Abstract
Migraine is a highly prevalent neurovascular disorder for which genome-wide association studies (GWAS) have identified over one hundred risk loci, yet the causal variants and genes remain mostly unknown. Here, we meta-analyzed three migraine GWAS including 98,374 cases and 869,160 controls and identified 122 independent risk loci of which 35 were new. Fine-mapping of a meta-analysis is challenging because some variants may be missing from some participating studies and accurate linkage disequilibrium (LD) information of the variants is often not available. Here, using the exact in-sample LD, we first investigated which statistics could reliably capture the quality of fine-mapping when only reference LD was available. We observed that the posterior expected number of causal variants best distinguished between the high- and low-quality results. Next, we performed fine-mapping for 102 autosomal risk regions using FINEMAP. We produced high-quality fine-mapping for 93 regions and defined 181 distinct credible sets. Among the high-quality credible sets were 7 variants with very high posterior inclusion probability (PIP > 0.9) and 2 missense variants with PIP > 0.5 (rs6330 in NGF and rs1133400 in INPP5A). For 35 association signals, we managed to narrow down the set of potential risk variants to at most 5 variants.
Introduction
Migraine is a common neurological disorder characterized by recurrent disabling episodes of severe headache that are typically one-sided, pulsating in nature, and accompanied by other symptoms such as nausea, and hypersensitivity to light and/or sound. It has two main subtypes, migraine without aura and migraine with aura. The aura is a reversible visual, sensory or speech disturbance, that typically occurs before the headache phase. Migraine attacks last usually from 4 to 72 hours, and can significantly harm daily life of patients1. Migraine was ranked as the second most disabling disease worldwide in terms of years lived with disability by Global Burden of Diseases Study in 20192. Its lifetime prevalence has been estimated to be about 15 to 20 % worldwide, and it is three times more common in females than in males2. Family and twin studies estimate the heritability to be about 40%3. To date, over 100 migraine associated loci have been reported by GWAS4,5,6,7,8,9,10,11,12,13,14. The genetic association of migraine has shown a general enrichment in genes highly expressed in vascular and central nervous system related tissues15,13 but we lack detailed information on specific genetic variants that affect the migraine risk.
Identification of causal genes and variants that have a biological effect on migraine is crucial for understanding the biology of migraine, and for developing new effective treatments for the disorder. Here, we aim to narrow down correlated genetic variation in migraine associated regions to a smaller number of candidate causal variants by applying statistical fine-mapping16. Fine-mapping methods evaluate how plausibly each variant in the region is among the causal variants by utilizing the observed association statistics and the LD structure of the region16. Multiple methods that can utilize GWAS summary statistics have been developed, including PAINTOR17, CAVIAR18, FINEMAP19, JAM20 and SuSIE21. The optimal way to apply fine-mapping is to compute the LD information from the original GWAS data (in-sample LD), but when the original genotype data are unavailable, approximate LD information is often obtained from a reference genotype panel (reference LD). However, when reference LD is used, the discrepancy from the in-sample LD can cause errors in fine-mapping and this problem becomes more severe as the GWAS sample size grows22.
Even though large meta-analyses have become a successful way to increase statistical power of GWAS, they remain difficult to fine-map reliably for several reasons23. First, meta-analyses are combinations of multiple studies and typically no single analyst has access to the exact in-sample LD of the whole meta-analysis, which means that reference LD must be used. Second, differences in genotyping platforms and genotype imputation pipelines between the meta-analyzed studies can bias the fine-mapping results. Third, some variants included in the meta-analysis may be present in only a subset of the studies, which leads to variation in information content of the association statistics of different variants. In a landmark fine-mapping study on schizophrenia, Trubetskoy et al. (2022)24 avoided these problems by collecting all genotype-phenotype data into a single analysis site. Unfortunately, to our knowledge, no other international disease consortium has been able to create a comparable analysis environment that would allow an in-sample fine-mapping of a large meta-analysis. Given that fine-mapping of meta-analysis results typically relies on reference LD, a crucial question is how we can assess when the results of fine-mapping based on reference LD are reliable.
So far, the largest GWAS meta-analysis on migraine contained 102,084 cases and 771,257 controls from 25 study collections13. Unfortunately, we cannot perform reliable fine-mapping for that meta-analysis, since the in-sample LD is not available. Instead, we conducted a migraine meta-analysis with 98,374 migraine cases and 869,160 controls by combining data from three sources: 23andMe, Inc., FinnGen, and UK Biobank (UKB). Of these data sets, 23andMe and UKB were included in the earlier meta-analysis of Hautakangas et al. (2022) while FinnGen was not. Statistical power of our meta-analysis was comparable to the previous migraine meta-analysis of Hautakangas et al. (2022), with effective sample sizes of 339,000 and 326,000, respectively. Importantly, we have the full in-sample LD available for 26 risk loci and for the remaining risk loci we have the in-sample LD for FinnGen and UKB but not for 23andMe (Table 1). This set-up allowed us to investigate how different LD reference panels perform compared to the in-sample LD. In particular, we evaluated different statistics that could be used to assess fine-mapping quality when only reference LD is available. Finally, we utilized our results to fine-map 102 migraine risk loci to narrow down the putative causal variants behind the associations. We were able to get reliable fine-mapping results for 93 out of 102 regions and identified 7 variants with a high probability (>90%) of being causal and two missense variants, rs6330 in NGF and rs1133400 in INPP5A, with a probability > 50% of being causal.
Table 1.
Three study collections included in the migraine meta-analysis.
| Study | Ancestry | Cases | Controls | N | Case % | Migraine definition | LD availability |
|---|---|---|---|---|---|---|---|
| UK Biobank | European, British | 10,881 | 330,169 | 341,050 | 0.03 | Self-reported | In-sample |
| 23andMe, Inc | European descent | 53,109 | 230,876 | 283,985 | 0.19 | Self-reported | In-sample for 26/102 fine-map regions |
| FinnGen R8 | European, Finnish | 34,385 | 308,114 | 342,499 | 0.10 | Medication purchases | In-sample |
| Meta-analysis | European descent | 98,374 | 869,160 | 967,534 | 0.10 | Self-reported, medication purchases | In-sample 26/102, reference LD 76/102 |
Results
We conducted an inverse-variance weighted meta-analysis on migraine by combining results from the three GWAS (Table 1): UK Biobank (UKB; 10,881 cases and 330,169 controls), 23andMe, Inc. (53,109 cases and 230,876 controls), and FinnGen Release 8 (34,385 cases and 308,114 controls). The total sample size is 98,374 migraine cases and 869,160 controls. Before meta-analyzing the data, we estimated pairwise genetic correlations between the study collections by LD Score regression (LDSC)25. The estimated genetic correlations were 1.00 (s.e. 0.04) between UKB and 23andMe, 0.84 (s.e. 0.05) between UKB and FinnGen, and 0.87 (s.e. 0.03) between 23andMe and FinnGen. The lower genetic correlation between FinnGen and the other two studies could be due to differences in the case definitions (triptan purchases in FinnGen vs. self-reporting in UKB and 23andMe). A comparable level of genetic correlation (0.81) has been reported before between primary care and self-reported migraine cases within UKB26. Another source of possible heterogeneity in effect sizes is the difference in genetic ancestry (Finnish in FinnGen vs. Non-Finnish European in the other two).
The genomic inflation factor of the migraine meta-analysis was 1.38. There was a linear relationship between the association statistic and the LD-score (Supplementary Fig 1) indicating that the polygenic background of migraine was the main source of the genomic inflation. However, as the intercept from LDSC was elevated to 1.09 (s.e. 0.01) from its null value of 1.0, some inflation could also be due to confounding factors such as cryptic relatedness, population stratification or other model misspecification. Consequently, we further checked the LDSC intercepts for the individual studies: 1.03 (s.e. 0.01) for 23andMe, 1.00 (s.e. 0.01) for UKB and 1.10 (s.e. 0.01) for FinnGen. The higher intercept for FinnGen could be due to a different GWAS analysis method (whole genome-regression by REGENIE27 including related samples) compared to UKB and 23andMe (logistic regression excluding related samples). Estimated SNP-heritability was 11.49% (s.e. 0.47%) from LDSC when population prevalence was assumed to be 16%.
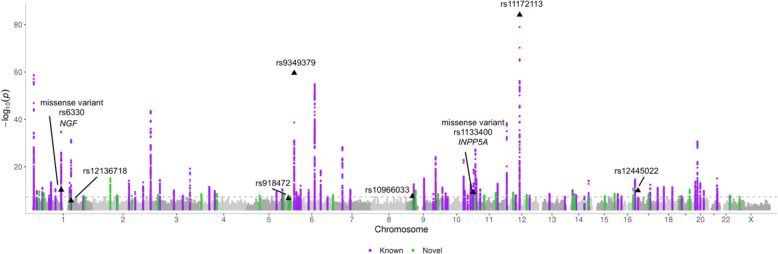
We followed the locus definition of Hautakangas et al. (2022) and defined the LD-independent genome-wide significant (GWS; P < 5 × 10−8) risk loci from the meta-analysis iteratively by choosing the variant with the smallest P-value as an index variant and excluding all other GWS variants with LD r2 > 0.1 to that index variant from further considerations until no GWS variants remained. Next, we formed a high LD region around each index variant extending to the level of r2 > 0.6, and merged regions that were closer than 250 kb. Lastly, all other GWS variants were included in their closest region, and the region boundaries were updated, and once again regions closer than 250 kb were merged (see further details in Methods). Based on this locus definition, we identified 122 LD-independent risk loci, of which 35 were new (Table 2), and 87 overlapped with the previously known risk loci (Fig 1, Supplementary Table 1, Supplementary Figs 2–4)4,5,6,7,8,9,10,11,12,13,14. We observed statistically significant heterogeneity (P < 0.05/122) in effect sizes between the study collections only for two lead variants, both of which resided in the previously known migraine loci (PRDM16 and near ZCCHC14)(Supplementary Table 1, Supplementary Fig 3). As external replication data of 34,807 cases and 193,475 controls, we meta-analyzed data from the Trøndelag Health Study (HUNT)28 and IHGC16 migraine meta-analysis excluding the Finnish cohorts and the 23andMe data9. Of the 35 lead variants of our new loci, 32 were consistent in direction (P = 2.1 × 10−7, one-sided binomial test) and 17 replicated with P < 0.05 (one-sided test; Supplementary Table 2) in the replication data. When we meta-analyzed the discovery and the replication data, 28 out of the 35 novel loci remained GWS (Supplementary Table 2).
Table 2.
New 35 migraine risk loci identified from the meta-analysis of 98,374 migraine cases and 869,160 controls.
| Locus name | RSID | Chrom osome | Position GRCh37 | Effect allele | Other allele | Effect allele frequency | Log-odds ratio | S.e. | P-value |
|---|---|---|---|---|---|---|---|---|---|
| near RUNX3 | rs71014329 | 1 | 25348950 | I | D | 0.604 | 0.034 | 0.005 | 2.57E-10 |
| ST3GAL3 | rs783302 | 1 | 44366341 | G | A | 0.878 | 0.047 | 0.008 | 1.68E-09 |
| SF3B4 | rs7544531 | 1 | 149897217 | T | C | 0.084 | 0.072 | 0.012 | 5.08E-09 |
| near DTL | rs61830764 | 1 | 212289976 | A | G | 0.382 | 0.031 | 0.006 | 3.71E-08 |
| near APLF | rs112706954 | 2 | 68819969 | G | A | 0.023 | 0.137 | 0.017 | 7.88E-16 |
| TMEM131 | rs2305142 | 2 | 98375722 | G | A | 0.322 | 0.031 | 0.005 | 1.18E-08 |
| near GPD2 | rs74482068 | 2 | 157560108 | D | I | 0.039 | 0.076 | 0.014 | 1.76E-08 |
| near RANP7 | rs11386839 | 3 | 22929430 | D | I | 0.500 | 0.029 | 0.005 | 7.68E-09 |
| ADD1 | rs10026792 | 4 | 2862190 | G | A | 0.687 | 0.032 | 0.005 | 2.79E-09 |
| EPHA5 | rs147908403 | 4 | 66362482 | C | T | 0.054 | 0.069 | 0.012 | 2.80E-09 |
| ITGA1 | rs4865540 | 5 | 52184268 | C | A | 0.820 | 0.037 | 0.007 | 1.41E-08 |
| near GLRA1 | rs372257780 | 5 | 151200938 | I | D | 0.599 | 0.033 | 0.006 | 2.27E-09 |
| KCNIP1 | rs78151838 | 5 | 170108683 | A | G | 0.905 | 0.054 | 0.010 | 1.82E-08 |
| MAML1 | rs10794701 | 5 | 179181061 | A | G | 0.119 | 0.043 | 0.008 | 3.57E-08 |
| near COX19 | rs117303395 | 7 | 1001963 | A | G | 0.019 | 0.122 | 0.022 | 4.40E-08 |
| MAD1L1 | rs10479762 | 7 | 2045351 | T | C | 0.419 | 0.029 | 0.005 | 8.01E-09 |
| ELAVL2 | rs10966033 | 9 | 23705736 | G | T | 0.617 | 0.029 | 0.005 | 2.70E-08 |
| near ZCCHC7 | rs10973207 | 9 | 37100525 | T | G | 0.187 | 0.042 | 0.007 | 1.04E-10 |
| near LMX1B | rs4358894 | 9 | 129464802 | C | G | 0.513 | 0.030 | 0.005 | 3.33E-09 |
| near DENND5A | rs34494849 | 11 | 9287030 | C | T | 0.768 | 0.034 | 0.006 | 1.17E-08 |
| near MTCH2 | rs11039324 | 11 | 47665686 | G | A | 0.601 | 0.030 | 0.005 | 9.76E-09 |
| MRE11A | rs639311 | 11 | 94205747 | C | T | 0.681 | 0.033 | 0.005 | 9.02E-10 |
| IPO8 | rs12369125 | 12 | 30807195 | A | C | 0.251 | 0.036 | 0.006 | 7.08E-10 |
| MGAT4C | rs73187675 | 12 | 86409247 | T | A | 0.193 | 0.037 | 0.006 | 6.08E-09 |
| RP11-562L8.1 | rs1957110 | 14 | 29777492 | T | C | 0.409 | 0.029 | 0.005 | 1.59E-08 |
| INSM2 | rs2296919 | 14 | 36005659 | T | C | 0.807 | 0.038 | 0.006 | 3.44E-09 |
| RPS6KA5 | rs117151272 | 14 | 91415550 | A | T | 0.026 | 0.097 | 0.018 | 3.59E-08 |
| near ONECUT1 | rs1899730 | 15 | 53166138 | T | G | 0.707 | 0.032 | 0.006 | 2.11E-08 |
| FAM174B | rs12910861 | 15 | 93218540 | C | T | 0.227 | 0.037 | 0.006 | 2.15E-09 |
| FAM65A | rs9934328 | 16 | 67573367 | C | G | 0.137 | 0.049 | 0.007 | 1.32E-11 |
| TUBG2 | rs2292750 | 17 | 40811781 | C | T | 0.452 | 0.030 | 0.005 | 3.53E-09 |
| near NRTN | rs76899991 | 19 | 5822370 | G | T | 0.963 | 0.077 | 0.014 | 2.89E-08 |
| SYMPK | rs74821481 | 19 | 46320041 | G | T | 0.678 | 0.036 | 0.005 | 4.59E-11 |
| near SERHL2 | rs141478056 | 22 | 42939927 | G | A | 0.120 | 0.046 | 0.008 | 2.23E-08 |
| near FTHL17 | rs149675702 | 23 | 31063624 | C | T | 0.945 | 0.079 | 0.014 | 4.56E-08 |
RSID = reference SNP ID, GRCh37 = Genome Reference Consortium Human Build 37, s.e. = standard error. Alleles D and I refer to deletion and insertion, respectively.
Figure 1.
A Manhattan plot of the inverse-variance weighted fixed effects migraine meta-analysis including 98,374 cases and 869,160 controls. X-axis presents the chromosomal location and y-axis the −log10(P-value). Known loci are highlighted in purple and new loci in green. Variants with posterior inclusion probability (PIP) > 0.9 and missense variants with PIP > 0.5 in high-quality fine-mapping regions are annotated.
To define the fine-map regions, we merged together the risk loci that were closer than 1.5 Mb. This resulted in 102 fine-map regions. To avoid problems due to varying sample sizes across the variants, we included in fine-mapping only autosomal SNPs that were available in all three cohorts. This criterion reduced the number of common variants (MAF>0.05) per regions on average by 19%.
Comparison of different LD panels in fine-mapping
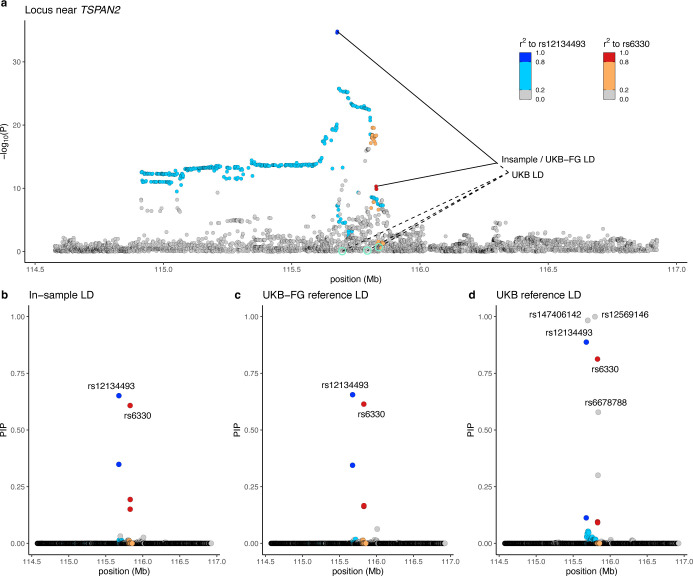
A common problem in meta-analyses is that the in-sample LD is not available, and use of reference LD may lead to biased results. Figure 2 demonstrates this problem at the locus around TSPAN2 where fine-mapping using the in-sample LD disagrees strongly with the UKB reference LD but agrees well with a more accurate UKB-FG reference LD. This shows that, in our setting, fine-mapping based on the UKB-FG reference LD has a potential to yield reliable results but that we need some way to assess, for each region, whether the reference LD has provided reliable results. Therefore, we evaluated whether some statistics, either derived from the GWAS results or from the fine-mapping results, could flag the regions where the reference LD produced unreliable fine-mapping results compared to the in-sample LD. We did this comparison in the 26 regions where the in-sample LD was available. As candidate statistics, we considered: (1) posterior expectation of the number of causal variants (PENC), and, from the top variant(s) of the credible sets, (2) maximum pairwise r2, (3) maximum marginal P-value, and (4) minimum INFO value. We used the maximum difference of the variant-specific posterior inclusion probabilities (maxΔ) between the reference LD and the in-sample LD to assess the quality of the refence LD results. A small maxΔ value (close to 0) indicates high quality (the reference LD produces similar results to the in-sample LD), and a large value (close to 1) indicates low quality (the reference LD produces different results from the in-sample LD).
Figure 2.
Fine-mapping a region near TSPAN2 at chromosome 1 using three different LD sources. a) Plot of the GWAS results with the chromosomal location on x-axis and the strength of the association as −log10 P-values from the inverse-variance weighted fixed-effect meta-analysis with 98,374 migraine cases and 869,160 controls on y-axis. Variants are colored based on the squared correlation (r2) to the two variants in the top configuration suggested by FINEMAP with the in-sample LD. The suggested top configurations based on three LD panels are marked by lines with the in-sample LD and the UKB-FG reference LD giving the same top configuration and the UKB reference LD including three additional variants (highlighted in green). Posterior inclusion probabilities (PIPs) for the variants based on b) in-sample LD, c) UKB-FG reference LD and d) UKB reference LD.
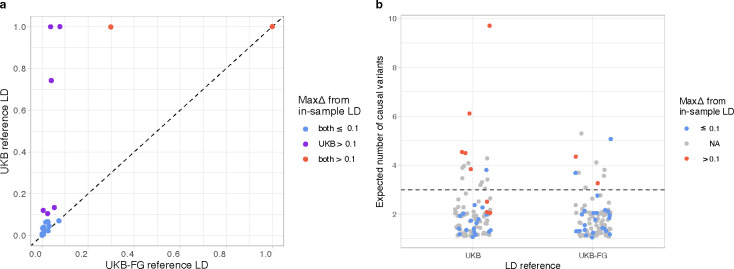
In general, both LD reference panels performed well in most of the 26 regions available for this comparison, but, as expected22, the more accurate UKB-FG panel performed clearly better than the UKB panel alone. For example, maxΔ was above 0.1 only in 2/26 regions with the UKB-FG panel but in 8/26 regions with the UKB panel (Fig 3a).
Figure 3.
a) Scatter plot comparing the maximum PIP differences (maxΔ) between the in-sample and reference LD for 26 fine-map regions. X-axis shows the UKB-FG reference LD and y-axis the UKB reference LD. b) Strip chart shows the posterior expected number of causal variants (PENC) from fine-mapping for the two LD reference panels for the 102 fine-map regions. Red dots indicate large differences from the in-sample LD (maxΔ > 0.1), and grey color indicates regions for which only reference LD is available and therefore maxΔ is not known. Horizontal line shows PENC = 3 that we use as a threshold to define reliable results with the UKB-FG panel.
We then investigated how well the four different statistics could separate the regions with low-quality fine-mapping results from those with high-quality results for the two LD reference panels (Supplementary Fig 5). First, when PENC was used, both LD reference panels performed similarly for the regions where FINEMAP suggested only one or two causal variants (Supplementary Fig 5a). Those results were also close to the in-sample results (maxΔ < 0.07). All low-quality regions (with maxΔ > 0.1) had PENC > 2 with the UKB panel and PENC > 3 with the UKB-FG panel. Thus, we used these PENC thresholds to define low-quality regions when the in-sample LD was not available. We expect that these thresholds have a high sensitivity for low-quality results but will simultaneously exclude some of the regions that truly have many causal variants. The other three statistics are not able to distinguish the low-quality regions as clearly as PENC (Supplementary Figs 5b–d). First, the maximum r2 among the top configuration variants does not distinguish both of the low-quality regions with the UKB-FG panel (Supplementary Fig 5b). Additionally, neither the maximum P nor the minimum INFO within the top credible set variants separates well the low-quality regions from the good-quality regions (Supplementary Figs 5c,d). We conclude that PENC gives the best separation among the statistics investigated. Previously, PENC has been used to filter FINEMAP results in the schizophrenia fine-mapping study24.
Next, we evaluated how PENC classifies the 76 fine-map regions where only reference LD was available to us. The 76 grey points in Figure 3b show that the fine-map regions without the in-sample LD are typically having PENC < 2.5 and, with the UKB-FG LD, only 6 of the 76 regions have PENC > 3.
FINEMAP results overview
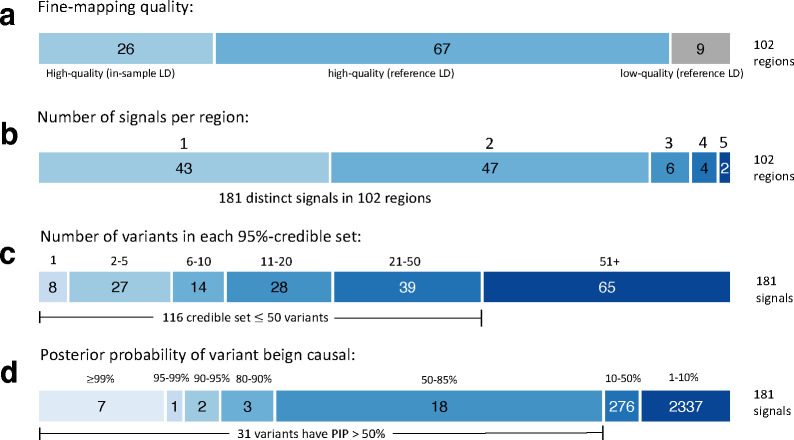
Overall, for a majority of the fine-map regions, FINEMAP suggested one (42%) or two (46%) causal variants (Supplementary Table 3, Fig 4.). The 102 fine-map regions together had 181 distinct signals when the signals were defined by the number of causal variants per region with the highest posterior probability. Among the 76 regions without the in-sample LD, 6 had PENC above 3. We flagged these regions to be of low-quality, and their interpretation requires extra caution. The largest PENC observed was 5 and it occurred for two fine-map regions: PRDM16 (index variant rs10218452) and HOXB3 (index variant rs2555111). Of these, HOXB3 region is flagged as low-quality because there is no in-sample LD available. The sizes of 95%-credible sets ranged from 1 to 2,787 variants, and 49 credible sets had 10 variants or less. A very high PIP (≥ 0.9) was observed for 10 variants (Supplementary Table 4), of which seven were in the high-quality fine-map regions (Table 3). We conducted a look-up from Variant Effect Predictor (VEP) database for all credible sets to search for variants that could have an impact on the gene transcript. In total, 149 unique missense variants were found of which 3 had PIP > 0.5: rs6330 (PIP=0.59) in NGF located at chromosome 1, rs1133400 (PIP=0.93) in INPP5A located at chromosome 10 and rs28929474 (PIP=0.64) in SERPINA1 located in a low-quality fine-map region at chromosome 14 (Table 3, Supplementary Table 5). Of these, rs6330 is a significant cis-eQTL for NGF-AS1 expressed in atrial appendage of heart and rs28929474 for IFI27L2 expressed in tibial artery and in left ventricle of heart in GTEx v.08 data.
Figure 4.
Summary of the fine-mapping results across the 102 migraine risk regions.
Table 3.
Variants with high (>0.9) posterior inclusion probability (PIP) and missense variants with PIP > 0.5 among the 93 high-confidence fine-map regions.
| Gene (VEP) | Predicted consequence (VEP) | RSID | Chromosome | Position GRCh37 | Effect allele | Other allele | PIP | Minor allele frequency | Log-odds ratio | S.E. | P-value | LDsource |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PHACTR1 | Intron variant | rs9349379 | 6 | 12903957 | G | A | 1.000 | 0.422 | -0.084 | 0.005 | 2.59E-60 | in-sample |
| LRP1 | Intron variant | rs11172113 | 12 | 57527283 | C | T | 1.000 | 0.404 | -0.101 | 0.005 | 7.27E-85 | in-sample |
| - | Intergenic variant | rs12445022 | 16 | 87575332 | A | G | 1.000 | 0.333 | -0.035 | 0.005 | 1.04E-10 | in-sample |
| - | Intergenic variant | rs12136718 | 1 | 156409585 | A | G | 0.999 | 0.072 | 0.046 | 0.010 | 1.95E-06 | in-sample |
| ELAVL2 | Intron variant | rs10966033 | 9 | 23705736 | T | G | 0.954 | 0.383 | -0.029 | 0.005 | 2.70E-08 | UKB-FG |
| TLX3 | 3' UTR variant | rs918472 | 5 | 170738836 | G | A | 0.932 | 0.292 | -0.029 | 0.006 | 1.95E-07 | UKB-FG |
| INPP5A | missense variant | rs1133400 | 10 | 134459388 | G | A | 0.926 | 0.198 | 0.039 | 0.006 | 5.06E-10 | UKB-FG |
| NGF | missense variant | rs6330 | 1 | 115829313 | A | G | 0.593 | 0.461 | 0.033 | 0.005 | 4.97E-11 | in-sample |
NGF encodes protein nerve growth factor beta (NGHβ) that is important in the development and survival of neurons, and involved in transmission of pain, temperature, and touch sensations via sensory neurons. It binds to two receptors, NTRK1 encoded by NTRK1 and NGFR/p75NTR encoded by NGFR. Of note, two additional missense variants among the credible sets, rs6339 (PIP= 0.48) and rs6336 (PIP=0.39), are located in NTRK1 in a separate locus. The missense variant rs6330 shows association with multiple diseases of the musculoskeletal system and connective tissue including spinal stenosis, spondylosis, spondylopathies and hallux valgus in FinnGen R10 PheWAS scan, all to the opposite direction compared to the migraine risk (Supplementary Table 6).
INPP5A encodes a membrane-associated type I inositol 1,4,5-trisphosphate 5-phosphate protein, which hydrolyzes Ins(1,4,5)P3 leading to the mobilization of intracellular calcium. It has a central role in various cellular signaling processes including neurotransmission, hormone secretion, cell proliferation and muscle contraction. INPP5A is highly expressed in Purkinje cells of cerebellum, and in mice studies its deletion have been shown to cause ataxia and cerebellar degeneration29,30.
SERPINA1 encodes an alpha-1 antitrypsin, a serine protease inhibitor protein, that belongs to the serpin superfamily. Its primary target is elastase, and other targets are plasmin and thrombin. Several mutations, including our high-PIP variant rs28929474C>T, in SERPINA1 can cause an autosomal co-dominant genetic disorder alpha-1 antitrypsin (AAT) deficiency, which can lead to lung or liver disease due to reduced alpha-1 antitrypsin levels31. A missense variant rs28929474 is highly pleiotropic and shows associations to multiple disease categories in PheWAS of FinnGen R10 data including, for example, diseases of the respiratory system, diseases of the circulatory system, diseases of digestive system, pregnancy related diseases, diseases of the nervous system, and diseases of musculoskeletal system and connective tissue (Supplementary Tables 6–8).
Five additional high-impact variants on protein function (1 stop gained, 2 start lost, and 2 splice acceptor variants) were among the credible sets, but only with modest PIPs below 0.01 (Supplementary Table 5), and another 5 variants with high-impact on something else than protein coding function (long non-coding RNA, antisense or nonsense mediated decay) with PIPs below 0.02.
Our results provided new information on two of the strongest known migraine risk loci by estimating PIPs of 1.00 for the intronic variants rs9349379 in PHACTR1 and rs11172113 in LRP1. We were able to fine-map both of these loci by using the in-sample LD. The candidate variant in PHACTR1 is also associated with many vascular diseases and its effects on gene expression of the genes in the locus have been studied in detail but with contradicting results32,33. Also, the candidate variant in LRP1 is associated with several vascular diseases, such as sporadic thoracic aortic dissection, fibromuscular dysplacia and spontaneous coronary artery dissection34,35,36. The LDL receptor-related protein 1 (LRP1) is a cell surface receptor and has an important role in vascular and blood brain barrier integrity37,38,39. It is expressed in almost every tissue, and most studied in liver and brain. LRP1 is also involved in vascular calcium signaling by regulating smooth muscle cell contractility38. A recent study suggested that LRP1 expression is regulated by allele-specific mechanism of intronic rs11172113 located in an enhancer region through two transcription factors (MECP2 and SNAIL)40.
Due to the restriction of including in fine-mapping only the variants that are available in all three data sets, the original lead variant was missing in 17/102 fine-map regions (Supplementary Table 3b). In 14/17 of these regions, the original lead variant was represented by one of the top credible set variants (defined as being in LD with r2 > 0.1 in the UKB data). For the remaining 3 regions, the signal related to the original lead variant may be missing from the fine-mapping results, and we flagged these regions to be of low-quality. Among the fine-map regions for which the lead variant was included in the analysis, the lead variant was within the 95% credible sets in 83/85 fine-map regions and within the top configuration in 73/85 of the regions.
Phenome-wide association scans for the credible set variants
We conducted three separate phenome-wide association studies (PheWAS) by using data from FinnGen Data Freeze 10 including 429,209 individuals. First, by a PheWAS for the 181 credible set top variants and the list of 2,399 FinnGen endpoints excluding the migraine endpoints, we identified 404 variant-disease associations with P < 1 × 10−5 (Supplementary Table 6, phewas_app). Of these, 108 variant-disease associations belonged to diseases of the circulatory system, including, for example, hypertension and ischemic heart disease, followed by 39 variant-trait associations in a category of quantitative endpoints, including, e.g., height and BMI, 34 in diseases of the musculoskeletal system and connective tissue category, including, e.g., spinal stenosis and rheumatoid arthritis, and 28 associations in diseases of the respiratory system, including, e.g., asthma and COPD.
Second, for the 159 functional variants among the credible sets, we conducted a targeted PheWAS scan within neurological and cardiovascular endpoints, and identified 122 variant-disease associations with P < 1 × 10−4 (Supplementary Table 7, phewas_app), including traits such as sleep apnea and stroke. Third, for the 307 variants with PIP > 0.1, with a similar targeted PheWAS scan within the neurological and cardiovascular endpoints, we identified 330 variant-disease associations with P < 1 × 10−4 (Supplementary Table 8, phewas_app), including, e.g., focal epilepsy and hydrocephalus.
Discussion
Well over one hundred risk loci for migraine have been reported from GWAS, but the causal variants and genes are still mostly unknown4,5,6,7,8,9,10,11,12,13,14. Statistical fine-mapping of the GWAS results at the risk loci is a natural next step but reliable fine-mapping of large meta-analysis data has turned out to be very difficult. Our recent migraine meta-analysis of 25 studies13 illustrated these difficulties as the accurate LD information was not available and the sample size varied considerably across variants. In this study, our goal was to provide reliable fine-mapping for migraine by creating a new migraine meta-analysis for which accurate LD information was available and sample size across variants was more stable. Despite the more stringent selection criteria, the effective sample size of our new meta-analysis (339,000) turned out to be comparable to that of the earlier meta-analysis (326,000).
A key question in fine-mapping a GWAS meta-analysis is how to assess the reliability of the results. We were able to study this question by directly comparing results between accurate in-sample LD and approximate reference panel LD. We observed that the posterior expected number of causal variants (PENC) as reported by FINEMAP distinguished well the regions with high-quality fine-mapping results from those with low-quality results. We also observed that an appropriate PENC threshold depends on the quality of the reference panel. In our case, we were able to use an upper limit of 3.0 for PENC. While this upper limit restricts our ability to fine-map the migraine risk regions that truly have more than 3 causal signals, we expect that the proportion of such regions is small, as only 3/26 (12%) of the migraine loci with the in-sample LD had PENC over 3 in our analysis.
Here, we performed the first systematic fine-mapping of a migraine meta-analysis and provided high-quality fine-mapping results for 91% of the migraine risk regions identified by the meta-analysis. Our high-quality results highlight two missense variants with high PIPs: rs6330 (PIP=0.59) in NGF and rs1133400 (PIP=0.93) in INPP5A.
The variant rs6330 is only in weak LD (r2 = 0.04) with the lead variant (rs12134493) of its locus and was identified as a secondary signal in our fine-mapping. A recent study14 has also reported that the migraine association of rs6330 remained statistically significant in a conditional analysis after adjusting for the stronger signal (rs2078371) within the same risk locus. NGF has been reported to be highly expressed in hippocampus and cortex41,42 although according to the GTEx v8 data, NGF does not show statistically significant expression in any brain tissue but shows high expression in multiple other tissues, including, for example, ovary, tibial nerve, arteries, visceral adipose, and heart. NGF levels have been reported to be elevated in cerebrospinal fluid in chronic migraine patients compared to controls43, and decreased in blood serum of episodic migraine patients compared to controls and chronic migraine patients44. In addition, we observed two additional missense variants with considerable PIPs, rs6339 (PIP=0.48) and rs6336 (PIP=0.39), located in NTRK1 which encodes one of the two receptors for NGF. NGF and its receptors have a central role in the pain perception, and elevated NGF levels have been observed also in many other chronic pain conditions, such as osteoarthritis and low back pain45,46,47. Multiple antibodies of NGF or small molecular inhibitors of the NGF receptors have been developed and tested in clinical studies to treat chronic pain conditions, including low back pain and osteoarthritis48,49,50,51,52. Even though some candidate drugs have shown potential benefit relating to pain relief, an increased risk of progressive osteoarthritis has been observed in a small group of the treated patients52, and therefore none of the drugs have yet received FDA approval. Currently, other type of drug classes (p75 neurotrophin receptor fusion protein, LEVI-04 (ClinicalTrials.gov ID: NCT05618782) and anti-NGF PEGylated Fab’ antibody53), are being developed and in pre-clinical or clinical testing. In adults, after pain stimuli, NGF activates overexpression of other neuronal molecules, including calcitonin gene-related peptide (CGRP) and substance P52. CGRP is involved in migraine pain, and several effective monoclonal antibodies targeting either CGRP or its receptors have been developed to treat migraine54,55,56.
Gene INPP5A is highly expressed in Purkinje cells of cerebellum57 and involved in multiple cellular signaling processes including neurotransmission, hormone secretion, cell proliferation and muscle contraction through its role in the pathway regulating intracellular calcium levels. The missense variant rs1133400 is in modest LD (r2 = 0.36) with the lead variant of the locus (rs200314499) that was filtered out from fine-mapping due to QC. For this locus, FINEMAP suggested two causal variants (PENC = 1.65). PheWAS showed no other significant associations with this missense variant.
Another important finding is in the PHACTR1 locus, which is one of the strongest known migraine risk loci. There our fine-mapping suggested one causal variant (PENC = 1.29), with the lead variant rs9349379 being a clear candidate for being causal with PIP of 1.00. In our FinnGen PheWAS, we detected also strong associations between the variant and, for example, major coronary disease events (P = 8.22 × 10−52), ischemic heart disease (P = 1.18 × 10−38) and angina pectoris (P = 7.71 × 10−26), all to the opposite directions compared to migraine risk. Because of these well-known associations with multiple vascular diseases, this locus has been previously studied in detail but with contradicting results. Gupta et al. (2017)32 reported that rs9349379 regulates upstream gene EDN1, whereas Wang et al. (2018)33 reported that they failed to replicate this endothelial rs9349379-EDN1 eQTL, but instead showed that rs9349379 regulates the closest gene PHACTR1, confirming previous vascular rs9349379-PHACTR1 eQTLs. Further, Rubin et al (2022)58 observed that a loss of PHACTR1 gene does not seem to have any effect on the endothelial or smooth muscle cells of the transgenic mice, and suggested that PHACTR1 has no contribution to pathological vascular phenotype in mice through cells involved in vascular physiology. Our fine-mapping has provided strong evidence that the lead variant rs9349379 is causal for migraine, but given that the variant is intronic, our fine-mapping results alone do not provide direct evidence through which gene or mechanism this association affects the disease risk.
Our study has some limitations. First, since reliable fine-mapping requires that we exclude variants that are not present in all three component studies of our meta-analysis, it is possible that we exclude also some of the true causal variants. This is a potential problem especially when some of the top variants of the fine-map region have been filtered out from fine-mapping. To identify the regions that are likely to be affected by this problem, we studied the LD patterns between the fine-mapped variants and those top variants from the fine-map regions that were not included in the fine-mapping analysis. For most (14/17) regions where the top variants were missing from fine-mapping, the signal of the top variant was at least partly represented by another variant in LD with the top variant. Additionally, since very rare variants were not included in our analysis, we miss the true causal variants that are rare. Since our variant set is not comprehensive, we must keep in mind that also variants that have a very high probability of being causal in our analysis may still have such variants in high LD that were not included in our analysis. A valid calibration of the PIPs would require that all potential causal variants were included in the analysis. In practice, for common variants, this would require comprehensively imputed data sets with no missing variants in any of the meta-analyzed studies, and, for rare variants, availability of high coverage sequencing data. Currently, we do not yet have such resources available in typical GWAS meta-analyses of common diseases such as migraine.
Another limitation of our study relates to the phenotype definitions of different substudies. First, both the UKB and 23andMe GWAS are based on self-reported migraine status, and therefore some other conditions, such as tension headache, may have been wrongly reported as migraine for some cases. Second, the FinnGen GWAS is based on triptan purchase data, which may represent a specific subset of migraine patients. Triptans are not suitable for all migraineurs and, especially, they are contraindicated in patients with cardiovascular diseases. Overrepresentation of migraineurs without any cardiovascular diseases could lead some FinnGen PheWAS associations where migraine risk alleles seem to have protective effect on cardiovascular phenotypes. Observational studies have reported that both migraine and cardiovascular disease risk in women are positively associated59.
To conclude, we performed a migraine GWAS meta-analysis with 98,375 migraine cases and 869,159 controls and identified 122 risk loci of which 35 were new. We followed up the meta-analysis by the first systematic fine-mapping analysis of migraine risk loci and identified 7 variants with a high probability of being causal. In addition to providing new information about genetic risk of migraine, we also proposed how one could, in general, evaluate whether the fine-mapping results of each risk loci seem reliable based only on the output from the fine-mapping software FINEMAP. While a definitive fine-mapping analyses will require more comprehensive data than are currently available for the GWAS meta-analyses of common diseases, our study shows how reliable and novel fine-mapping results can be extracted already from the currently available data sets by a suitable analysis approach.
Methods
Data
We performed a new migraine meta-analysis by combining summary statistics from three migraine GWAS: UK Biobank (N= 341,050, 10,881 cases and 330,169 controls), 23andMe (N=283,985, 53,109 cases and 230,876 controls), and FinnGen R8 (N= 342,499, 34,385 cases and 308,114 controls). By meta-analyzing the three studies, the total sample size was 967,534 including 98,375 migraine cases and 869,159 controls.
UK Biobank:
The UK Biobank project is a population-based prospective cohort study that consists of over 500,000 participants aged 40–69 at recruitment collected from several regions across the United Kingdom. The participants completed questionnaires and attended interviews and clinal examinations by a trained staff member. A detailed description of UK Biobank is provided elsewhere60, and detailed genotyping, quality control and imputation procedures are described at the UK Biobank website (https://www.ukbiobank.ac.uk/). We used the migraine GWAS data described in13 with self-reported migraine as the phenotype. UK Biobank received ethical approval from the North West Multi-centre Research Ethics Committee (MREC) and informed consent has been obtained from all participants.
23andMe:
23andMe migraine GWAS was performed by a personal genomics company 23andMe, Inc. (https://www.23andme.com/) and detailed description of the migraine GWAS is provided elsewhere8. All participants have provided informed consent and filled an online survey according to 23andMe’s human subjects protocol, which was reviewed and approved by Ethical & Independent Review Services, a private institutional review board. Briefly, migraine cases were assessed from the participants that had reported migraine or answered “Yes” to any of the questions related to migraine, and controls from participants that did not report having migraine or answered “No” to all of the questions related to migraine, excluding participants with discordant answers.
FinnGen:
FinnGen (https://www.finngen.fi/en) is a large biobank study that has collected and genotyped 500,000 Finns and combined these data with longitudinal registry data including The National Hospital Discharge Registry, Causes of Death Registry and medication reimbursement registries, all of these linked by unique national personal identification codes. FinnGen includes prospective and retrospective epidemiological and disease-based cohorts and hospital biobank samples. A detailed description of FinnGen is provided in61. We used the 8th Data Freeze for the migraine GWAS. The migraine cases were defined as the individuals who had at least one triptan purchase and the remaining individuals without any triptan purchases were defined as controls from the social insurance institution of Finland (KELA) registry including medication reimbursement and drug purchases (https://r8.risteys.finngen.fi/phenocode/MIGRAINE_TRIPTAN).
FinnGen participants provided informed consent under the Finnish Biobank Act. Older cohorts with study-specific consents were transferred to the Finnish biobanks after approval by Fimea, the National Supervisory Authority for Welfare and Health. Recruitment protocols followed the biobank protocols approved by Fimea. The Coordinating Ethics Committee of the Hospital District of Helsinki and Uusimaa (HUS) approved the FinnGen study protocol (Nr HUS/990/2017).
The FinnGen study is approved by Finnish Institute for Health and Welfare (permit numbers: THL/2031/6.02.00/2017, THL/1101/5.05.00/2017, THL/341/6.02.00/2018, THL/2222/6.02.00/2018, THL/283/6.02.00/2019, THL/1721/5.05.00/2019 and THL/1524/5.05.00/2020), Digital and population data service agency (permit numbers: VRK43431/2017–3, VRK/6909/2018–3, VRK/4415/2019–3), the Social Insurance Institution (permit numbers: KELA 58/522/2017, KELA 131/522/2018, KELA 70/522/2019, KELA 98/522/2019, KELA 134/522/2019, KELA 138/522/2019, KELA 2/522/2020, KELA 16/522/2020), Findata permit numbers THL/2364/14.02/2020, THL/4055/14.06.00/2020,,THL/3433/14.06.00/2020, THL/4432/14.06/2020, THL/5189/14.06/2020, THL/5894/14.06.00/2020, THL/6619/14.06.00/2020, THL/209/14.06.00/2021, THL/688/14.06.00/2021, THL/1284/14.06.00/2021, THL/1965/14.06.00/2021, THL/5546/14.02.00/2020, THL/2658/14.06.00/2021, THL/4235/14.06.00/2021 and Statistics Finland (permit numbers: TK-53–1041-17 and TK/143/07.03.00/2020 (earlier TK-53–90-20) TK/1735/07.03.00/2021).
The Biobank Access Decisions for FinnGen samples and data utilized in FinnGen Data Freeze 8 include: THL Biobank BB2017_55, BB2017_111, BB2018_19, BB_2018_34, BB_2018_67, BB2018_71, BB2019_7, BB2019_8, BB2019_26, BB2020_1, Finnish Red Cross Blood Service Biobank 7.12.2017, Helsinki Biobank HUS/359/2017, Auria Biobank AB17–5154 and amendment #1 (August 17 2020), AB20–5926 and amendment #1 (April 23 2020), Biobank Borealis of Northern Finland_2017_1013, Biobank of Eastern Finland 1186/2018 and amendment 22 § /2020, Finnish Clinical Biobank Tampere MH0004 and amendments (21.02.2020 & 06.10.2020), Central Finland Biobank 1–2017, and Terveystalo Biobank STB 2018001.
We have access to the complete in-sample LD information for the UK Biobank and FinnGen samples via the individual-level genotype data. Additionally, we have access to the in-sample LD-matrices in 23andMe data for 26 of our fine-map regions. Thus, for the 26 fine-map regions, we are able to do a high-quality fine-mapping based on the in-sample LD while, for the remaining 76 regions, we need to apply an LD reference panel that does not perfectly match the LD information corresponding to our GWAS summary statistics. To assess the effect of the LD reference panel, we formed two reference panels from the available LD information: one including data only from the UK Biobank (UKB), and the other combining the LD matrices from UK Biobank and FinnGen (UKB-FG), as explained in section “Fine-mapping”.
Genetic association analyses
The UK Biobank and 23andMe GWAS had been conducted by logistic regression on migraine (using PLINK262 or custom software of the 23andMe Research Team, respectively), and the FinnGen GWAS by a whole-genome regression model for a binary trait with REGENIE27.
All the samples were of European descent. Related individuals had been excluded by using a kinship value threshold of 0.0442 computed by KING63 from UK Biobank, and by using a minimal expected amount of sharing between first cousins from a segmental identity-by descent algorithm from 23andMe. For the FinnGen GWAS analysis, REGENIE accounted for the genetic relatedness by default, and therefore no relatedness exclusions were applied.
We excluded multi-allelic variants, and variants with minor allele frequency (MAF) < 0.01, IMPUTE2 info or MACH r2 < 0.6, and when available, missingness > 0.05 and Hardy-Weinberg equilibrium (HWE) P < 1 × 10−6 from each study. Consequently, we are only considering biallelic common variants in this work. We recoded indels as insertions (I) and deletions (D). We mapped the FinnGen GWAS summary statistics positions from hg38 to hg37 by UCSC LiftOver64. We excluded the SNPs with an effect allele frequency (EAF) discrepancy of >0.30 and indels with an EAF discrepancy of >0.20 compared to UK Biobank from each study following Hautakangas et al. 2022.
We conducted an inverse-variance weighted fixed-effects meta-analysis to combine the three studies by GWAMA65 with 11,316,120 variants, of which 7,062,924 variants were available in all three studies.
Genetic correlation and SNP-heritability using LD Score regression
We estimated genetic correlations between the three GWAS and SNP-heritability from the migraine meta-analysis by LD Score regression v1.0.066,25 with precomputed 1000 Genomes European LD Scores (https://data.broadinstitute.org/alkesgroup/LDSCORE/) limiting the analysis to the HapMap3 SNPs. We used munge-tool to reformat and perform additional quality control for all GWAS summary statistics prior to the genetic correlation estimation. We obtained a liability scale SNP-heritability estimate67 by using a population prevalence of 16% for migraine.
Locus definition
We followed the locus definition of Hautakangas et al. (2022) and defined an LD-independent genome-wide significant (GWS, P < 5 × 10−8) risk locus from the meta-analysis by using the UKB LD. Iteratively, we chose the variant with the smallest P-value as the index variant and excluded all variants that had r2 ≥ 0.1 with the index variant, until no variant had P < 5 × 10−8. Next, we formed high LD regions around each index variant based on the combined UKB-FG LD and r2 threshold of 0.6. The start of the high LD region was the smallest position, and the end of the region was the largest position where any variant had r2>0.6 with the index variant. Next, we formed the loci by adding ± 250 kb around the high LD region and merged the overlapping regions. Further, we iteratively added all other GWS variants to their closest loci, and updated the loci boundaries if any of the variants added were outside the existing locus boundaries. Again, the overlapping loci were merged. We named each locus by the lead variant, i.e., the variant with the smallest P-value of the locus.
Replication in HUNT All-in Headache and IHGC16
To replicate our new loci, we used two independent data sets with no overlaps with our GWAS data: HUNT All-in Headache28 (N=40,224, 7,801 cases, 32,423 controls) and IHGC16 migraine meta-analysis9 excluding 23andMe and the Finnish cohorts (N = 189,000, 27,006 migraine cases and 161,994 controls). The meta-analysis of the replication data thus contained N=229,224 samples (34,807 cases and 194,417 controls). We used a one-sided P-value threshold of 0.05 to denote a replication and assessed consistency of the effect directions by a sign test. We also reported the two-sided P-value of a combined analysis of our discovery and replication results to determine which of the new loci remained GWS after observing the replication data.
Fine-mapping
For fine-mapping, we first merged loci that were closer than 1.5 Mb leading to 102 fine-map regions. We performed fine-mapping for each fine-map region with FINEMAP v1.419,22. FINEMAP is a Bayesian method that uses summary statistics from a GWAS together with LD information to infer which variants are most likely causal within the genomic region. We used the default prior parameters and set the maximum number of causal variants to 10.
We estimated the in-sample LD correlations for the individual GWAS cohorts by using LDStore222. We combined the in-sample LD correlations for the meta-analysis data set by combining the study-specific LD matrices by weighting each matrix in proportion to its effective sample size as follows:
| (F1) |
where is the LD correlation matrix of study , is the effective sample size of study , with being the total sample size (i.e., the sum of cases and controls) and being the proportion of cases in study , and is the sum of the effective sample sizes.
For the UK Biobank reference LD (UKB-LD), we used the in-sample LD estimated from the individuals included in the UKB GWAS.
For the combined UKB-FG LD reference panel, we combined the UKB and FG in-sample LD matrices by weighting FG in proportion to its effective sample size, and UKB in proportion to the combined UKB+23andMe effective sample size using the above formula (F1).
LD reference panel sensitivity analyses
We compared the performance of different LD refence panels (UKB LD, UKB-FG LD and in-sample LD) on the FINEMAP results for the 26 fine-map regions for which the in-sample LD was available. We used the maximum difference between the posterior inclusion probabilities (PIPs) from different panels (maxΔ) to compare the performance of the three LD panels.
In addition, we examined the following candidate statistics which could be used for separating the fine-map regions for which the fine-mapping with the reference LD performs poorly when compared to the use of the in-sample LD: 1) the posterior expectation of the number of causal variants (PENC), and, from the top variant(s) of the credible set(s) determined by FINEMAP, 2) the maximum pairwise r2, 3) the maximum marginal P-value from the meta-analysis, or 4) the minimum INFO value.
Variant annotation by VEP and eQTL mapping
FINEMAP reports 95%-credible sets (CS). We searched for coding variants among the CS from the Ensembl VEP (http://grch37.ensembl.org/Homo_sapiens/Tools/VEP) database by using a default of 5 kb window around the index variant.
For the follow-up analyses, we formed a functional variant group among the CS variants by including the variants that were predicted by VEP to have a moderate or high impact on the transcript (https://www.ensembl.org/info/genome/variation/prediction/predicted_data.html). This includes transcript ablation, splice acceptor or donor variants, stop gained, frameshift variant, stop lost, start lost, transcript amplification, inframe insertion or deletion, and missense variant.
We mapped the functional variant set, and also another set including all variants with PIP > 0.1 (highPIP), to significant eQTLs of the 49 tissues from GTEx v.8 (https://gtexportal.org/home/).
Phenome-wide association scans
We performed three phenome-wide association scans (PheWAS). First, we scanned all 181 candidate variants of the risk loci (top variants of the credible sets) among the 2,399 FinnGen Data Freeze 10 (R10) GWAS endpoints (excluding 9 migraine endpoints) at significance level 1 × 10−5. Second, we scanned all variants annotated as functional variants with a moderate to high impact on protein function by VEP among neurological and cardiovascular endpoints from FinnGen R10, including the FinnGen endpoint categories Neurological endpoints, VI Diseases of the nervous system (G6_), and IX Diseases of the circulatory system (I9_) with at significance level 1 × 10−4.
Third, we scanned all variants with PIP > 0.1 among the same FinnGen neurological and cardiovascular endpoints at significance level 1 × 10−4.
Results can be browsed from PheWAS app https://hhautakangas.github.io/phewas_migraine_tables.html.
Supplementary Material
Acknowledgements
We would like to thank the research participants and employees of 23andMe, Inc. for making this work possible. We thank all the study participants, employees, and investigators of FinnGen and the UK Biobank for their contribution to this research. This research has been conducted using the UK Biobank Resource under Application Number 22627. This work was supported by grants no. 336825, 338507, 352795 from the Research Council of Finland to M.P., by Sigrid Jusélius foundation (M.P. and A.P.) and by the Doctoral School of University of Helsinki (H.H.). The FinnGen project is funded by two grants from Business Finland (HUS 4685/31/2016 and UH 4386/31/2016) and the following industry partners: AbbVie Inc., AstraZeneca UK Ltd, Biogen MA Inc., Bristol Myers Squibb (and Celgene Corporation & Celgene International II Sàrl), Genentech Inc., Merck Sharp & Dohme LCC, Pfizer Inc., GlaxoSmithKline Intellectual Property Development Ltd., Sanofi US Services Inc., Maze Therapeutics Inc., Janssen Biotech Inc, Novartis AG, and Boehringer Ingelheim International GmbH. Following biobanks are acknowledged for delivering biobank samples to FinnGen: Auria Biobank (www.auria.fi/biopankki), THL Biobank (www.thl.fi/biobank), Helsinki Biobank (www.helsinginbiopankki.fi), Biobank Borealis of Northern Finland (https://www.ppshp.fi/Tutkimus-ja-opetus/Biopankki/Pages/Biobank-Borealis-briefly-in-English.aspx), Finnish Clinical Biobank Tampere (www.tays.fi/en-US/Research_and_development/Finnish_Clinical_Biobank_Tampere), Biobank of Eastern Finland (www.ita-suomenbiopankki.fi/en), Central Finland Biobank (www.ksshp.fi/fi-FI/Potilaalle/Biopankki), Finnish Red Cross Blood Service Biobank (www.veripalvelu.fi/verenluovutus/biopankkitoiminta), Terveystalo Biobank (www.terveystalo.com/fi/Yritystietoa/Terveystalo-Biopankki/Biopankki/) and Arctic Biobank (https://www.oulu.fi/en/university/faculties-and-units/faculty-medicine/northern-finland-birth-cohorts-and-arctic-biobank). All Finnish Biobanks are members of BBMRI.fi infrastructure (www.bbmri.fi). Finnish Biobank Cooperative - FINBB (https://finbb.fi/) is the coordinator of BBMRI-ERIC operations in Finland. The Finnish biobank data can be accessed through the Fingenious® services (https://site.fingenious.fi/en/) managed by FINBB.
The Trøndelag Health Study (HUNT) is a collaboration between HUNT Research Centre (Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology NTNU), Trøndelag County Council, Central Norway Regional Health Authority, and the Norwegian Institute of Public Health. The genotyping was financed by the National Institute of health (NIH), University of Michigan, The Norwegian Research council, and Central Norway Regional Health Authority and the Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology (NTNU). The genotype quality control and imputation has been conducted by the K.G. Jebsen center for genetic epidemiology, Department of public health and nursing, Faculty of medicine and health sciences, Norwegian University of Science and Technology (NTNU).
Consortia
A full list of FinnGen members and their affiliations appears in the Supplementary Data 1.
International Headache Genetics Consortium
Verneri Anttila1,2,3, Ville Artto4, Andrea C Belin5, Anna Bjornsdottir6, Gyda Bjornsdottir7, Dorret I Boomsma8, Sigrid Børte9,10,11, Mona A Chalmer12, Daniel I Chasman13,14, Bru Cormand15, Ester Cuenca-Leon16, George Davey-Smith17, Irene de Boer18, Martin Dichgans19,20, Tonu Esko21, Tobias Freilinger22,23, Padhraig Gormley24, Lyn R Griffiths25, Eija Hämäläinen26, Thomas F Hansen12,27, Aster VE Harder18,28, Heidi Hautakangas26, Marjo Hiekkala29, Maria G Hrafnsdottir30, M. Arfan Ikram31, Marjo-Riitta Järvelin32,33,34,35, Risto Kajanne26, Mikko Kallela4, Jaakko Kaprio26, Mari Kaunisto29, Lisette JA Kogelman12, Espen S Kristoffersen36,37,38, Christian Kubisch39, Mitja Kurki40, Tobias Kurth41, Lenore Launer42, Terho Lehtimäki43, Davor Lessel39, Lannie Ligthart8, Sigurdur H Magnusson7, Rainer Malik19, Bertram Müller-Myhsok44, Carrie Northover45, Dale R Nyholt46, Jes Olesen12, Aarno Palotie26,47, Priit Palta26, Linda M Pedersen48, Nancy Pedersen49, Matti Pirinen26,50,51, Danielle Posthuma52, Patricia Pozo-Rosich53, Alice Pressman54, Olli Raitakari55,56,57, Caroline Ran5, Gudrun R Sigurdardottir6, Hreinn Stefansson7, Kari Stefansson7, Olafur A Sveinsson30, Gisela M Terwindt18, Thorgeir E Thorgeirsson7, Arn MJM van den Maagdenberg18,28, Cornelia van Duijn58, Maija Wessman26,29, Bendik S Winsvold9,48,59, John-Anker Zwart9,10,48
1Analytical and Translational Genetics Unit, Department of Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA; 2Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA; 3Stanley Center for Psychiatric Research, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA; 4Department of Neurology, Helsinki University Central Hospital, Helsinki, Finland; 5Department of Neuroscience, Karolinska Institutet, Stockholm, Sweden; 6Neurology private practice, Laeknasetrid, Reykjavik, Iceland; 7deCODE genetics/Amgen Inc., Reykjavik, Iceland; 8Netherlands Twin Register, Department of Biological Psychology, Vrije Universiteit, Amsterdam, the Netherlands; 9K.G. Jebsen Center for Genetic Epidemiology, Department of Public Health and Nursing, Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology, Trondheim, Norway; 10Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway; 11Research and Communication Unit for Musculoskeletal Health, Department of Research, Innovation and Education, Division of Clinical Neuroscience, Oslo University Hospital, Oslo, Norway; 12Danish Headache Center, Department of Neurology, Copenhagen University Hospital, Copenhagen, Denmark; 13Department of Medicine, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, Massachusetts, USA; 14Harvard Medical School, Boston, Massachusetts, USA; 15Department of Genetics, Spain Centre for Biomedical Network Research on Rare Diseases, University of Barcelona, Barcelona, Spain; 16Pediatric Neurology Research Group, Vall d’Hebron Research Institute, Barcelona, Spain; 17University of Bristol/Medical Research Council Integrative Epidemiology Unit, University of Bristol, Bristol, UK; 18Department of Neurology, Leiden University Medical Centre, Leiden, the Netherlands; 19Institute for Stroke and Dementia Research, University Hospital, LMU Munich, Munich, Germany; 20Munich Cluster for Systems Neurology, Munich, Germany; 21Estonian Biobank Registry, the Estonian Genome Center, University of Tartu, Tartu, Estonia; 22Department of Neurology, Klinikum Passau, Passau, Germany; 23Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research, University of Tuebingen, Tuebingen, Germany; 24GSK Inc., Cambridge, Massachusetts, USA; 25Centre for Genomics and Personalised Health, Queensland University of Technology, Brisbane, Queensland, Australia; 26Institute for Molecular Medicine Finland, Helsinki Institute of Life Science, University of Helsinki, Helsinki, Finland; 27Novo Nordic Foundation Center for Protein Research, Copenhagen University, Copenhagen, Denmark; 28Department of Human Genetics, Leiden University Medical Centre, Leiden, the Netherlands; 29Folkhälsan Research Center, Helsinki, Finland; 30Landspitali University Hospital, Reykjavik, Iceland; 31Department of Epidemiology, Erasmus University Medical Center, Rotterdam, the Netherlands; 32Department of Epidemiology and Biostatistics, MRC-PHE Centre for Environment and Health, School of Public Health, Imperial College London, London, UK; 33Center for Life Course Health Research, Faculty of Medicine, University of Oulu, Oulu, Finland; 34Unit of Primary Health Care, Oulu University Hospital, OYS, Oulu, Finland; 35Department of Life Sciences, College of Health and Life Sciences, Brunel University London, London, UK; 36Research and Communication Unit for Musculoskeletal Health, Department of Research, Innovation and Education, Division of Clinical Neuroscience, Akershus University Hospital and University of Oslo, Oslo, Norway; 37Department of General Practice, Institute of Health and Society, University of Oslo, Oslo, Norway; 38Department of Neurology, Akershus University Hospital, Lørenskog, Norway; 39Institute of Human Genetics, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; 40Psychiatric and Neurodevelopmental Genetics Unit, Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts, USA; 41Institute of Public Health, Charité – Universitätsmedizin, Berlin; 42Laboratory of Epidemiology and Population Sciences, Intramural Research Program, National Institute on Aging, Bethesda, Maryland, USA; 43Department of Clinical Chemistry, Fimlab Laboratories, and Finnish Cardiovascular Research Center - Tampere, Faculty of Medicine and Health Technology, Tampere University, Tampere, Finland; 44Max Planck Institute of Psychiatry, Munich, Germany; 4523&Me Inc., Mountain View, California, USA; 46School of Biomedical Sciences, Faculty of Health, Centre for Genomics and Personalised Health, Centre for Data Science, Queensland University of Technology, Brisbane, Queensland, Australia; 47University of Helsinki, Helsinki, Finland; 48Department of Research, Innovation and Education, Division of Clinical Neuroscience, Oslo University Hospital, Oslo, Norway; 49Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden; 50Department of Mathematics and Statistics, University of Helsinki, Helsinki, Finland; 51Department of Public Health, University of Helsinki, Helsinki, Finland; 52Department of Complex Trait Genetics, Center for Neurogenomics and Cognitive Research, Neuroscience Campus Amsterdam, VU University, Amsterdam, The Netherlands; 53Headache Unit, Neurology Department, Vall d’Hebron University Hospital, Barcelona, Spain; 54Sutter Health, Sacramento, California, USA; 55Centre for Population Health Research, University of Turku, Turku University Hospital, Turku, Finland; 56Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland; 57Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland; 58Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, the Netherlands; 59Department of Neurology, Oslo University Hospital, Oslo, Norway
HUNT All-in Headache
Amy E Martinsen1,2,3, Anne Heidi Skogholt3, Ben M Brumpton3, Bendik S Winsvold4,5,6, Cristen J Willer7, Erling Tronvik8,9, Espen Saxhaug Kristoffersen1,10,11, John-Anker Zwart1,2,3, Jonas B Nielsen3,7,12, Knut Hagen8, Kristian Hveem3,13,14, Kristian Bernhard Nilsen8,15, Lars G Fritsche16, Lars Jacob Stovner8,17, Laurent F Thomas3,18,19,20, Linda M Pedersen1, Maiken E Gabrielsen3, Marianne B Johnsen3,21, Marie U Lie2,21, Oddgeir L Holmen13, Sigrid Børte2,3,21, Wei Zhou22,23
1Department of Research and Innovation, Division of Clinical Neuroscience, Oslo University Hospital, Oslo, Norway, 2Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway, 3K. G. Jebsen Center for Genetic Epidemiology, Department of Public Health and Nursing, Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology (NTNU), Trondheim, Norway, 4Department of Research and Innovation, Division of Clinical Neuroscience, Oslo University Hospital, Oslo, Norway, 5K. G. Jebsen Center for Genetic Epidemiology, Department of Public Health and Nursing, Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology (NTNU), Trondheim, Norway, 6Department of Neurology, Oslo University Hospital, Oslo, Norway, 7Department of Internal Medicine, Division of Cardiovascular Medicine, University of Michigan, Ann Arbor, MI, 48109, USA, 8Department of Neuromedicine and Movement Science, Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology (NTNU), Trondheim, Norway, 9Department of Neurology and Clinical Neurophysiology, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway, 10Department of General Practice, University of Oslo, Oslo, Norway, 11Department of Neurology, Akershus University Hospital, Lørenskog, Norway, 12Department of Epidemiology Research, Statens Serum Institut, Copenhagen, Denmark, 13HUNT Research Center, Department of Public Health and Nursing, Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology (NTNU), Trondheim, Norway, 14Department of Research, Innovation and Education, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway, 15Department of Neurology, Oslo University Hospital, Oslo, Norway, 16Center for Statistical Genetics, Department of Biostatistics, University of Michigan, Ann Arbor, MI, 48109, USA, 17Norwegian Advisory Unit on Headaches, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway, 18Department of Clinical and Molecular Medicine, Norwegian University of Science and Technology (NTNU), Trondheim, Norway, 19BioCore - Bioinformatics Core Facility, Norwegian University of Science and Technology (NTNU), Trondheim, Norway, 20Clinic of Laboratory Medicine, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway, 21Research and Communication Unit for Musculoskeletal Health (FORMI), Department of Research and Innovation, Division of Clinical Neuroscience, Oslo University Hospital, Oslo, Norway, 22Department of Computational Medicine and Bioinformatics, University of Michigan, Ann Arbor, MI, 48109, USA, 23Analytic and Translational Genetics Unit, Massachusetts General Hospital, Boston, MA, USA
Footnotes
Competing interests
A.P. is the Scientific Director of the public-private partnership project FinnGen that has 12 industry partners that provide funding for the FinnGen project. Other authors report no conflicts of interests.
Data availability
The access to the UK biobank data can be applied through https://www.ukbiobank.ac.uk/
The GWAS summary statistics for FinnGen R8 are publicly available through https://www.finngen.fi/en/access_results. The Finnish biobank data can be accessed through the Fingenious® services (https://site.fingenious.fi/en/) managed by FINBB. Finnish Health register data can be applied from Findata (https://findata.fi/en/data/). The GWAS summary statistics for the 23andMe data set will be made available through 23andMe to qualified researchers under an agreement with 23andMe that protects the privacy of the 23andMe participants. Please visit https://research.23andme.com/collaborate/#publication for more information and to apply to access the data.
References:
- 1.IHS H.C.C.o.t.I.H.S. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 38, 1–211 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Vos T. et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet 396, 1204–1222 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gervil M., Ulrich V., Kaprio J., Olesen J. & Russell M.B. The relative role of genetic and environmental factors in migraine without aura. Neurology 53, 995–999 (1999). [DOI] [PubMed] [Google Scholar]
- 4.Anttila V. et al. Genome-wide association study of migraine implicates a common susceptibility variant on 8q22.1. Nature Genetics 42, 869–873 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chasman D.I. et al. Genome-wide association study reveals three susceptibility loci for common migraine in the general population. Nature Genetics 43, 695–U116 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freilinger T. et al. Genome-wide association analysis identifies susceptibility loci for migraine without aura. Nature Genetics 44, 777–782 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anttila V. et al. Genome-wide meta-analysis identifies new susceptibility loci for migraine. Nature Genetics 45, 912–U255 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pickrell J.K. et al. Detection and interpretation of shared genetic influences on 42 human traits. Nature Genetics 48, 709–717 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gormley P. et al. Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nature Genetics 48, 856–866 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S.-P. et al. Genome-wide association study identifies novel susceptibility loci for migraine in Han Chinese resided in Taiwan. Cephalalgia 38, 466–475 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Chang X. et al. Common variants at 5q33.1 predispose to migraine in African-American children. Journal of Medical Genetics 55, 831 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choquet H. et al. New and sex-specific migraine susceptibility loci identified from a multiethnic genome-wide meta-analysis. Communications Biology 4, 864 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hautakangas H. et al. Genome-wide analysis of 102,084 migraine cases identifies 123 risk loci and subtype-specific risk alleles. Nature Genetics 54, 152–160 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bjornsdottir G. et al. Rare variants with large effects provide functional insights into the pathology of migraine subtypes, with and without aura. Nature Genetics 55, 1843–1853 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finucane H.K. et al. Heritability enrichment of specifically expressed genes identifies disease-relevant tissues and cell types. Nature Genetics 50, 621–629 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaid D.J., Chen W. & Larson N.B. From genome-wide associations to candidate causal variants by statistical fine-mapping. Nature Reviews Genetics 19, 491–504 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kichaev G. et al. Integrating functional data to prioritize causal variants in statistical fine-mapping studies. PLoS Genet 10, e1004722 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hormozdiari F., Kostem E., Kang E.Y., Pasaniuc B. & Eskin E. Identifying causal variants at loci with multiple signals of association. Genetics 198, 497–508 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benner C. et al. FINEMAP: efficient variable selection using summary data from genome-wide association studies. Bioinformatics 32, 1493–1501 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newcombe P.J., Conti D.V. & Richardson S. JAM: A Scalable Bayesian Framework for Joint Analysis of Marginal SNP Effects. Genetic Epidemiology 40, 188–201 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang G., Sarkar A., Carbonetto P. & Stephens M. A Simple New Approach to Variable Selection in Regression, with Application to Genetic Fine Mapping. Journal of the Royal Statistical Society Series B: Statistical Methodology 82, 1273–1300 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benner C. et al. Prospects of Fine-Mapping Trait-Associated Genomic Regions by Using Summary Statistics from Genome-wide Association Studies. American Journal of Human Genetics 101, 539–551 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanai M. et al. Meta-analysis fine-mapping is often miscalibrated at single-variant resolution. Cell Genomics 2(2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trubetskoy V. et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 604, 502–508 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bulik-Sullivan B. et al. An atlas of genetic correlations across human diseases and traits. Nature Genetics 47, 1236–1241 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isgut M., Song K., Ehm M.G., Wang M.D. & Davitte J. Effect of case and control definitions on genome-wide association study (GWAS) findings. Genetic Epidemiology 47, 394–406 (2023). [DOI] [PubMed] [Google Scholar]
- 27.Mbatchou J. et al. Computationally efficient whole-genome regression for quantitative and binary traits. Nature Genetics 53, 1097–1103 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Krokstad S. et al. Cohort Profile: The HUNT Study, Norway. International Journal of Epidemiology 42, 968–977 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Yang A.W., Sachs A.J. & Nystuen A.M. Deletion of Inpp5a causes ataxia and cerebellar degeneration in mice. neurogenetics 16, 277–285 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Liu Q. et al. Cerebellum-enriched protein INPP5A contributes to selective neuropathology in mouse model of spinocerebellar ataxias type 17. Nature Communications 11, 1101 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zorzetto M. et al. SERPINA1 Gene Variants in Individuals from the General Population with Reduced α1-Antitrypsin Concentrations. Clinical Chemistry 54, 1331–1338 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Gupta R.M. et al. A Genetic Variant Associated with Five Vascular Diseases Is a Distal Regulator of Endothelin-1 Gene Expression. Cell 170, 522–533.e15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X. & Musunuru K. Confirmation of Causal rs9349379-PHACTR1 Expression Quantitative Trait Locus in Human-Induced Pluripotent Stem Cell Endothelial Cells. Circulation: Genomic and Precision Medicine 11, e002327 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo D. c. et al. Genetic Variants in LRP1 and ULK4 Are Associated with Acute Aortic Dissections. The American Journal of Human Genetics 99, 762–769 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Georges A. et al. Genetic investigation of fibromuscular dysplasia identifies risk loci and shared genetics with common cardiovascular diseases. Nature Communications 12, 6031 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turley T.N. et al. Identification of Susceptibility Loci for Spontaneous Coronary Artery Dissection. JAMA Cardiology 5, 929–938 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Storck S.E., Kurtyka M. & Pietrzik C.U. Brain endothelial LRP1 maintains blood–brain barrier integrity. Fluids and Barriers of the CNS 18, 27 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Z., Andraska E., Akinbode D., Mars W. & Alvidrez R.I.M. LRP1 in the Vascular Wall. Current Pathobiology Reports 10, 23–34 (2022). [Google Scholar]
- 39.Lee J. et al. ANKS1A regulates LDL receptor-related protein 1 (LRP1)-mediated cerebrovascular clearance in brain endothelial cells. Nature Communications 14, 8463 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu L. et al. Regulatory mechanisms in multiple vascular diseases locus LRP1 involve repression by SNAIL and extracellular matrix remodeling. bioRxiv, 2023.05.09.539992 (2023). [Google Scholar]
- 41.Korsching S., Auburger G., Heumann R., Scott J. & Thoenen H. Levels of nerve growth factor and its mRNA in the central nervous system of the rat correlate with cholinergic innervation. The EMBO Journal 4, 1389–1393 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Connor B. & Dragunow M. The role of neuronal growth factors in neurodegenerative disorders of the human brain. Brain Research Reviews 27, 1–39 (1998). [DOI] [PubMed] [Google Scholar]
- 43.van Dongen R.M. et al. Migraine biomarkers in cerebrospinal fluid: A systematic review and meta-analysis. Cephalalgia 37, 49–63 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Mozafarihashjin M. et al. Assessment of peripheral biomarkers potentially involved in episodic and chronic migraine: a case-control study with a focus on NGF, BDNF, VEGF, and PGE2. The Journal of Headache and Pain 23, 3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aloe L., Tuveri M.A., Carcassi U. & Levi-Montalcini R. Nerve growth factor in the synovial fluid of patients with chronic arthritis. Arthritis & Rheumatism 35, 351–355 (1992). [DOI] [PubMed] [Google Scholar]
- 46.Freemont A.J. et al. Nerve growth factor expression and innervation of the painful intervertebral disc. The Journal of Pathology 197, 286–292 (2002). [DOI] [PubMed] [Google Scholar]
- 47.Walsh D.A. et al. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology (Oxford) 49, 1852–61 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanga P. et al. Efficacy, safety, and tolerability of fulranumab, an anti-nerve growth factor antibody, in the treatment of patients with moderate to severe osteoarthritis pain. Pain 154, 1910–1919 (2013). [DOI] [PubMed] [Google Scholar]
- 49.Tiseo P.J., Ren H. & Mellis S. Fasinumab (REGN475), an antinerve growth factor monoclonal antibody, for the treatment of acute sciatic pain: results of a proof-of-concept study. Journal of Pain Research 7, 523–30 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watt F.E. et al. Tropomyosin-related kinase A (TrkA) inhibition for the treatment of painful knee osteoarthritis: results from a randomized controlled phase 2a trial. Osteoarthritis Cartilage 27, 1590–1598 (2019). [DOI] [PubMed] [Google Scholar]
- 51.Berenbaum F. et al. Subcutaneous tanezumab for osteoarthritis of the hip or knee: efficacy and safety results from a 24-week randomised phase III study with a 24-week follow-up period. Ann Rheum Dis 79, 800–810 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wise B.L., Seidel M.F. & Lane N.E. The evolution of nerve growth factor inhibition in clinical medicine. Nature Reviews Rheumatology 17, 34–46 (2021). [DOI] [PubMed] [Google Scholar]
- 53.Koya Y. et al. A novel anti-NGF PEGylated Fab’ provides analgesia with lower risk of adverse effects. mAbs 15, 2149055 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Detke H.C. et al. Galcanezumab in chronic migraine: The randomized, double-blind, placebo-controlled REGAIN study. Neurology 91, e2211–e2221 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dodick D.W. et al. ARISE: A Phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia 38, 1026–1037 (2018). [DOI] [PubMed] [Google Scholar]
- 56.Ferrari M.D. et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial. The Lancet 394, 1030–1040 (2019). [DOI] [PubMed] [Google Scholar]
- 57.De Smedt F., Verjans B., Mailleux P. & Erneux C. Cloning and expression of human brain type I inositol 1,4,5-trisphosphate 5-phosphatase High levels of mRNA in cerebellar Purkinje cells. FEBS Letters 347, 69–72 (1994). [DOI] [PubMed] [Google Scholar]
- 58.Rubin S. et al. PHACTR-1 (Phosphatase and Actin Regulator 1) Deficiency in Either Endothelial or Smooth Muscle Cells Does Not Predispose Mice to Nonatherosclerotic Arteriopathies in 3 Transgenic Mice. Arteriosclerosis, Thrombosis, and Vascular Biology 42, 597–609 (2022). [DOI] [PubMed] [Google Scholar]
- 59.Kurth T. et al. Migraine and risk of cardiovascular disease in women: prospective cohort study. BMJ 353, i2610 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bycroft C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kurki M.I. et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 613, 508–518 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang C.C. et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience 4, s13742–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Manichaikul A. et al. Robust relationship inference in genome-wide association studies. Bioinformatics 26, 2867–2873 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hinrichs A.S. et al. The UCSC Genome Browser Database: update 2006. Nucleic Acids Research 34, D590–8 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mägi R. & Morris A.P. GWAMA: software for genome-wide association meta-analysis. BMC bioinformatics 11, 288 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bulik-Sullivan B. et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nature genetics 47, 291–295 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee S.H., Wray N.R., Goddard M.E. & Visscher P.M. Estimating missing heritability for disease from genome-wide association studies. American Journal of Human Genetics 88, 294–305 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The access to the UK biobank data can be applied through https://www.ukbiobank.ac.uk/
The GWAS summary statistics for FinnGen R8 are publicly available through https://www.finngen.fi/en/access_results. The Finnish biobank data can be accessed through the Fingenious® services (https://site.fingenious.fi/en/) managed by FINBB. Finnish Health register data can be applied from Findata (https://findata.fi/en/data/). The GWAS summary statistics for the 23andMe data set will be made available through 23andMe to qualified researchers under an agreement with 23andMe that protects the privacy of the 23andMe participants. Please visit https://research.23andme.com/collaborate/#publication for more information and to apply to access the data.