Abstract
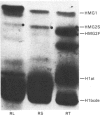
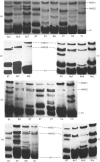
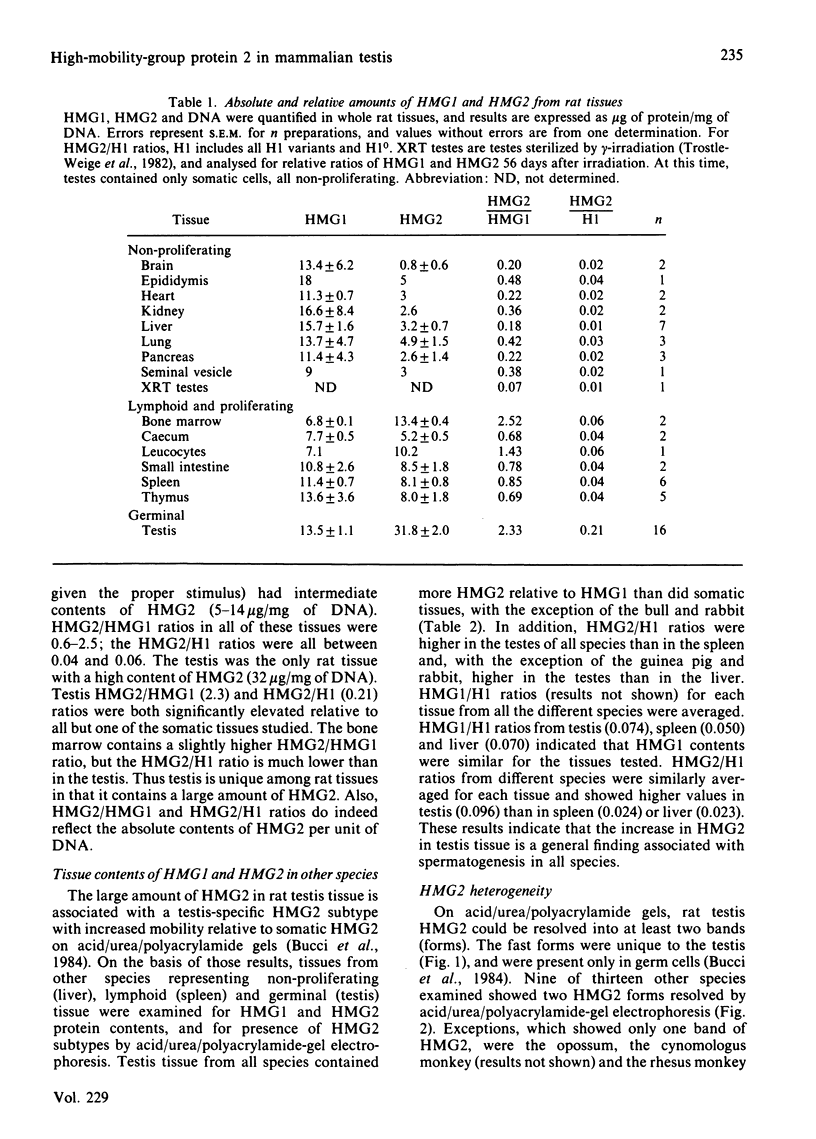
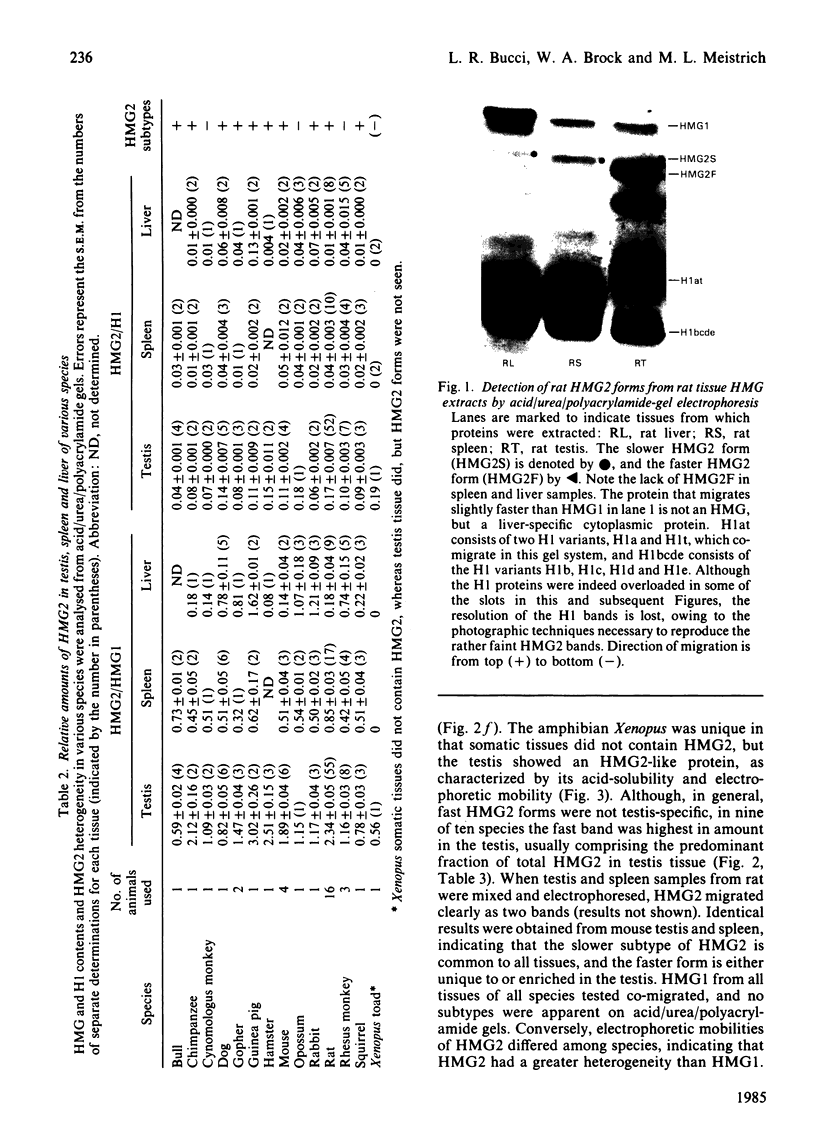
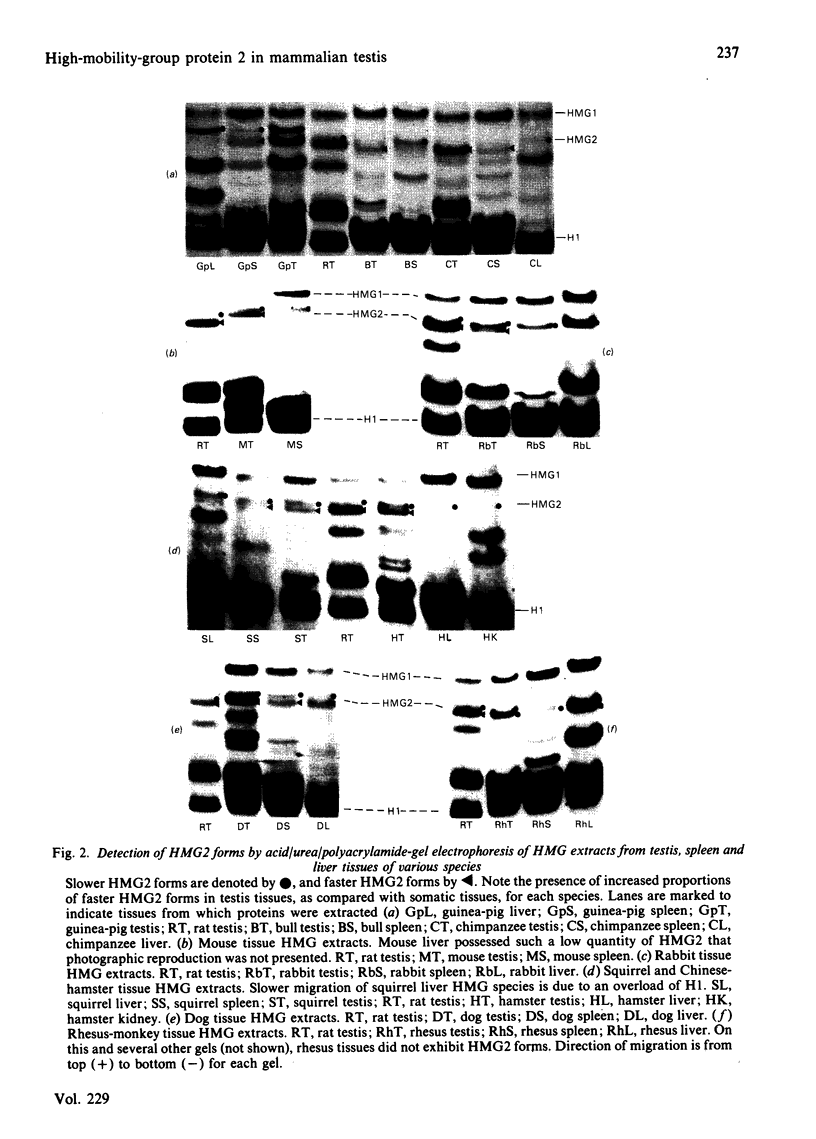
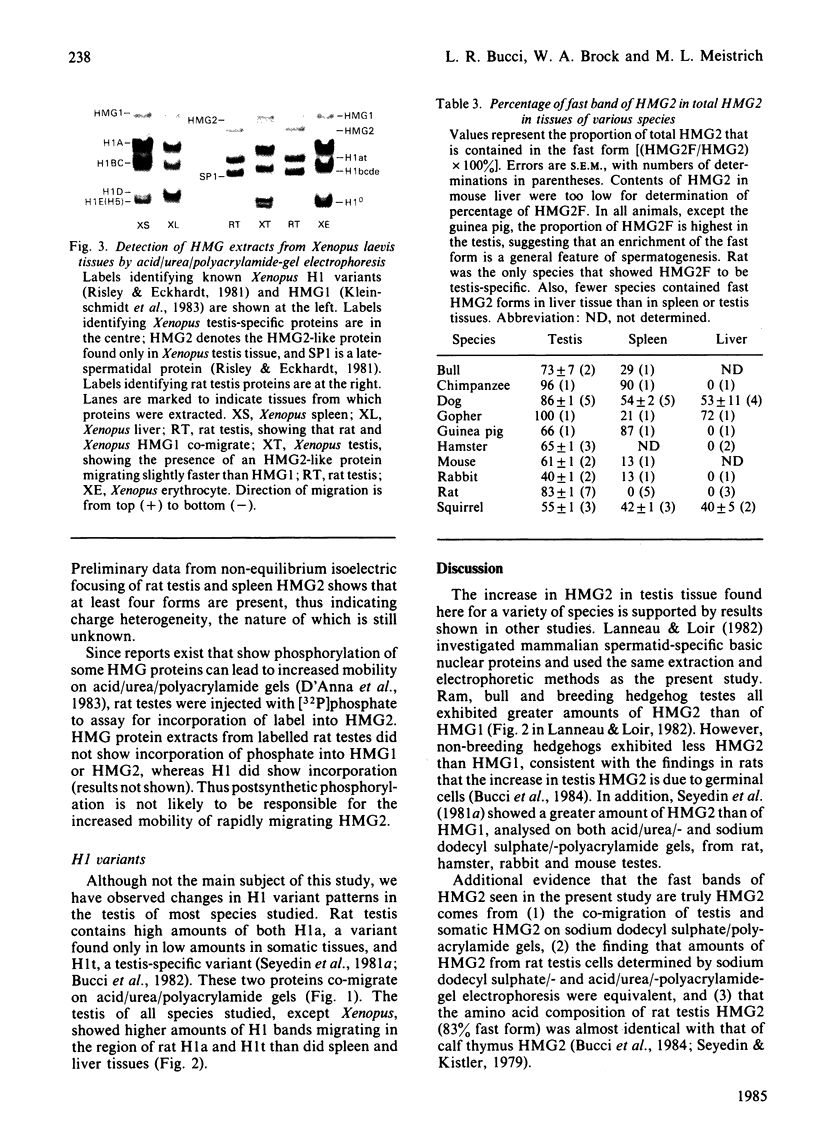
A determination of the absolute amounts of high-mobility-group proteins 1 and 2 (HMG1 and HMG2) in rat tissues demonstrated that amounts of HMG2 were low in non-proliferating tissues, somewhat higher in proliferating and lymphoid tissues, but were extremely elevated in the testis. This increase was due to a germ-cell-specific form of HMG2 with increased mobility relative to somatic HMG2 on acid/urea/polyacrylamide-gel electrophoresis. To determine if the findings in the rat were a general feature of spermatogenesis, testis (germinal), spleen (lymphoid), and liver (non-proliferating) tissues from various vertebrate species were examined for their relative amounts of HMG1 and HMG2, and for HMG2 heterogeneity. Bull, chimpanzee, cynomologus monkey, dog, gopher, guinea pig, hamster, mouse, opossum, rabbit, rat, rhesus monkey, squirrel and toad (Xenopus) tissues were analysed. Nearly all species showed relatively high contents of HMG2 in testis tissue, whereas HMG1 contents were similar in all species and tissues. Ten of thirteen species showed a rapidly migrating HMG2 subtype in testis tissue, separable by acid/urea/polyacrylamide-gel electrophoresis. Xenopus, which lacks HMG2 in somatic tissues, showed an HMG2-like protein in testis tissue. Although the rapidly migrating HMG2 subtype in species other than rat was not testis-specific, it was always enriched in the testis. This study indicates that increased amounts of HMG2 and the enrichment of a rapidly migrating HMG2 subtype are general features of spermatogenic cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allis C. D., Bowen J. K., Abraham G. N., Glover C. V., Gorovsky M. A. Proteolytic processing of histone H3 in chromatin: a physiologically regulated event in Tetrahymena micronuclei. Cell. 1980 May;20(1):55–64. doi: 10.1016/0092-8674(80)90234-2. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonne C., Sautiere P., Duguet M., de Recondo A. M. Identification of a single-stranded DNA binding protein from rat liver with high mobility group protein 1. J Biol Chem. 1982 Mar 25;257(6):2722–2725. [PubMed] [Google Scholar]
- Brown E., Goodwin G. H., Mayes E. L., Hastings J. R., Johns E. W. Heterogeneity of proteins resembling high-mobility-group protein HMG-T in trout testes nuclei. Biochem J. 1980 Nov 1;191(2):661–664. doi: 10.1042/bj1910661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci L. R., Brock W. A., Goldknopf I. L., Meistrich M. L. Characterization of high mobility group protein levels during spermatogenesis in the rat. J Biol Chem. 1984 Jul 25;259(14):8840–8846. [PubMed] [Google Scholar]
- Bucci L. R., Brock W. A., Meistrich M. L. Distribution and synthesis of histone 1 subfractions during spermatogenesis in the rat. Exp Cell Res. 1982 Jul;140(1):111–118. doi: 10.1016/0014-4827(82)90162-8. [DOI] [PubMed] [Google Scholar]
- Chiva M., Mezquita C. Quantitative changes of high mobility group non-histone chromosomal proteins HMG1 and HMG2 during rooster spermatogenesis. FEBS Lett. 1983 Oct 17;162(2):324–328. doi: 10.1016/0014-5793(83)80781-9. [DOI] [PubMed] [Google Scholar]
- D'Anna J. A., Becker R. R., Tobey R. A., Gurley L. R. Composition and synthesis during G1 and S phase of a high mobility group-E/G component from Chinese hamster ovary cells. Biochim Biophys Acta. 1983 Mar 10;739(2):197–206. doi: 10.1016/0167-4781(83)90030-1. [DOI] [PubMed] [Google Scholar]
- Goodwin G. H., Johns E. W. Are the high mobility group non-histone chromosomal proteins associated with 'active' chromatin? Biochim Biophys Acta. 1978 Jun 22;519(1):279–284. doi: 10.1016/0005-2787(78)90081-3. [DOI] [PubMed] [Google Scholar]
- Goodwin G. H., Walker J. M., Johns E. W. Studies on the degradation of high mobility group non-histone chromosomal proteins. Biochim Biophys Acta. 1978 Jun 22;519(1):233–242. doi: 10.1016/0005-2787(78)90076-x. [DOI] [PubMed] [Google Scholar]
- Gordon J. S., Bruno J., Lucas J. J. Heterogeneous binding of high mobility group chromosomal proteins to nuclei. J Cell Biol. 1981 Feb;88(2):373–379. doi: 10.1083/jcb.88.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann P. The H1 class of histone and diversity in chromosomal structure. Subcell Biochem. 1978;5:87–127. doi: 10.1007/978-1-4615-7942-7_2. [DOI] [PubMed] [Google Scholar]
- Hyde J. E., Igo-Kemenes T., Zachau H. G. The non-histone proteins of the rat liver nucleus and their distribution amongst chromatin fractions as produced by nuclease digestion. Nucleic Acids Res. 1979 Sep 11;7(1):31–48. doi: 10.1093/nar/7.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isackson P. J., Bidney D. L., Reeck G. R., Neihart N. K., Bustin M. High mobility group chromosomal proteins isolated from muclei and cytosol of cultured hepatoma cells are similar. Biochemistry. 1980 Sep 16;19(19):4466–4471. doi: 10.1021/bi00560a013. [DOI] [PubMed] [Google Scholar]
- Isackson P. J., Fishback J. L., Bidney D. L., Reeck G. R. Preferential affinity of high molecular weight high mobility group non-histone chromatin proteins for single-stranded DNA. J Biol Chem. 1979 Jul 10;254(13):5569–5572. [PubMed] [Google Scholar]
- Isackson P. J., Reeck G. R. Removal of degradation products from calf thymus high mobility group non-histone chromatin proteins by chromatography on immobilized double-stranded DNA. Biochim Biophys Acta. 1982 Jun 30;697(3):378–380. doi: 10.1016/0167-4781(82)90102-6. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Pollock J. M., Jr, Rill R. L. Chromatin fractionation procedure that yields nucleosomes containing near-stoichiometric amounts of high mobility group nonhistone chromosomal proteins. Biochemistry. 1979 Aug 21;18(17):3739–3748. doi: 10.1021/bi00584a015. [DOI] [PubMed] [Google Scholar]
- Javaherian K., Sadeghi M., Liu L. F. Nonhistone proteins HMG1 and HMG2 unwind DNA double helix. Nucleic Acids Res. 1979 Aug 10;6(11):3569–3580. doi: 10.1093/nar/6.11.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt J. A., Scheer U., Dabauvalle M. C., Bustin M., Franke W. W. High mobility group proteins of amphibian oocytes: a large storage pool of a soluble high mobility group-1-like protein and involvement in transcriptional events. J Cell Biol. 1983 Sep;97(3):838–848. doi: 10.1083/jcb.97.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl L., Lyness T., Watson D. C., Dixon G. H. Binding of HMG-T to trout testis chromatin. Biochem Biophys Res Commun. 1979 Sep 27;90(2):391–397. doi: 10.1016/0006-291x(79)91247-6. [DOI] [PubMed] [Google Scholar]
- Lanneau M., Loir M. An electrophoretic investigation of mammalian spermatid-specific nuclear proteins. J Reprod Fertil. 1982 May;65(1):163–170. doi: 10.1530/jrf.0.0650163. [DOI] [PubMed] [Google Scholar]
- Levy B. W., Connor W., Dixon G. H. A subset of trout testis nucleosomes enriched in transcribed DNA sequences contains high mobility group proteins as major structural components. J Biol Chem. 1979 Feb 10;254(3):609–620. [PubMed] [Google Scholar]
- Mathew C. G., Goodwin G. H., Gooderham K., Walker J. M., Johns E. W. A comparison of the high mobility group non-histone chromatin protein HMG 2 in chicken thymus and erythrocytes. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1243–1251. doi: 10.1016/s0006-291x(79)80040-6. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Peters E. H., Levy-Wilson B., Dixon G. H. Evidence for the location of high mobility group protein T in the internucleosomal linker regions of trout testis chromatin. J Biol Chem. 1979 May 10;254(9):3358–3361. [PubMed] [Google Scholar]
- Risley M. S., Eckhardt R. A. H1 histone variants in Xenopus laevis. Dev Biol. 1981 May;84(1):79–87. doi: 10.1016/0012-1606(81)90372-9. [DOI] [PubMed] [Google Scholar]
- Seyedin S. M., Kistler W. S. Levels of chromosomal protein high mobility group 2 parallel the proliferative activity of testis, skeletal muscle, and other organs. J Biol Chem. 1979 Nov 25;254(22):11264–11271. [PubMed] [Google Scholar]
- Seyedin S. M., Pehrson J. R., Cole R. D. Loss of chromosomal high mobility group proteins HMG1 and HMG2 when mouse neuroblastoma and Friend erythroleukemia cells become committed to differentiation. Proc Natl Acad Sci U S A. 1981 Oct;78(10):5988–5992. doi: 10.1073/pnas.78.10.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R., Wachtel E. J. The histones. Adv Protein Chem. 1981;34:1–60. doi: 10.1016/s0065-3233(08)60517-3. [DOI] [PubMed] [Google Scholar]
- Sterner R., Boffa L. C., Vidali G. Comparative structural analysis of high mobility group proteins from a variety of sources. Evidence for a high mobility group protein unique to avian erythrocyte nuclei. J Biol Chem. 1978 Jun 10;253(11):3830–3836. [PubMed] [Google Scholar]
- Trostle-Weige P. K., Meistrich M. L., Brock W. A., Nishioka K., Bremer J. W. Isolation and characterization of TH2A, a germ cell-specific variant of histone 2A in rat testis. J Biol Chem. 1982 May 25;257(10):5560–5567. [PubMed] [Google Scholar]
- Tyrell D., Isackson P. J., Reeck G. R. Two-dimensional gel electrophoresis of nonhistone chromatin proteins with nonequilibrium pH gradient electrophoresis as the first dimension. Anal Biochem. 1982 Jan 15;119(2):433–439. doi: 10.1016/0003-2697(82)90610-8. [DOI] [PubMed] [Google Scholar]
- Vidali G., Boffa L. C., Allfrey V. G. Selective release of chromosomal proteins during limited DNAase 1 digestion of avian erythrocyte chromatin. Cell. 1977 Oct;12(2):409–415. doi: 10.1016/0092-8674(77)90117-9. [DOI] [PubMed] [Google Scholar]
- Von Holt C., Strickland W. N., Brandt W. F., Strickland M. S. More histone structures. FEBS Lett. 1979 Apr 15;100(2):201–218. doi: 10.1016/0014-5793(79)80337-3. [DOI] [PubMed] [Google Scholar]
- Wu L., Rechsteiner M., Kuehl L. Comparative studies on microinjected high-mobility-group chromosomal proteins, HMG1 and HMG2. J Cell Biol. 1981 Nov;91(2 Pt 1):488–496. doi: 10.1083/jcb.91.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]