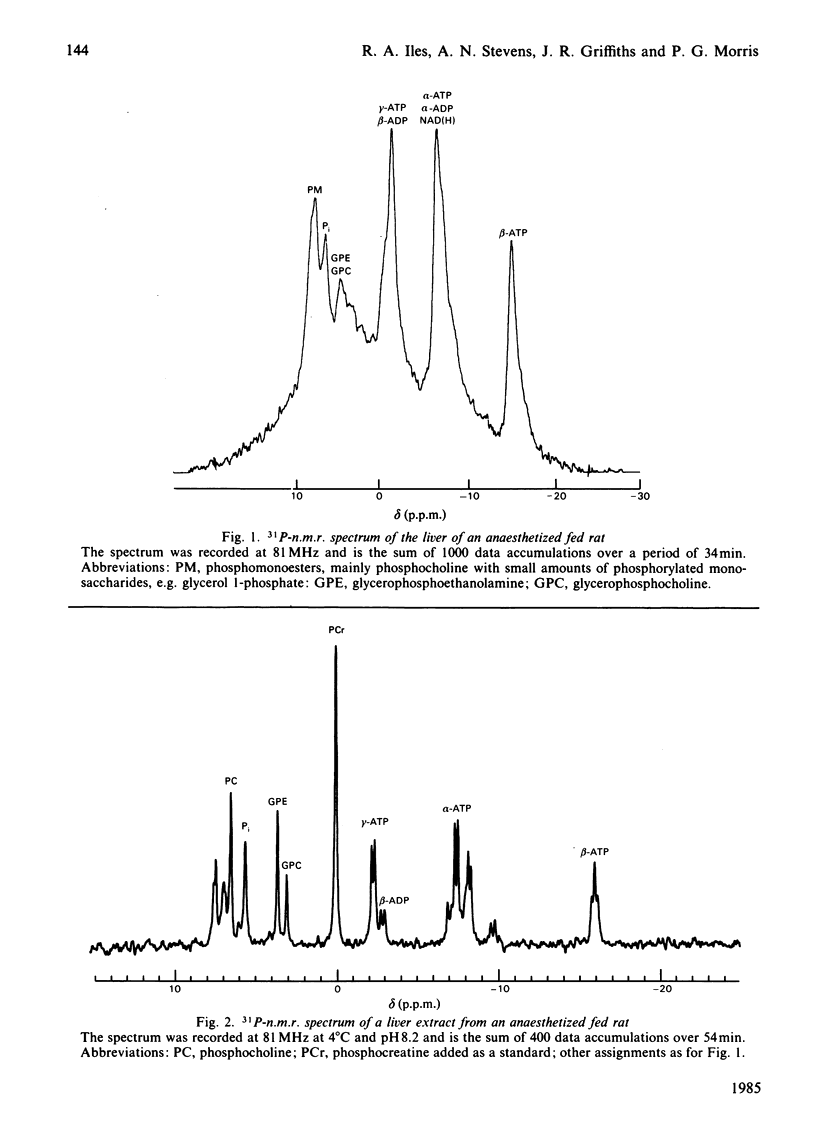
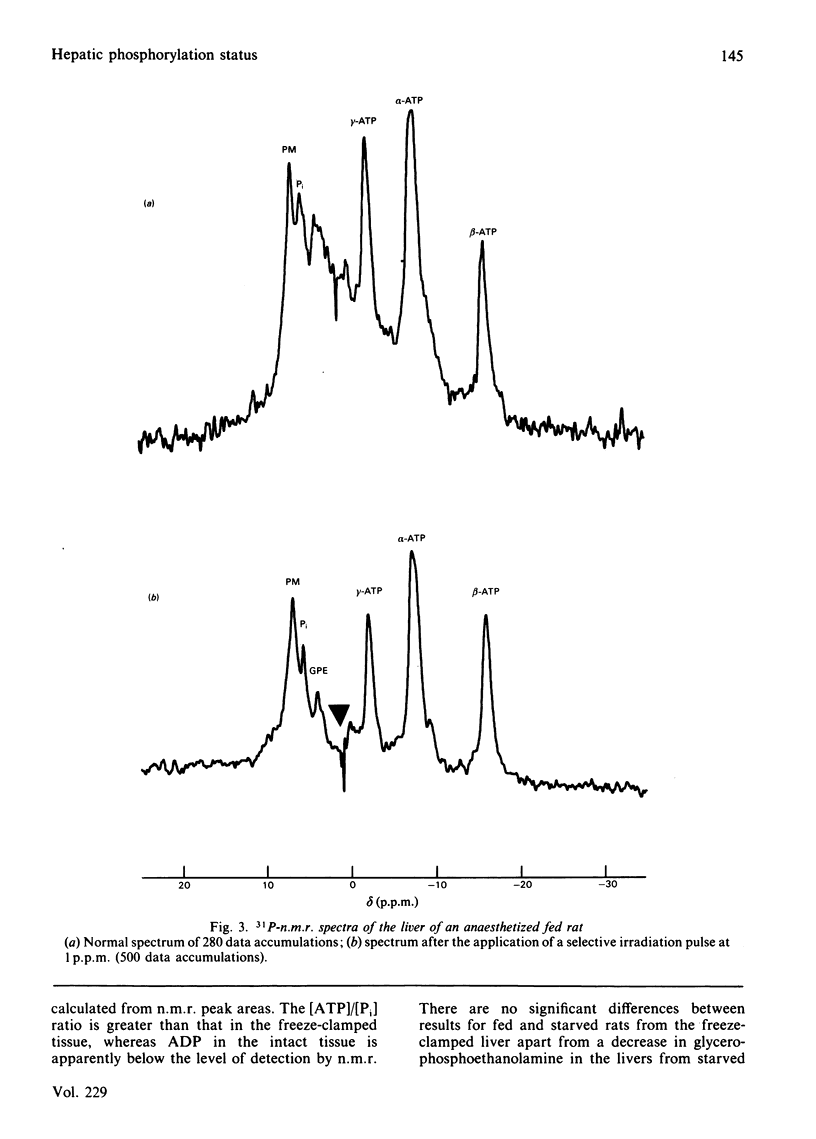
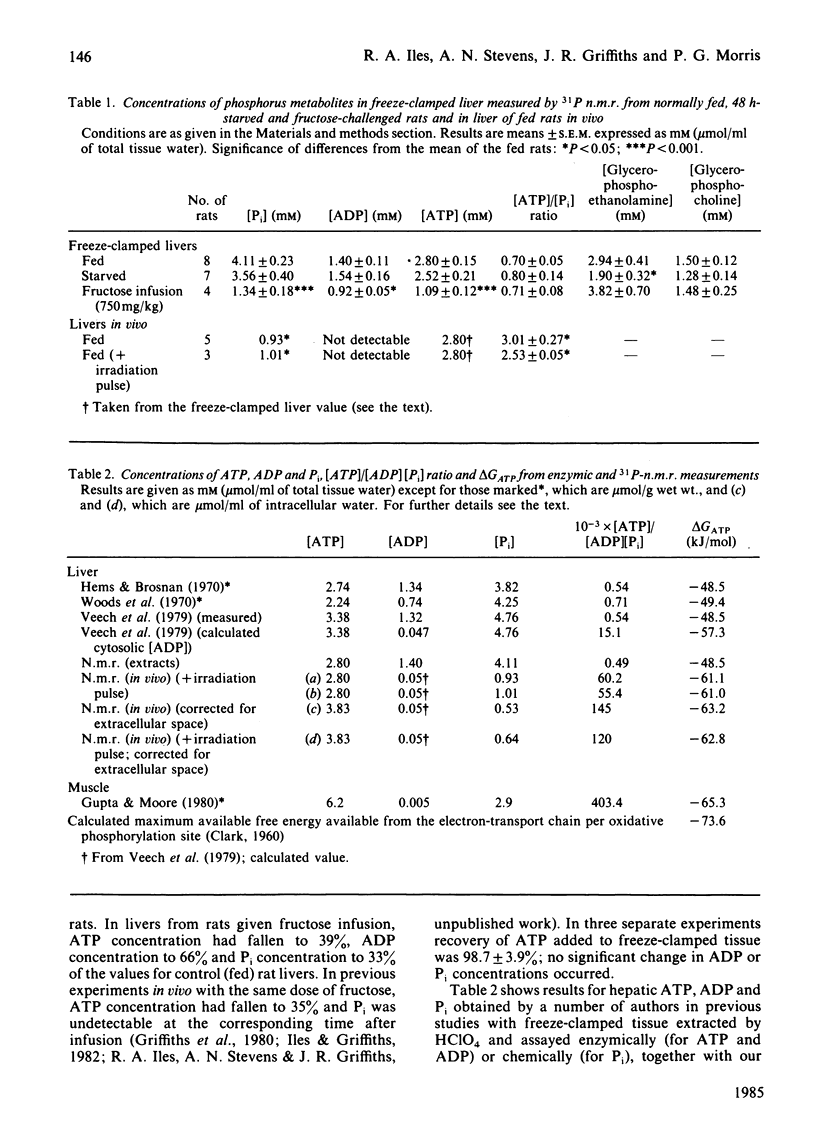
Abstract
An investigation into the measurement of Pi and ADP in rat liver in vivo and in freeze-clamped extracts by 31P-n.m.r. spectroscopy was carried out. The concentration of Pi estimated in vivo is less than 25% [1 mM (mumol/ml of cell water)] of the value obtained from freeze-clamped liver (4 mM), whereas ADP in vivo is undetectable (1.4 mM in vitro). At 5 min after infusion of 750 mg of fructose/kg, the Pi content of liver extracts fell to 1.3 mM, whereas Pi is undetectable in vivo under these conditions [Griffiths, Stevens, Gadian, Iles & Porteous (1980) Biochem. Soc. Trans. 8, 641]. The results indicate that the lower Pi and ADP concentrations found in vivo may be due to compartmentation or binding rather than to degradation of labile organic phosphates during extraction. The results are discussed with reference to previous measurements of liver phosphates and investigations of compartmentation in the liver, as are some of the possible consequences for metabolic control in the liver of low ADP and Pi concentrations.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman J. J., Grove T. H., Wong G. G., Gadian D. G., Radda G. K. Mapping of metabolites in whole animals by 31P NMR using surface coils. Nature. 1980 Jan 10;283(5743):167–170. doi: 10.1038/283167a0. [DOI] [PubMed] [Google Scholar]
- Akerboom T. P., Bookelman H., Zuurendonk P. F., van der Meer R., Tager J. M. Intramitochondrial and extramitochondrial concentrations of adenine nucleotides and inorganic phosphate in isolated hepatocytes from fasted rats. Eur J Biochem. 1978 Mar 15;84(2):413–420. doi: 10.1111/j.1432-1033.1978.tb12182.x. [DOI] [PubMed] [Google Scholar]
- Brocks D. G., Siess E. A., Wieland O. H. Validity of the digitonin method for metabolite compartmentation in isolated hepatocytes. Biochem J. 1980 Apr 15;188(1):207–212. doi: 10.1042/bj1880207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955 Nov;217(1):383–393. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Cohen S. M. Simultaneous 13C and 31P NMR studies of perfused rat liver. Effects of insulin and glucagon and a 13C NMR assay of free Mg2+. J Biol Chem. 1983 Dec 10;258(23):14294–14308. [PubMed] [Google Scholar]
- Coty W. A., Pedersen P. L. Phosphate transport in rat liver mitochondria. Kinetics and energy requirements. J Biol Chem. 1974 Apr 25;249(8):2593–2598. [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Contraction and recovery of living muscles studies by 31P nuclear magnetic resonance. J Physiol. 1977 Jun;267(3):703–735. doi: 10.1113/jphysiol.1977.sp011835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Muscular fatigue investigated by phosphorus nuclear magnetic resonance. Nature. 1978 Aug 31;274(5674):861–866. doi: 10.1038/274861a0. [DOI] [PubMed] [Google Scholar]
- Dawson M. J. Quantitative analysis of metabolite levels in normal human subjects by 31P topical magnetic resonance. Biosci Rep. 1982 Sep;2(9):727–733. doi: 10.1007/BF01114836. [DOI] [PubMed] [Google Scholar]
- Dawson M. J. Quantitative analysis of metabolite levels in normal human subjects by 31P topical magnetic resonance. Biosci Rep. 1982 Sep;2(9):727–733. doi: 10.1007/BF01114836. [DOI] [PubMed] [Google Scholar]
- Fiske C. H., Subbarow Y. THE NATURE OF THE "INORGANIC PHOSPHATE" IN VOLUNTARY MUSCLE. Science. 1927 Apr 22;65(1686):401–403. doi: 10.1126/science.65.1686.401. [DOI] [PubMed] [Google Scholar]
- Freeman D., Bartlett S., Radda G., Ross B. Energetics of sodium transport in the kidney. Saturation transfer 31P-NMR. Biochim Biophys Acta. 1983 Apr 5;762(2):325–336. doi: 10.1016/0167-4889(83)90087-3. [DOI] [PubMed] [Google Scholar]
- Fritz P. J., White E. L. 3-Phosphoglycerate kinase from rat tissues. Further characterization and developmental studies. Biochemistry. 1974 Jan 29;13(3):444–449. doi: 10.1021/bi00700a008. [DOI] [PubMed] [Google Scholar]
- Gordon D. J., Boyer J. L., Korn E. D. Comparative biochemistry of non-muscle actins. J Biol Chem. 1977 Nov 25;252(22):8300–8309. [PubMed] [Google Scholar]
- Griffiths J. R., Iles R. A. Nuclear magnetic resonance--a 'magnetic eye' on metabolism. Clin Sci (Lond) 1980 Oct;59(4):225–230. doi: 10.1042/cs0590225. [DOI] [PubMed] [Google Scholar]
- Griffiths J. R., Stevens A. N., Gadian D. G., Iles R. A., Porteous R. Hepatic fructose metabolism studied by 31P nuclear magnetic resonance in the anaesthetized rat. Biochem Soc Trans. 1980 Oct;8(5):641–641. doi: 10.1042/bst0080641. [DOI] [PubMed] [Google Scholar]
- Gupta R. K., Moore R. D. 31P NMR studies of intracellular free Mg2+ in intact frog skeletal muscle. J Biol Chem. 1980 May 10;255(9):3987–3993. [PubMed] [Google Scholar]
- Harris D. A., Rosing J., van de Stadt R. J., Slater E. C. Tight binding of adenine nucleotides to beef-heart mitochondrial ATPase. Biochim Biophys Acta. 1973 Aug 31;314(2):149–153. doi: 10.1016/0005-2728(73)90130-8. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Klingenberg M. Differences between the reactivity of endogenous and exogenous adenine nucleotides in mitochondria as studied at low temperature. Eur J Biochem. 1968 Mar;4(1):1–8. doi: 10.1111/j.1432-1033.1968.tb00165.x. [DOI] [PubMed] [Google Scholar]
- Hems D. A., Brosnan J. T. Effects of ischaemia on content of metabolites in rat liver and kidney in vivo. Biochem J. 1970 Nov;120(1):105–111. doi: 10.1042/bj1200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems R., Ross B. D., Berry M. N., Krebs H. A. Gluconeogenesis in the perfused rat liver. Biochem J. 1966 Nov;101(2):284–292. doi: 10.1042/bj1010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holian A., Owen C. S., Wilson D. F. Control of respiration in isolated mitochondria: quantitative evaluation of the dependence of respiratory rates on [ATP], [ADP], and [Pi]. Arch Biochem Biophys. 1977 May;181(1):164–171. doi: 10.1016/0003-9861(77)90494-5. [DOI] [PubMed] [Google Scholar]
- Iles R. A., Baron P. G., Cohen R. D. The effect of reduction of perfusion rate on lactate and oxygen uptake, glucose output and energy supply in the isolated perfused liver of starved rats. Biochem J. 1979 Dec 15;184(3):635–642. doi: 10.1042/bj1840635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles R. A., Griffiths J. R., Stevens A. N., Gadian D. G., Porteous R. Effects of fructose on the energy metabolism and acid-base status of the perfused starved-rat liver. A 31phosphorus nuclear magnetic resonance study. Biochem J. 1980 Oct 15;192(1):191–202. doi: 10.1042/bj1920191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg M. Metabolite transport in mitochondria: an example for intracellular membrane function. Essays Biochem. 1970;6:119–159. [PubMed] [Google Scholar]
- Kretzschmar K. M., Wilkie D. R. A new approach to freezing tissues rapidly. J Physiol. 1969 Jun;202(2):66P–67P. [PubMed] [Google Scholar]
- Krietsch W. K., Bücher T. 3-phosphoglycerate kinase from rabbit sceletal muscle and yeast. Eur J Biochem. 1970 Dec;17(3):568–580. doi: 10.1111/j.1432-1033.1970.tb01202.x. [DOI] [PubMed] [Google Scholar]
- Middleton M. C., Walker D. G. Comparison of the properties of two forms of pyruvate kinase in rat liver and determination of their separate activities during development. Biochem J. 1972 May;127(4):721–731. doi: 10.1042/bj1270721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navon G., Navon R., Shulman R. G., Yamane T. Phosphate metabolites in lymphoid, Friend erythroleukemia, and HeLa cells observed by high-resolution 31P nuclear magnetic resonance. Proc Natl Acad Sci U S A. 1978 Feb;75(2):891–895. doi: 10.1073/pnas.75.2.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S., Shulman R. G., Glynn P., Yamane T., Navon G. On the measurement of pH in Escherichia coli by 31P nuclear magnetic resonance. Biochim Biophys Acta. 1978 Apr 11;502(1):45–50. doi: 10.1016/0005-2728(78)90130-5. [DOI] [PubMed] [Google Scholar]
- Ottaway J. H., Mowbray J. The role of compartmentation in the control of glycolysis. Curr Top Cell Regul. 1977;12:107–208. doi: 10.1016/b978-0-12-152812-6.50010-x. [DOI] [PubMed] [Google Scholar]
- SERAYDARIAN K., MOMMAERTS W. F., WALLNER A., GUILLORY R. J. An estimation of the true inorganic phosphate content of frog sartorius muscle. J Biol Chem. 1961 Jul;236:2071–2075. [PubMed] [Google Scholar]
- Schwenke W. D., Soboll S., Seitz H. J., Sies H. Mitochondrial and cytosolic ATP/ADP ratios in rat liver in vivo. Biochem J. 1981 Nov 15;200(2):405–408. doi: 10.1042/bj2000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C., Boens C. C., Ogawa S. Steady state measurements of the internal phosphorylation potential and the cross membrane electrochemical potential for proton in respiring mitochondria. Biochem Biophys Res Commun. 1980 Mar 13;93(1):243–249. doi: 10.1016/s0006-291x(80)80272-5. [DOI] [PubMed] [Google Scholar]
- Smith C. M., Velick S. F. The glyceraldehyde 3-phosphate dehydrogenases of liver and muscle. Cooperative interactions and conditions for functional reversibility. J Biol Chem. 1972 Jan 10;247(1):273–284. [PubMed] [Google Scholar]
- Soboll S., Scholz R., Heldt H. W. Subcellular metabolite concentrations. Dependence of mitochondrial and cytosolic ATP systems on the metabolic state of perfused rat liver. Eur J Biochem. 1978 Jun 15;87(2):377–390. doi: 10.1111/j.1432-1033.1978.tb12387.x. [DOI] [PubMed] [Google Scholar]
- Stubbs M., Freeman D., Ross B. D. Formation of n.m.r.-invisible ADP during renal ischaemia in rats. Biochem J. 1984 Nov 15;224(1):241–246. doi: 10.1042/bj2240241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Berghe G., Hue L., Hers H. G. Effect of administration of the fructose on the glycogenolytic action of glucagon. An investigation of the pathogeny of hereditary fructose intolerance. Biochem J. 1973 Jun;134(2):637–645. doi: 10.1042/bj1340637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veech R. L., Lawson J. W., Cornell N. W., Krebs H. A. Cytosolic phosphorylation potential. J Biol Chem. 1979 Jul 25;254(14):6538–6547. [PubMed] [Google Scholar]
- Vincent M. F., Van den Berghe G., Hers H. G. The pathway of adenine nucleotide catabolism and its control in isolated rat hepatocytes subjected to anoxia. Biochem J. 1982 Jan 15;202(1):117–123. doi: 10.1042/bj2020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. F., Stubbs M., Veech R. L., Erecińska M., Krebs H. A. Equilibrium relations between the oxidation-reduction reactions and the adenosine triphosphate synthesis in suspensions of isolated liver cells. Biochem J. 1974 Apr;140(1):57–64. doi: 10.1042/bj1400057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods H. F., Eggleston L. V., Krebs H. A. The cause of hepatic accumulation of fructose 1-phosphate on fructose loading. Biochem J. 1970 Sep;119(3):501–510. doi: 10.1042/bj1190501. [DOI] [PMC free article] [PubMed] [Google Scholar]


