Abstract
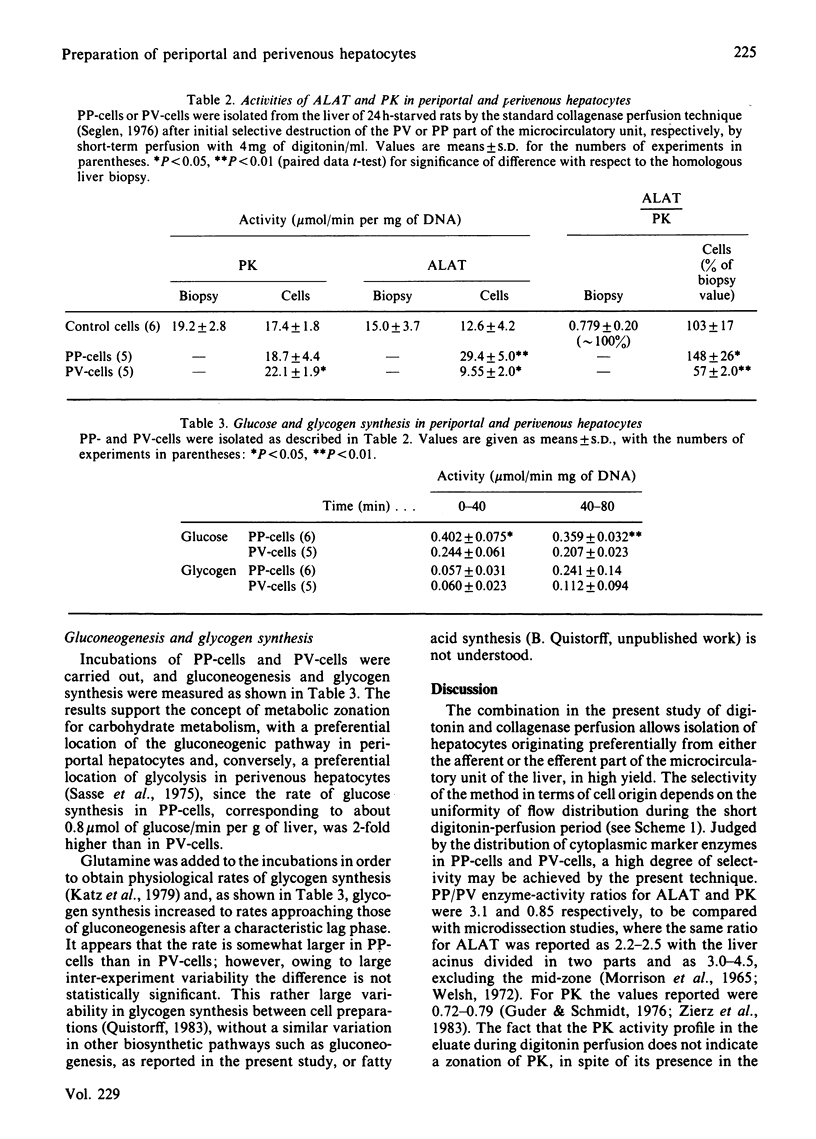
A technique is described which allows preparations of hepatocytes, enriched in either periportal or perivenous hepatocytes ('PP-cells' and 'PV-cells' respectively), in a yield of about 30-50% compared with control cell preparations. The liver is first perfused for 40-60s with digitonin (4 mg/ml) to destroy selectively either the periportal or the perivenous part of the microcirculatory unit, and then the remaining hepatocytes are isolated by the ordinary collagenase perfusion technique. In periportal cells the activities of alanine aminotransferase and pyruvate kinase were 29.4 and 18.7 mumol/min per mg of DNA respectively. The rate of gluconeogenesis was 0.402 mumol/min per mg of DNA. In perivenous cells the corresponding values were 9.55, 22.1 and 0.244 mumol/min per mg of DNA respectively. These data support the concept of a zonation of glucose metabolism within the microcirculatory unit of the liver, with the afferent part (periportal zone) having a 2-fold, more active gluconeogenesis than the efferent part (perivenous zone).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DANIEL P. M., PRICHARD M. M. L. Variations in the circulation of the portal venous blood within the liver. J Physiol. 1951 Aug;114(4):521–537. doi: 10.1113/jphysiol.1951.sp004642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guder W. G., Schmidt U. Liver cell heterogeneity. The distribution of pyruvate kinase and phosphoenolpyruvate carboxykinase (GTP) in the liver lobule of fed and starved rats. Hoppe Seylers Z Physiol Chem. 1976 Dec;357(12):1793–1800. doi: 10.1515/bchm2.1976.357.2.1793. [DOI] [PubMed] [Google Scholar]
- Gumucio J. J. Functional and anatomic heterogeneity in the liver acinus: impact on transport. Am J Physiol. 1983 Jun;244(6):G578–G582. doi: 10.1152/ajpgi.1983.244.6.G578. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Sies H., Gerok W. Functional hepatocyte heterogeneity in ammonia metabolism. The intercellular glutamine cycle. J Hepatol. 1985;1(1):3–14. doi: 10.1016/s0168-8278(85)80063-5. [DOI] [PubMed] [Google Scholar]
- Jungermann K., Heilbronn R., Katz N., Sasse D. The glucose/glucose-6-phosphate cycle in the periportal and perivenous zone of rat liver. Eur J Biochem. 1982 Apr 1;123(2):429–436. doi: 10.1111/j.1432-1033.1982.tb19786.x. [DOI] [PubMed] [Google Scholar]
- Jungermann K., Katz N. Functional hepatocellular heterogeneity. Hepatology. 1982 May-Jun;2(3):385–395. doi: 10.1002/hep.1840020316. [DOI] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Katz J., Golden S., Wals P. A. Glycogen synthesis by rat hepatocytes. Biochem J. 1979 May 15;180(2):389–402. doi: 10.1042/bj1800389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsumura T., Thurman R. G. Predominance of glycolysis in pericentral regions of the liver lobule. Eur J Biochem. 1984 Apr 16;140(2):229–234. doi: 10.1111/j.1432-1033.1984.tb08091.x. [DOI] [PubMed] [Google Scholar]
- Morrison G. R., Brock F. E., Karl I. E., Shank R. E. Quantitative analysis of regenerating and degenerating areas within the lobule of the carbon tetrachloride-injured liver. Arch Biochem Biophys. 1965 Aug;111(2):448–460. doi: 10.1016/0003-9861(65)90208-0. [DOI] [PubMed] [Google Scholar]
- Probst I., Schwartz P., Jungermann K. Induction in primary culture of 'gluconeogenic' and 'glycolytic' hepatocytes resembling periportal and perivenous cells. Eur J Biochem. 1982 Aug;126(2):271–278. doi: 10.1111/j.1432-1033.1982.tb06775.x. [DOI] [PubMed] [Google Scholar]
- Quistorff B., Grunnet N., Cornell N. W. Digitonin perfusion of rat liver. A new approach in the study of intra-acinar and intracellular compartmentation in the liver. Biochem J. 1985 Feb 15;226(1):289–297. doi: 10.1042/bj2260289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport A. M. Hepatic blood flow: morphologic aspects and physiologic regulation. Int Rev Physiol. 1980;21:1–63. [PubMed] [Google Scholar]
- Roehrig K. L., Allred J. B. Direct enzymatic procedure for the determination of liver glycogen. Anal Biochem. 1974 Apr;58(2):414–421. doi: 10.1016/0003-2697(74)90210-3. [DOI] [PubMed] [Google Scholar]
- Sasse D., Katz N., Jungermann K. Functional heterogeneity of rat liver parenchyma and of isolated hepatocytes. FEBS Lett. 1975 Sep 1;57(1):83–88. doi: 10.1016/0014-5793(75)80157-8. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Vänänen H., Lindros K. O., Salaspuro M. Selective isolation of intact periportal or perivenous hepatocytes by antero- or retrograde collagenase gradient perfusion. Liver. 1983 Jun;3(3):131–139. doi: 10.1111/j.1600-0676.1983.tb00861.x. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Stäubli W., Gnägi H. R., Hess F. A. Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol. 1969 Jul;42(1):68–91. doi: 10.1083/jcb.42.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh F. A. Changes in distribution of enzymes within the liver lobule during adaptive increases. J Histochem Cytochem. 1972 Feb;20(2):107–111. doi: 10.1177/20.2.107. [DOI] [PubMed] [Google Scholar]
- Zierz S., Katz N., Jungermann K. Distribution of pyruvate kinase type L and M2 in microdissected periportal and perivenous rat liver tissue with different dietary states. Hoppe Seylers Z Physiol Chem. 1983 Oct;364(10):1447–1453. doi: 10.1515/bchm2.1983.364.2.1447. [DOI] [PubMed] [Google Scholar]


