exhibit any difference in visceral hypersensitivity when given stool from post-inflammatory male donors compared to male control donors (Figure 9(d)). The degree of visceral hypersensitivity caused by FMT did not fully recapitulate the intensity of the effects observed in conventional mice (Figure 2(c)) when comparing the peak of the visceromotor response. Together, these data suggest that microbes and/or their products from post-inflammatory female mice are pro-nociceptive regardless of recipient sex, whereas female mice are resistant to the pro-nociceptive effects of post-inflammatory male stool.
We evaluated the engraftment rate of FMT in germ-free recipients using male and female donor microbiota by evaluating the number of total ASVs present.55 In female donors, we identified 342 taxa in the control group and 480 taxa in the DSS group [n = 20-21/group]. In recipients of control female stool, the engraftment percentage was 70% in males and 80% in females. In recipients of female DSS stool, the engraftment percentage was 82% in males and 77% in females. In male donors, we identified 303 taxa in the control group and 319 taxa in the DSS group [n = 18/group]. In recipients of control male stool, the engraftment percentage was 78% in males and 86% in females. In recipients of DSS male stool, the engraftment percentage was 81% in males and 83% in females. These data suggest that engraftment of fecal microbiota for these FMT experiments were similar between sex-appropriate and cross-sex FMT.
Discussion
Female IBD patients are at increased risk of developing chronic pain in the absence of inflammation,29,30 but this has not been previously examined in a mechanistic fashion. We have demonstrated that female mice exhibit increased visceral pain after recovery from DSS colitis, despite displaying less disease activity compared to their male counterparts. OVX reversed these changes on visceral pain. SCFA-producing species were increased in both sexes, but fecal SCFA content was only increased in the stool of post-inflammatory males. As SCFA can sensitize nociceptive neurons,18 this suggests that SCFA may play a role in visceral pain in a sex-specific manner. Crucially, FMT of female stool was pro-nociceptive to both male and female germ-free recipients, while male stool only produced a pro-nociceptive phenotype in male mice. Overall, these data demonstrate that both hormonal status as well as the gut microbiota play a role in nociception and highlight the importance of considering biological sex as an important variable in the evaluation of visceral pain.
Male mice treated with DSS showed a greater degree of weight loss and colitis severity than female mice, consistent with previous studies that have reported increased colitis severity in male mice.63 Moreover, our results suggest that OVX may have a protective effect in decreasing the severity of colitis, as OVX females displayed decreased colitis severity compared to sham females. Previous studies using OVX female mice have demonstrated that estrogen deficiency also decreases colitis severity, while estrogen supplementation increases colonic inflammation, as well as visceral hypersensitivity.63,64 This supports our own work, which suggests a role for sex hormones in modulating visceral sensitivity in the post-inflammatory state. Interestingly, another study which evaluated the role of hormonal status and the gut microbiome demonstrated that OVX increased visceral hypersensitivity in conventional female mice, but not in germ-free animals.39 However, we did not observe any differences in visceral pain responses between sham and OVX controls. It is possible that these differences are due to the mouse model used, or differences in the gut microbiota between the different facilities. Nevertheless, these data highlight the significance of investigating sex-specific differences in preclinical research. Future directions could include examination of orchiectomy on visceral pain and colitis severity.
As visceral and somatic pain thresholds are known to vary across the estrous cycle in rodents,39,68 we chose to perform both visceral and somatic sensitivity measurements in the diestrus phase as this is the longest phase of the estrous cycle. Interestingly, a previous study demonstrated that the gut microbiome remains stable throughout the estrous cycle in control mice.69 Thus, future directions should include evaluation of pain responses as well as gut microbial composition throughout the estrous cycle in post-inflammatory mice, which could provide valuable insight into the relative contribution of sex hormones vs the gut microbiome in the pathophysiology of visceral pain.
The role of estrogen in the development and severity of IBD is complex and not fully understood. The biological effects of estrogen are mediated by the binding and activation of its two nuclear receptors, estrogen receptor (ER) α and ERβ,70 as well as the membrane receptor, GPR30.71 The colon predominantly expresses ERβ, which plays an anti-inflammatory role in T cell function72 and maintains epithelial barrier integrity.73 Circulating estrogen is decreased in female mice treated with DSS,74 while estrogen pre-treatment improves the severity of DSS colitis.75 ERβ is markedly decreased in lamina propria CD4+ T cells in DSS colitis,76 while a decrease in ERβ expression and barrier dysfunction precedes the development of colonic inflammation in the IL-10 knockout mouse model,77 suggesting a protective role of estrogen receptor stimulation in colitis. Furthermore, patients with active IBD display decreased ERβ on epithelial cells, lamina propria lymphocytes and peripheral blood T lymphocytes,72,76 although no significant association between ER expression and patient sex was found. A study investigating the expression of estrogen receptors in DSS-induced colitis showed sex-dependent effects of ERα and ERβ signaling in colitis. ERβ expression protected female but not male mice from DSS-induced colitis.78 Together, these data indicate that the role of estrogen in IBD pathogenesis is multifaceted.
Although both ERα and ERβ are expressed on nociceptive dorsal root ganglia neurons expressing TRPV1,79,80 and nociceptor TRPV1 expression is modulated by estrogen receptors,81,82 previous studies have not examined sex-specific changes in dorsal root ganglia TRPV1 and ER expression in murine models of colitis. Previous studies have shown that estrogen increases neuronal excitability and visceral hyperalgesia via the upregulation of spinal N-methyl D-aspartic acid receptor expression, primarily through an ERα-dependent pathway in murine models of colonic inflammation.83–85 A recent study demonstrated that female mice had a significantly increased proportion of TRPV1-expressing nociceptors in their dorsal root ganglia when compared to males.86 A significant decrease in TRPV1 expression was seen in mice genetically deficient in ERα and ERβ.81 Thus, the decreased disease severity and pain we observed in OVX females compared to cycling females could be attributed to reduced circulating estrogen levels in OVX females and/or changes in receptor stimulation possibly at the level of the dorsal root ganglia or spinal cord.
Both post-inflammatory male and female mice exhibited somatic hyperalgesia, similar to our previous data.18 As we previously did not observe effects of microbial manipulation on somatic sensitivity, we did not examine this further in our cross-sex and sex-appropriate FMT experiments. It is likely that somatic hyperalgesia in our model results from central sensitization due to the initial inflammatory insult, as has been previously demonstrated.87–89 Interestingly, somatic hyperalgesia was not seen in OVX or sham treated mice, suggesting that central sensitization could have been affected by the administration of analgesia/anesthesia with surgery.
We observed sex-specific differences in the microbiota composition between males and cycling females in the post-inflammatory model, with many differentially abundant ASVs in post-inflammatory males and females being SCFA-producing bacteria.90 In control male and females, fecal SCFA content were similar. This suggests that the gut microbiota in a healthy state can produce SCFA equally in both male and female mice. However, in the post-inflammatory state, fecal SCFA levels increased in male mice but decreased in female mice, despite the presence of SCFA-producing taxa in the female post-inflammatory microbiome, including Rumminococcaceae and Lachnospiraceae families.90 It is possible that this represents impaired SCFA production, perhaps due to changes in the post-inflammatory gut microenvironment vs alterations in colonic epithelial uptake or absorption in females.91,92 It is important to note that fecal SCFA are not necessarily representative of SCFA production in the proximal colon.93 Thus, it is also possible that SCFA production is unchanged in the proximal colon of post-inflammatory females or is shifted distally in post-inflammatory males. A similar SCFA profile has been noted in patients with ulcerative colitis in remission, where changes in SCFA-producing genera and a decrease in fecal SCFA levels have been observed.94 However, it is important to note that this study did not evaluate sex differences. Interestingly, a recent study in IBS patients demonstrated that psyllium fiber improved visceral pain in boys, but not in girls. Curiously, microbial composition was not a significant determinant in the effects of fiber, suggesting alterations in bacterial fermentation, and possibly SCFA production, between sexes.95 Further investigation is necessary to ascertain whether the decrease in fecal SCFA is due to sex-specific shifts in SCFA production between the proximal and distal colon, impaired bacterial metabolism vs changes in colonic epithelial uptake of SCFA in females. Moreover, it is essential to explore whether SCFA modulation plays a role in visceral hypersensitivity in females or if different metabolites are involved. SCFA have multiple roles besides modulation of visceral sensitivity, including mucosal repair and the suppression of inflammation.96 As microbial metabolites are not the only cause of visceral hypersensitivity in the post-inflammatory DSS model,40 it is possible that these shifts in the post-inflammatory male microbiome are protective against visceral hypersensitivity through increases in SCFA production, which may in turn play a protective role in mucosal repair. Future studies should evaluate these relative contributions of SCFA.
During our semi-targeted metabolite analysis, we identified multiple metabolites that were different between the post-inflammatory males and females but did not show a significant difference between control groups. Of these, adenine and tryptophan are known to play a role in visceral pain. Further studies are needed to fully characterize the role of the other metabolites in visceral pain.
Post-inflammatory females exhibited significantly higher adenine levels compared to controls and post-inflammatory males. Adenine is a purine base that forms part of the structure of nucleic acids, such as DNA and RNA. It is derived from both host and bacteria and can exist both as a free compound or a nucleoside, such as adenosine.97 Interestingly, a recent study has demonstrated sex-dependency of microbial purine metabolism, potentially due to a hormonal effect.38 Adenine nucleotides, such as ADP and ATP, play important physiological roles in the central and peripheral nervous systems through the activation of purinergic P2X and P2Y receptors, while adenosine can also bind adenosine receptors.98,99 Nociceptors, as well as microglia, express multiple purinoceptor P2X receptor subtypes, as well as adenosine A3 receptors, which have been shown to play a role in visceral hypersensitivity.99–103 Thus, it is plausible that this metabolite may contribute to visceral hypersensitivity in a sex-dependent manner.
Tryptophan is an essential amino acid which can be metabolized by both the host and gut bacteria, serving as a precursor for the synthesis of bioactive compounds such as tryptamine, indoles and serotonin.104 Tryptophan metabolites are known to regulate host immunity and modulate the development of experimental colitis.105 Genera such as Alistipes, Enterobacter, Clostridium, Lactobacillus, and Lachnospiraceae are known to metabolize tryptophan into various metabolites.105,106 These genera are present in both males and females in the post-inflammatory state. In the stool of post-inflammatory females, tryptophan levels were found to be decreased compared to the control group. Conversely, the opposite trend was observed in males, with increased tryptophan in the post-inflammatory group, although the difference was not statistically significant. These findings indicate possible changes in tryptophan metabolism between post-inflammatory males and females. Interestingly, a recent study demonstrated tonic engagement of a serotonergic enterochromaffin cell/mucosal afferent circuit in female but not male mice.26 It is possible that our observed decrease in stool tryptophan in post-inflammatory females is due to changes in tryptophan uptake or metabolism by enterochromaffin cells or adherent gut bacteria, thus contributing to visceral hypersensitivity in a sex-dependent manner. However, since tryptophan acts as a precursor for various other metabolites, further analysis of tryptophan metabolism at the mucosal level is needed to determine whether this metabolite directly or indirectly contributes to the modulation of visceral hypersensitivity.
We demonstrated that stool from post-inflammatory females transferred visceral hypersensitivity to both germ-free female and male mice, whereas post-inflammatory male stool only transferred visceral hypersensitivity to male recipients. This sex dichotomy in FMT has been previously observed in patient studies. One such study showed that female recipients with IBS demonstrated better resolution of symptoms, including visceral pain, after receiving an FMT from a male donor, compared to male recipients; a female donor, however, was not used for comparison in this study.107 Another recent study demonstrated that patients who received a cross-sex FMT for the treatment of C. difficile were more likely to develop post-infectious IBS.108 These data suggest that there is an interaction between sex-specific microbiota and/or sex-specific microbial metabolites on the pathogenesis of visceral hypersensitivity.
Strengths and limitations
We have shown that both hormonal status and the gut microbiota play a role in the pathophysiology of visceral pain. We have used a combination of multiple behavioral assessments and -omics based analysis to demonstrate evidence of sex-dependent differences in both host and microbiome-associated responses.
We acknowledge that our work does have limitations in its interpretation. Regarding the severity of colitis when comparing males and females (Figure 1(c)), water consumption between cages was not measured. Thus, it is possible that female mice may exhibit less severe colitis due to differences in DSS intake when compared to males. We only evaluated visceral and somatic sensitivity in diestrus; future studies should include evaluation of pain responses as well as microbial composition throughout the estrous cycle in this mouse model. The degree of visceral hypersensitivity which occurs post-FMT in germ-free recipients did not fully recapitulate the intensity of the effects observed in conventional mice (Figure 9 vs Figure 2(c)) when comparing the peak of the visceromotor response. These data suggest that the although the post-inflammatory microbiome is sufficient to cause visceral sensitivity, the initial insult caused by DSS results in increased visceral hypersensitivity compared to FMT, possibly through the physical damage induced by DSS, subsequent inflammation and nociceptor activation,109 and/or through central sensitization, such as enhanced microglial activation.101 Future studies could evaluate the relative contribution of these pathways in inducing visceral hypersensitivity in addition to the gut microbiome in this model. Finally, sensory afferent distribution is known to be sex-specific,110 and it is known that neuroanatomical changes occur in sensory afferents and SCFA receptor expression after acute DSS-induced colitis in male mice.111 Thus, future studies should evaluate sex-specific neuroanatomical projections of nociceptors in the post-inflammatory state.
Conclusion
Our work plays an important role in addressing an existing research gap, which has predominantly focused on male animals. By specifically investigating possible bacterial-derived factors modulating pain differences between males and females, we provide insights into sex-specific differences in chronic pain.
Supplementary Material
Acknowledgments
The authors acknowledge Berton Guo, Stephanie Raptis, Cameron Fielding, Helvira Cavalcante Melo, and Anowara Islam for their outstanding technical expertise. Portions of this manuscript have been presented in abstract form at Digestive Diseases Week (DDW) and Canadian Digestive Diseases Week (CDDW).
Funding Statement
Supported by the Canadian Institutes of Health Research (CIHR - YN), the Weston Family Microbiome Initiative (YN), Crohn’s and Colitis Canada (YN), IMAGINE-SPOR (MA), the Department of Medicine (MA), MITACS Globalink Fellowship (MA). Metabolomics data were acquired at the Calgary Metabolomics Research Facility (CMRF), which is part of the Alberta Centre for Advanced Diagnostics (ACAD; PrairiesCan RIE #22734) and is supported by the International Microbiome Centre and the Canada Foundation for Innovation [CFI-JELF 34986].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
MJA, KAS, KM, CA, MB, and YN designed the study; MJA, YBH, MD, AS, CHB, RC, CO, DGB performed the experiments; IAL and MBG provided material/experimental support; MJA, YBH, AS, CHB, DB, and YN analyzed and interpreted the data; MJA and YN drafted the manuscript; IAL, KAS, KDM, CA, MBG critically revised the manuscript for important intellectual content. All authors had access to the study data and have reviewed and approved the final manuscript.
Data availability statement
The authors confirm that data supporting the findings of this study are available as follows: data containing summary statistics at different steps of the analysis pipeline can be found in Supplementary data 1. Raw sequencing data are available at http://www.ncbi.nlm.nih.gov/bioproject/1132548, while the mapping file can be found in Supplementary data 2. Raw metabolomic data can be found in Supplementary data 3.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19490976.2024.2409207
References
- 1.Coward S, Benchimol EI, Kuenzig ME, Windsor JW, Bernstein CN, Bitton A, Jones JL, Lee K, Murthy SK, Targownik LE, et al. The 2023 impact of inflammatory bowel disease in Canada: epidemiology of IBD. J Can Assoc Gastroenterol. 2023;6(Supplement_2):S9–24. doi: 10.1093/jcag/gwad004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, Keefe FJ, Mogil JS, Ringkamp M, Sluka KA, et al. The revised international association for the study of pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976–1982. doi: 10.1097/j.pain.0000000000001939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fairbrass KM, Costantino SJ, Gracie DJ, Ford AC.. Prevalence of irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease in remission: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(12):1053–1062. doi: 10.1016/S2468-1253(20)30300-9. [DOI] [PubMed] [Google Scholar]
- 4.Fairbrass KM, Hamlin PJ, Gracie DJ, Ford AC. Natural history and impact of irritable bowel syndrome-type symptoms in inflammatory bowel disease during 6 years of longitudinal follow-up. Aliment Pharmacol Ther. 2022;56(8):1264–1273. doi: 10.1111/apt.17193. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K, Khwaja IG, Schreyer JR, Bulmer D, Peiris M, Terai S, Aziz Q. Post-inflammatory abdominal pain in patients with inflammatory bowel disease during remission: a comprehensive review. Crohn’s & Colitis 360. 2021;3(4):otab073. doi: 10.1093/crocol/otab073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurtado-Lorenzo A, Honig G, Weaver SA, Larkin PB, Heller C. Chronic abdominal pain in IBD research initiative: unraveling biological mechanisms and patient heterogeneity to personalize treatment and improve clinical outcomes. Crohn’s & Colitis 360. 2021;3(3):otab034. doi: 10.1093/crocol/otab034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regueiro M, Greer JB, Szigethy E. Etiology and treatment of pain and psychosocial issues in patients with inflammatory bowel diseases. Gastroenterology. 2017;152(2):430–439.e4. doi: 10.1053/j.gastro.2016.10.036. [DOI] [PubMed] [Google Scholar]
- 8.Targownik LE, Nugent Z, Singh H, Bugden S, Bernstein CN. The prevalence and predictors of opioid use in inflammatory bowel disease: a population-based analysis. Am J Gastroenterol. 2014;109(10):1613–1620. doi: 10.1038/ajg.2014.230. [DOI] [PubMed] [Google Scholar]
- 9.Chhibba T, Guizzetti L, Seow CH, Lu C, Novak KL, Ananthakrishnan AN, Bernstein CN, Kaplan GG, Panaccione R, Ma C, et al. Frequency of opioid prescription at emergency department discharge in patients with inflammatory bowel disease: a nationwide analysis. Clin Gastroenterol Hepatol. 2021;19(10):2064–2071.e1. doi: 10.1016/j.cgh.2020.07.020. [DOI] [PubMed] [Google Scholar]
- 10.DeGruttola AK, Low D, Mizoguchi A, Mizoguchi E. Current understanding of dysbiosis in disease in human and animal models. Inflamm Bowel Dis. 2016;22(5):1137–1150. doi: 10.1097/MIB.0000000000000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halfvarson J, Brislawn CJ, Lamendella R, Vázquez-Baeza Y, Walters WA, Bramer LM, D’Amato M, Bonfiglio F, McDonald D, Gonzalez A, et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol. 2017;2(5):17004. doi: 10.1038/nmicrobiol.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shutkever O, Gracie DJ, Young C, Wood HM, Taylor M, John Hamlin P, Ford AC, Quirke P. No significant association between the fecal microbiome and the presence of irritable bowel syndrome-type symptoms in patients with quiescent inflammatory bowel disease. Inflamm Bowel Dis. 2018;24(7):1597–1605. doi: 10.1093/ibd/izy052. [DOI] [PubMed] [Google Scholar]
- 13.Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, Nalin R, Jarrin C, Chardon P, Marteau P, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55:205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Siles M, Enrich-Capo N, Aldeguer X, Sabat-Mir M, Duncan SH, Garcia-Gil LJ, Martinez-Medina M. Alterations in the abundance and Co-occurrence of akkermansia muciniphila and faecalibacterium prausnitzii in the colonic mucosa of inflammatory bowel disease subjects. Front Cell Infect Microbiol. 2018;8:281. doi: 10.3389/fcimb.2018.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez C, Antolin M, Santos J, Torrejon A, Casellas F, Borruel N, Guarner F, Malagelada J-R. Unstable composition of the fecal microbiota in ulcerative colitis during clinical remission. Am J Gastroenterol. 2008;103(3):643–648. doi: 10.1111/j.1572-0241.2007.01592.x. [DOI] [PubMed] [Google Scholar]
- 16.Shin A, Kashyap PC. Multi-omics for biomarker approaches in the diagnostic evaluation and management of abdominal pain and irritable bowel syndrome: what lies ahead. Gut Microbes. 2023;15(1):2195792. doi: 10.1080/19490976.2023.2195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amaral FA, Sachs D, Costa VV, Fagundes CT, Cisalpino D, Cunha TM, Ferreira SH, Cunha FQ, Silva TA, Nicoli JR, et al. Commensal microbiota is fundamental for the development of inflammatory pain. Proc Natl Acad Sci USA. 2008;105(6):2193–2197. doi: 10.1073/pnas.0711891105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esquerre N, Basso L, Defaye M, Vicentini FA, Cluny N, Bihan D, Hirota SA, Schick A, Jijon HB, Lewis IA, et al. Colitis-induced microbial perturbation promotes post-inflammatory visceral hypersensitivity. Cellular And Mol Gastroenterol Hepatol. 2020;10(2):225–244. doi: 10.1016/j.jcmgh.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nohr MK, Egerod KL, Christiansen SH, Gille A, Offermanns S, Schwartz TW, Møller M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience. 2015;290:126–137. doi: 10.1016/j.neuroscience.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 20.Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A, Tsujimoto G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci USA. 2011;108(19):8030–8035. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ochoa-Cortes F, Ramos-Lomas T, Miranda-Morales M, Spreadbury I, Ibeakanma C, Barajas-Lopez C, Vanner S. Bacterial cell products signal to mouse colonic nociceptive dorsal root ganglia neurons. Am J Physiol Gastrointest Liver Physiol. 2010;299(3):G723–32. doi: 10.1152/ajpgi.00494.2009. [DOI] [PubMed] [Google Scholar]
- 22.Meseguer V, Alpizar YA, Luis E, Tajada S, Denlinger B, Fajardo O, Manenschijn J-A, Fernández-Peña C, Talavera A, Kichko T, et al. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat Commun. 2014;5(1):3125. doi: 10.1038/ncomms4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501(7465):52–57. doi: 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellono NW, Bayrer JR, Leitch DB, Castro J, Zhang C, O’Donnell TA, Brierley SM, Ingraham HA, Julius D. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell. 2017;170(1):185–198.e16. doi: 10.1016/j.cell.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobson A, Yang D, Vella M, Chiu IM. The intestinal neuro-immune axis: crosstalk between neurons, immune cells, and microbes. Mucosal Immunol. 2021;14(3):555–565. doi: 10.1038/s41385-020-00368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bayrer JR, Castro J, Venkataraman A, Touhara KK, Rossen ND, Morrie RD, Maddern J, Hendry A, Braverman KN, Garcia-Caraballo S, et al. Gut enterochromaffin cells drive visceral pain and anxiety. Nature. 2023;616(7955):137–142. doi: 10.1038/s41586-023-05829-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruau D, Liu LY, Clark JD, Angst MS, Butte AJ. Sex differences in reported pain across 11,000 patients captured in electronic medical records. J Pain. 2012;13(3):228–234. doi: 10.1016/j.jpain.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, Tack J, Whitehead WE, Dumitrascu DL, Fang X, Fukudo S, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome foundation global study. Gastroenterology. 2021;160(1):99–114.e3. doi: 10.1053/j.gastro.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Janssen LM, Rezazadeh Ardabili A, Romberg-Camps MJL, Winkens B, van den Broek RJ, Hulst J, Verwijs HJA, Keszthelyi D, Jonkers DMAE, van Bodegraven AA, et al. Abdominal pain in patients with inflammatory bowel disease in remission: a prospective study on contributing factors. Aliment Pharmacol Ther. 2023;58(10):1041–1051. doi: 10.1111/apt.17718. [DOI] [PubMed] [Google Scholar]
- 30.Gracie DJ, Williams CJ, Sood R, Mumtaz S, Bholah MH, Hamlin PJ, Ford AC. Negative effects on psychological health and quality of life of genuine irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2017;15(3):376–384.e5. doi: 10.1016/j.cgh.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Mogil JS. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat Rev Neurosci. 2020;21(7):353–365. doi: 10.1038/s41583-020-0310-6. [DOI] [PubMed] [Google Scholar]
- 32.Pujo J, De Palma G, Lu J, Galipeau HJ, Surette MG, Collins SM, Bercik P. Gut microbiota modulates visceral sensitivity through calcitonin gene-related peptide (CGRP) production. Gut Microbes. 2023;15(1):2188874. doi: 10.1080/19490976.2023.2188874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francis-Malave AM, Martinez Gonzalez S, Pichardo C, Wilson TD, Rivera-García LG, Brinster LR, Carrasquillo Y. Sex differences in pain-related behaviors and clinical progression of disease in mouse models of colonic pain. Pain. 2023;164(1):197–215. doi: 10.1097/j.pain.0000000000002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Min Y, Ma X, Sankaran K, Ru Y, Chen L, Baiocchi M, Zhu S. Sex-specific association between gut microbiome and fat distribution. Nat Commun. 2019;10(1):2408. doi: 10.1038/s41467-019-10440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding T, Schloss PD. Dynamics and associations of microbial community types across the human body. Nature. 2014;509(7500):357–360. doi: 10.1038/nature13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valeri F, Endres K. How biological sex of the host shapes its gut microbiota. Front Neuroendocrinol. 2021;61:100912. doi: 10.1016/j.yfrne.2021.100912. [DOI] [PubMed] [Google Scholar]
- 37.Org E, Mehrabian M, Parks BW, Shipkova P, Liu X, Drake TA, Lusis AJ. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. 2016;7(4):313–322. doi: 10.1080/19490976.2016.1203502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown K, Thomson CA, Wacker S, Drikic M, Groves R, Fan V, Lewis IA, McCoy KD. Microbiota alters the metabolome in an age- and sex- dependent manner in mice. Nat Commun. 2023;14(1):1348. doi: 10.1038/s41467-023-37055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tramullas M, Collins JM, Fitzgerald P, Dinan TG, O’ Mahony SM, Cryan JF. Estrous cycle and ovariectomy-induced changes in visceral pain are microbiota-dependent. iScience. 2021;24(8):102850. doi: 10.1016/j.isci.2021.102850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lapointe TK, Basso L, Iftinca MC, Flynn R, Chapman K, Dietrich G, Vergnolle N, Altier C. TRPV1 sensitization mediates postinflammatory visceral pain following acute colitis. Am J Physiol Gastrointest Liver Physiol. 2015;309(2):G87–99. doi: 10.1152/ajpgi.00421.2014. [DOI] [PubMed] [Google Scholar]
- 41.Park YH, Kim N, Shim YK, Choi YJ, Nam RH, Choi YJ, Ham MH, Suh JH, Lee SM, Lee CM, et al. Adequate dextran sodium sulfate-induced colitis model in mice and effective outcome measurement method. J Cancer Prev. 2015;20(4):260–267. doi: 10.15430/JCP.2015.20.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCafferty DM, Wallace JL, Sharkey KA. Effects of chemical sympathectomy and sensory nerve ablation on experimental colitis in the rat. Am J Physiol. 1997;272(2):G272–80. doi: 10.1152/ajpgi.1997.272.2.G272. [DOI] [PubMed] [Google Scholar]
- 43.Erben U, Loddenkemper C, Doerfel K, Spieckermann S, Haller D, Heimesaat MM, Zeitz M, Siegmund B, Kühl AA. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int J Clin Exp Pathol. 2014;7(8):4557–4576. [PMC free article] [PubMed] [Google Scholar]
- 44.Kim JJ, Shajib MS, Manocha MM, Khan WI. Investigating intestinal inflammation in dss-induced model of IBD. J Vis Exp. 2012;(60). doi: 10.3791/3678-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hasegawa A, Mochida K, Ogonuki N, Hirose M, Tomishima T, Inoue K, Ogura A. Efficient and scheduled production of pseudopregnant female mice for embryo transfer by estrous cycle synchronization. J Reprod Dev. 2017;63(6):539–545. doi: 10.1262/jrd.2017-068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dewar AD. Effects of hysterectomy on corpus luteum activity in the cyclic, pseudopregnant and pregnant mouse. J Reprod Fertil. 1973;33(1):77–89. doi: 10.1530/jrf.0.0330077. [DOI] [PubMed] [Google Scholar]
- 47.Ajayi AF, Akhigbe RE. Staging of the estrous cycle and induction of estrus in experimental rodents: an update. Fertil Res And Pract. 2020;6(1):5. doi: 10.1186/s40738-020-00074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Varol AB, Esen EC, Kocak EE. Repeated collection of vaginal smear causes stress in mice. Noro Psikiyatr Ars. 2022;59(4):325–329. doi: 10.29399/npa.28099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Becegato M, Meurer YSR, Paiva-Santos MA, Lima AC, Marinho GF, Bioni VS, Soares MBL, Leão AHFF, Suchecki D, Silva RH, et al. Impaired discriminative avoidance and increased plasma corticosterone levels induced by vaginal lavage procedure in rats. Physiol Behav. 2021;232:113343. doi: 10.1016/j.physbeh.2021.113343. [DOI] [PubMed] [Google Scholar]
- 50.Strom JO, Theodorsson A, Ingberg E, Isaksson I-M, Theodorsson E. Ovariectomy and 17β-estradiol replacement in rats and mice: a visual demonstration. J Vis Exp. 2012;(64):e4013. doi: 10.3791/4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCoy KD, Ohland CL. Innate responses to gut microbiota; critical assessment of the necessary experimental controls. Curr Opin Microbiol. 2021;59:34–41. doi: 10.1016/j.mib.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 52.Choo JM, Rogers GB. Establishment of murine gut microbiota in gnotobiotic mice. iScience. 2021;24(2):102049. doi: 10.1016/j.isci.2021.102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barrot M. Tests and models of nociception and pain in rodents. Neuroscience. 2012;211:39–50. doi: 10.1016/j.neuroscience.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 54.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naïve bayesian Classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73(16):5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gopalakrishnan V, Dozier EA, Glover MS, Novick S, Ford M, Morehouse C, Warrener P, Caceres C, Hess S, Sellman BR, et al. Engraftment of bacteria after fecal microbiota transplantation is dependent on both frequency of dosing and duration of preparative antibiotic regimen. Microorganisms. 2021;9(7):1399. doi: 10.3390/microorganisms9071399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ianiro G, Puncochar M, Karcher N, Porcari S, Armanini F, Asnicar F, Beghini F, Blanco-Míguez A, Cumbo F, Manghi P, et al. Variability of strain engraftment and predictability of microbiome composition after fecal microbiota transplantation across different diseases. Nat Med. 2022;28(9):1913–1923. doi: 10.1038/s41591-022-01964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bihan DG, Rydzak T, Wyss M, Pittman K, McCoy KD, Lewis IA. Method for absolute quantification of short chain fatty acids via reverse phase chromatography mass spectrometry. PLoS One. 2022;17(4):e0267093. doi: 10.1371/journal.pone.0267093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Groves RA, Mapar M, Aburashed R, Ponce LF, Bishop SL, Rydzak T, Drikic M, Bihan DG, Benediktsson H, Clement F, et al. Methods for quantifying the metabolic boundary fluxes of cell cultures in large cohorts by high-resolution hydrophilic liquid chromatography mass spectrometry. Anal Chem. 2022;94(25):8874–8882. doi: 10.1021/acs.analchem.2c00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clasquin MF, Melamud E, Rabinowitz JD. LC-MS data processing with MAVEN: a metabolomic analysis and visualization engine. Curr Protoc Bioinf. 2012;14(1):14 11. doi: 10.1002/0471250953.bi1411s37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Melamud E, Vastag L, Rabinowitz JD. Metabolomic analysis and visualization engine for LC−MS data. Anal Chem. 2010;82(23):9818–9826. doi: 10.1021/ac1021166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McMurdie PJ, Holmes S, Watson M. Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8(4):e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for rna-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Babickova J, Tothova L, Lengyelova E, Bartoňová A, Hodosy J, Gardlík R, Celec P. Sex differences in experimentally induced colitis in mice: a role for estrogens. Inflammation. 2015;38(5):1996–2006. doi: 10.1007/s10753-015-0180-7. [DOI] [PubMed] [Google Scholar]
- 64.Ji Y, Murphy AZ, Traub RJ. Estrogen modulates the visceromotor reflex and responses of spinal dorsal horn neurons to colorectal stimulation in the rat. J Neurosci. 2003;23(9):3908–3915. doi: 10.1523/JNEUROSCI.23-09-03908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wen J, Zhao Y, Huang C, Li S, Li P, Zhou Y, Yan Z, Zhang G. Estrogen inhibits colonic smooth muscle contractions by regulating BKβ1 signaling. PLoS One. 2023;18(11):e0294249. doi: 10.1371/journal.pone.0294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain axis? Neurochem Int. 2016;99:110–132. doi: 10.1016/j.neuint.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 67.Buhnik-Rosenblau K, Danin-Poleg Y, Kashi Y. Predominant effect of host genetics on levels of lactobacillus johnsonii bacteria in the mouse gut. Appl Environ Microbiol. 2011;77(18):6531–6538. doi: 10.1128/AEM.00324-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vinogradova EP, Zhukov DA, Batuev AS. The effects of stages of the estrous cycle on pain thresholds in female white rats. Neurosci Behav Physiol. 2003;33(3):269–272. doi: 10.1023/A:1022155432262. [DOI] [PubMed] [Google Scholar]
- 69.Wallace JG, Potts RH, Szamosi JC, Surette MG, Sloboda DM. The murine female intestinal microbiota does not shift throughout the estrous cycle. PLoS One. 2018;13(7):e0200729. doi: 10.1371/journal.pone.0200729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paterni I, Granchi C, Katzenellenbogen JA, Minutolo F. Estrogen receptors alpha (ERα) and beta (ERβ): subtype-selective ligands and clinical potential. Steroids. 2014;90:13–29. doi: 10.1016/j.steroids.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Filardo EJ, Quinn JA, Frackelton AR Jr., Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16(1):70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- 72.Pierdominici M, Maselli A, Varano B, Barbati C, Cesaro P, Spada C, Zullo A, Lorenzetti R, Rosati M, Rainaldi G, et al. Linking estrogen receptor β expression with inflammatory bowel disease activity. Oncotarget. 2015;6(38):40443–40451. doi: 10.18632/oncotarget.6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wada-Hiraike O, Imamov O, Hiraike H, Hultenby K, Schwend T, Omoto Y, Warner M, Gustafsson J-Å. Role of estrogen receptor β in colonic epithelium. Proc Natl Acad Sci USA. 2006;103(8):2959–2964. doi: 10.1073/pnas.0511271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Z, Chen C, Yu W, Xu L, Jia H, Wang C, Pei N, Liu Z, Luo D, Wang J, et al. Colitis-mediated dysbiosis of the intestinal Flora and impaired vitamin a absorption reduce ovarian function in mice. Nutrients. 2023;15(11):15. doi: 10.3390/nu15112425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Verdu EF, Deng Y, Bercik P, Collins SM. Modulatory effects of estrogen in two murine models of experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2002;283(1):G27–36. doi: 10.1152/ajpgi.00460.2001. [DOI] [PubMed] [Google Scholar]
- 76.Guo D, Liu X, Zeng C, Cheng L, Song G, Hou X, Zhu L, Zou K. Estrogen receptor β activation ameliorates dss-induced chronic colitis by inhibiting inflammation and promoting treg differentiation. Int Immunopharmacol. 2019;77:105971. doi: 10.1016/j.intimp.2019.105971. [DOI] [PubMed] [Google Scholar]
- 77.Looijer-van Langen M, Hotte N, Dieleman LA, Albert E, Mulder C, Madsen KL. Estrogen receptor-β signaling modulates epithelial barrier function. Am J Physiol Gastrointest Liver Physiol. 2011;300(4):G621–6. doi: 10.1152/ajpgi.00274.2010. [DOI] [PubMed] [Google Scholar]
- 78.Goodman WA, Havran HL, Quereshy HA, Kuang S, De Salvo C, Pizarro TT. Estrogen receptor α loss-of-function protects female mice from DSS-Induced experimental colitis. Cell Mol Gastroenterol Hepatol. 2018;5(4):630–633.e1. doi: 10.1016/j.jcmgh.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Papka RE, Storey-Workley M. Estrogen receptor-α and -β coexist in a subpopulation of sensory neurons of female rat dorsal root ganglia. Neurosci Lett. 2002;319(2):71–74. doi: 10.1016/S0304-3940(01)02562-9. [DOI] [PubMed] [Google Scholar]
- 80.Cho T, Chaban VV. Interaction between P2X3 and oestrogen receptor (ER)α/ERβ in ATP-Mediated calcium signalling in mice sensory neurones. J Neuroendocrinol. 2012;24(5):789–797. doi: 10.1111/j.1365-2826.2011.02272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cho T, Chaban VV. Expression of P2X3 and TRPV1 receptors in primary sensory neurons from estrogen receptors-α and estrogen receptor-β knockout mice. Neuroreport. 2012;23(9):530–534. doi: 10.1097/WNR.0b013e328353fabc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen Q, Zhang W, Sadana N, Chen X. Estrogen receptors in pain modulation: cellular signaling. Biol Sex Differ. 2021;12(1):22. doi: 10.1186/s13293-021-00364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ji Y, Bai G, Cao DY, Traub RJ. Estradiol modulates visceral hyperalgesia by increasing thoracolumbar spinal GluN2B subunit activity in female rats. Neurogastroenterol Motil. 2015;27(6):775–786. doi: 10.1111/nmo.12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ji Y, Tang B, Traub RJ. Spinal estrogen receptor alpha mediates estradiol-induced pronociception in a visceral pain model in the rat. Pain. 2011;152(5):1182–1191. doi: 10.1016/j.pain.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tang B, Ji Y, Traub RJ. Estrogen alters spinal NMDA receptor activity via a PKA signaling pathway in a visceral pain model in the rat. Pain. 2008;137(3):540–549. doi: 10.1016/j.pain.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ji J, He Q, Luo X, Bang S, Matsuoka Y, McGinnis A, Nackley AG, Ji R-R. IL-23 enhances C-Fiber-mediated and blue light-induced spontaneous pain in female mice. Front Immunol. 2021;12:787565. doi: 10.3389/fimmu.2021.787565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoban AE, Moloney RD, Golubeva AV, McVey Neufeld KA, O’Sullivan O, Patterson E, Stanton C, Dinan TG, Clarke G, Cryan JF, et al. Behavioural and neurochemical consequences of chronic gut microbiota depletion during adulthood in the rat. Neuroscience. 2016;339:463–477. doi: 10.1016/j.neuroscience.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 88.Gustafsson JK, Greenwood-Van Meerveld B. Amygdala activation by corticosterone alters visceral and somatic pain in cycling female rats. Am J Physiol Gastrointest Liver Physiol. 2011;300(6):G1080–5. doi: 10.1152/ajpgi.00349.2010. [DOI] [PubMed] [Google Scholar]
- 89.Johnson AC, Tran L, Greenwood-Van Meerveld B. Knockdown of corticotropin-releasing factor in the central amygdala reverses persistent viscerosomatic hyperalgesia. Transl Psychiatry. 2015;5(3):e517. doi: 10.1038/tp.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vacca M, Celano G, Calabrese FM, Portincasa P, Gobbetti M, De Angelis M. The controversial role of human gut lachnospiraceae. Microorganisms. 2020;8(4):8. doi: 10.3390/microorganisms8040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lucarini E, Di Pilato V, Parisio C, Micheli L, Toti A, Pacini A, Bartolucci G, Baldi S, Niccolai E, Amedei A, et al. Visceral sensitivity modulation by faecal microbiota transplantation: the active role of gut bacteria in pain persistence. Pain. 2022;163(5):861–877. doi: 10.1097/j.pain.0000000000002438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19(1):29–41. doi: 10.1111/1462-2920.13589. [DOI] [PubMed] [Google Scholar]
- 93.Sakata T. Pitfalls in short-chain fatty acid research: a methodological review. Anim Sci J. 2019;90(1):3–13. doi: 10.1111/asj.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.James SL, Christophersen CT, Bird AR, Conlon MA, Rosella O, Gibson PR, Muir JG. Abnormal fibre usage in UC in remission. Gut. 2015;64(4):562–570. doi: 10.1136/gutjnl-2014-307198. [DOI] [PubMed] [Google Scholar]
- 95.So SY, Badu S, Wu Q, Yalcinkaya N, Mirabile Y, Castaneda R, Musaad S, Heitkemper M, Savidge TC, Shulman RJ, et al. Sex-dependent efficacy of dietary fiber in pediatric functional abdominal pain. Gastroenterology. 2023;166(4):645–657.e14. doi: 10.1053/j.gastro.2023.12.011. [DOI] [PubMed] [Google Scholar]
- 96.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119. [DOI] [PubMed] [Google Scholar]
- 97.Moffatt BA, Ashihara H. Purine and pyrimidine nucleotide synthesis and metabolism. Arabidopsis Book. 2002;1:e0018. doi: 10.1199/tab.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brown SG, King BF, Kim YC, Jang SY, Burnstock G, Jacobson KA. Activity of novel adenine nucleotide derivatives as agonists and antagonists at recombinant rat P2X receptors. Drug Dev Res. 2000;49(4):253–259. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Burnstock G. Purinergic mechanisms and pain. Adv Pharmacol. 2016;75:91–137. [DOI] [PubMed] [Google Scholar]
- 100.Wu YY, Wang Q, Zhang PA, Zhu C, Xu G-Y. miR-1306-3p directly activates P2X3 receptors in primary sensory neurons to induce visceral pain in rats. Pain. 2023;164(7):1555–1565. doi: 10.1097/j.pain.0000000000002853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Defaye M, Abdullah NS, Iftinca M, Hassan A, Agosti F, Zhang Z, Cumenal M, Zamponi GW, Altier C. Gut-innervating TRPV1+ neurons drive chronic visceral pain via microglial P2Y12 receptor. Cell Mol Gastroenterol Hepatol. 2022;13(4):977–999. doi: 10.1016/j.jcmgh.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen L, Liu YW, Yue K, Ru Q, Xiong Q, Ma B-M, Tian X, Li C-Y. Differential expression of atp-gated P2X receptors in DRG between chronic neuropathic pain and visceralgia rat models. Purinergic Signal. 2016;12(1):79–87. doi: 10.1007/s11302-015-9481-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lucarini E, Coppi E, Micheli L, Parisio C, Vona A, Cherchi F, Pugliese AM, Pedata F, Failli P, Palomino S, et al. Acute visceral pain relief mediated by A3AR agonists in rats: involvement of N-type voltage-gated calcium channels. Pain. 2020;161(9):2179–2190. doi: 10.1097/j.pain.0000000000001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Friedman M. Analysis, nutrition, and health benefits of tryptophan. Int J Tryptophan Res. 2018;11:1178646918802282. doi: 10.1177/1178646918802282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li S. Modulation of immunity by tryptophan microbial metabolites. Front Nutr. 2023;10:1209613. doi: 10.3389/fnut.2023.1209613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kaur H, Bose C, Mande SS. Tryptophan metabolism by gut microbiome and gut-Brain-Axis: an in silico analysis. Front Neurosci. 2019;13:1365. doi: 10.3389/fnins.2019.01365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.El-Salhy M, Casen C, Valeur J, Hausken T, Hatlebakk JG. Responses to faecal microbiota transplantation in female and male patients with irritable bowel syndrome. World J Gastroenterol. 2021;27(18):2219–2237. doi: 10.3748/wjg.v27.i18.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sehgal K, Yadav D, Saha S, Mara K, Grover M, Khanna S. Sex-discordant fecal microbiota transplantation for clostridioides difficile May increase risk of postinfection irritable bowel syndrome. Gastroenterology. 2023;166(4):704–706.e2. doi: 10.1053/j.gastro.2023.11.295. [DOI] [PubMed] [Google Scholar]
- 109.Chassaing B, Aitken JD, Malleshappa M, Vijay‐Kumar M. Dextran sulfate sodium (dss)-induced colitis in mice. Curr Protoc Immunol. 2014;104(1):15251–152514. doi: 10.1002/0471142735.im1525s104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Makowska K, Gonkowski S. Age and sex-dependent differences in the neurochemical characterization of calcitonin gene-related peptide-like immunoreactive (CGRP-LI) nervous structures in the porcine descending colon. Int J Mol Sci. 2019;20(5):20. doi: 10.3390/ijms20051024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hertati A, Hayashi S, Ogata H, Miyata K, Kato R, Yamamoto T, Kadowaki M. Morphological elucidation of short-chain fatty acid receptor GPR41-positive enteric sensory neurons in the colon of mice with dextran sulfate sodium-induced colitis. Heliyon. 2020;6(12):e05647. doi: 10.1016/j.heliyon.2020.e05647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that data supporting the findings of this study are available as follows: data containing summary statistics at different steps of the analysis pipeline can be found in Supplementary data 1. Raw sequencing data are available at http://www.ncbi.nlm.nih.gov/bioproject/1132548, while the mapping file can be found in Supplementary data 2. Raw metabolomic data can be found in Supplementary data 3.


