Abstract
A prototype Shigella human immunodeficiency virus type 1 (HIV-1) gp120 DNA vaccine vector was constructed and evaluated for immunogenicity in a murine model. For comparative purposes, mice were also vaccinated with a vaccinia virus-env (vaccinia-env) vector or the gp120 DNA vaccine alone. Enumeration of the CD8+-T-cell responses to gp120 after vaccination using a gamma interferon enzyme-linked spot assay revealed that a single intranasal dose of the Shigella HIV-1 gp120 DNA vaccine vector elicited a CD8+ T-cell response to gp120, the magnitude of which was comparable to the sizes of the analogous responses to gp120 that developed in mice vaccinated intraperitoneally with the vaccinia-env vector or intramuscularly with the gp120 DNA vaccine. In addition, a single dose of the Shigella gp120 DNA vaccine vector afforded significant protection against a vaccinia-env challenge. Moreover, the number of vaccinia-env PFU recovered in mice vaccinated intranasally with the Shigella vector was about fivefold less than the number recovered from mice vaccinated intramuscularly with the gp120 DNA vaccine. Since the Shigella vector did not express detectable levels of gp120, this report confirms that Shigella vectors are capable of delivering passenger DNA vaccines to host cells and inducing robust CD8+ T-cell responses to antigens expressed by the DNA vaccines. Furthermore, to our knowledge, this is the first documentation of antiviral protective immunity following vaccination with a live Shigella DNA vaccine vector.
It is widely agreed that human immunodeficiency virus type 1 (HIV-1)-specific effector CD8+ T cells play a substantive role in controlling HIV-1 replication in infected individuals and are a prognostic determinant of HIV-1 infection outcome (3, 18, 22, 31). Although the mechanisms underlying the initiation and maintenance of effector CD8+ T-cell responses during HIV-1 infection are still unclear, vaccination strategies that are proficient at priming effector CD8+ T-cell responses against HIV-1 antigens have been developed (5, 11, 15). In this vein, a growing number of macaque studies have reported compelling evidence showing an association between CD8+ T-cell responses to HIV-1 antigens and antiviral protection against the progression of simian/human immunodeficiency virus (SHIV) or HIV-1 infections in nonhuman primates (5, 15, 19, 25).
The central tenet of our HIV-1 vaccine development strategy is that the induction of high-level antiviral protection against sexually transmitted HIV-1 will be achieved only if the priming immunogen is targeted to mucosal lymphoid tissues (8, 33). Indeed, there is convincing evidence that mucosal immunity against HIV-1 will play a crucial role in protection against sexually acquired HIV-1 (6, 19). However, while it is possible to boost mucosal responses with parenteral immunogens in humans, the induction of strong mucosal immune responses requires that the priming immunogen be given mucosally (12). Thus, the aforementioned HIV-1 vaccines, which are administered parenterally, do not induce strong local cell-mediated immune responses in the mucosal lymphoid compartment (5, 11, 15, 25). On the other hand, Wu et al. (33) and Valentine et al. (28) have demonstrated that live oral bacterial vectors deliver HIV-1 immunogens to mucosal lymphoid tissues and induce mucosal immune responses against HIV-1 antigens. Unfortunately, these first-generation bacterial HIV vectors did not induce measurable HIV-specific CD8+ T-cell responses or antiviral immunity in laboratory animals (9, 27, 33).
More recently, an alternative bacterial vector modality that utilizes attenuated derivatives of Shigella flexneri to deliver DNA vaccines was reported. In this capacity, attenuated Shigella DNA vaccine vectors deliver passenger DNA vaccines to rodent (26) and human (24) cells and stimulate cytotoxic T-cell responses against DNA vaccine-encoded antigens in mice (7). These observations suggest that attenuated Shigella strains may serve as vectors for the delivery of HIV-1 DNA vaccines.
The purpose of the studies described in this report, therefore, was to determine whether a prototype Shigella HIV-1 DNA vaccine vector elicits CD8+ T-cell responses to HIV-1. Since shigellae are host adapted, we made use of an experimental murine model in which attenuated Shigella vectors are inoculated intranasally (29) throughout the studies in this report. Although infection by Shigella in intranasally inoculated mice does not directly involve the gastrointestinal tract, as is the case in human shigellosis, this model is useful in determining the relative immunopotency of attenuated Shigella vector strains. We show that vaccination of mice with the Shigella gp120 DNA vaccine induces robust CD8+ T-cell responses to gp120 and significant levels of antiviral protective immunity against a vaccinia virus-env (vaccinia-env) challenge.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Attenuated Shigella flexneri 2a ΔaroA ΔiscA strain CVD1203 has been described elsewhere (21). CVD1203 is capable of invading epithelial cells but undergoes minimal intracellular proliferation (due to the aroA mutation) and exhibits defective cell-to-cell spread (due to the ascA mutation). (21). In a phase 1 volunteer trial, CVD1203 was found to be attenuated and well tolerated at a dose of 106 bacilli (17). Escherichia coli strain Stable2R was purchased from Life Technologies (Gaithersburg, Md.). Eukaryotic expression vector pcDNA3.1ZEO was purchased from Invitrogen Inc. (Carlsbad, Calif.). Plasmid pEF1αsyngp120MN, which served as the source of DNA encoding HIV-1MN gp120, is described elsewhere (2) and was kindly provided by Brian Seed, Department of Molecular Biology, Massachusetts General Hospital, Harvard Medical School, Boston, Mass. All bacterial strains were grown on tryptic soy agar (Difco, Detroit, Mich.) or in tryptic soy broth (Difco). Shigella strains H1016 (carries pcDNA3.1ZEO) and H1012 (carries pOGL1) were cultured in solid and liquid media supplemented with 100 μg of ampicillin (Sigma, St. Louis, Mo.) per ml.
BALB/c mastocytoma cell line P815 (ATCC no. TIB-64) and human adenocarcinoma cell line HeLa (ATCC no. CCL-2) were obtained from the American Type Culture Collection (Manassas, Va.). These cell lines were maintained in complete medium (CM), which was comprised of RPMI 1640 medium (Life Technologies) supplemented with 10 mM HEPES (pH 7.3) (Life Technologies), 10% (vol/vol) fetal calf serum (Gemini Bioproducts, Calabasas, Calif.), 4 mM glutamine (Life Technologies), 1 mM sodium pyruvate (Life Technologies), and 100 μg each of penicillin and streptomycin (Life Technologies) per ml.
DNA vaccine construction.
gp120-encoding DNA was obtained by PCR amplification of the synthetic HIV-1MN gp120 gene (syngp120) in plasmid pEF1αsyngp120 (2) using forward (5′-GGGGGGGGATCCATGCCCATGGGGTCTCTGCAACCGCTG) and reverse (5′-GGGGGCGGCCGCTTATTAGGCGCGCTTCTCGCGCTGCACCACGCG) primers specific for the 5′ and 3′ ends of syngp120, and standard PCR procedures (4). The resultant PCR-generated DNA fragment was digested with restriction endonucleases BamHI and NotI and annealed by ligation with T4 DNA ligase (New England Biolabs Inc., Beverly, Mass.) to BamHI- and NotI-digested pcDNA3.1ZEO DNA. Following ligation, the chimeric plasmid DNA was introduced into transformation-competent E. coli strain Stable2R and cultured overnight. Plasmid DNA was prepared from 2 ml of liquid cultures of individual colonies and screened for the presence of recombinant plasmids with the appropriate restriction endonuclease digestion pattern. One isolate, designated H1058, containing the modified pcDNA3.1ZEO with the BamHI-NotI syngp120 fragment (designated pOGL1), was stored at −80°C. Additional characterization of the cloned syngp120 DNA in pOGL1, including various restriction endonuclease digestions and dideoxynucleotide sequencing, was conducted to verify that no significant alterations occurred during the construction of pOGL1.
In addition, the expression of gp120 by P815 cells transiently transfected with pOGL1 was assessed by quantitative capture enzyme-linked immunosorbent assay (1), using half-log serial dilutions of purified glycosylated HIV-1MN gp120 (Virostat, Portland, Oreg.) to generate a standard curve. The results showed that plasmid pOGL1 expressed about 278 ± 55 pg of gp120 per 104 P815 cells.
A prototype Shigella gp120 DNA vaccine vector, designated strain H1012, was obtained by introducing plasmid pOGL1 into strain CVD1203 by electroporation (21). Similarly, negative-control strain H1016 was constructed by introducing plasmid pcDNA3.1ZEO into CVD1203 by electroporation.
Animal housing and handling.
Specific-pathogen free, 18- to 20-g female BALB/cAnNCrlBR mice, purchased from the Charles River Laboratories (Wilmington, Mass.), were maintained in a specific-pathogen free, microisolator environment, and allowed to drink and eat ad lib. All murine studies were conducted in accordance with Institutional Animal Care and Use Committee-approved protocol no. 004-97 and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (20a).
Vaccination procedures.
Shigella vector strains were cultured in 20 ml of tryptic soy broth at 37°C until the optical density at 600 nm reached 1.0 relative to sterile tryptic soy broth control. The bacterial suspensions were centrifuged at 5,000 × g for 15 min, and the bacterial pellets were washed twice in 20 ml of phosphate-buffered saline (PBS). Finally, the inocula were suspended in PBS at a density of 106 CFU per ml. These suspensions were used immediately to vaccinate groups of six mice intranasally as described previously (29).
In parallel, groups of six mice were vaccinated intraperitoneally with 107, 3 × 107, or 108 PFU of vaccinia-env vector vP1174, or 108 PFU of vaccinia-lacZ vector vSC8. The vaccinia vectors vP1174 and vSC8 were obtained from AIDS Research & Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health (Bethesda, Md.). Inocula of vP1174 and vSC8 were prepared by propagating the vaccinia virus constructs in HeLa cells, which were cultured in CM (see above) at 37°C in 5% CO2 (23).
Two additional groups of six mice were vaccinated intramuscularly with 20 μg of endotoxin-free (<0.5 endotoxin units [EU] per mg of DNA) pcDNA3.1ZEO or pOGL1 DNA suspended in normal saline (0.85% [wt/vol] NaCl). The gp120 DNA vaccine, pOGL1, and control DNA vaccine pcDNA3.1ZEO were formulated for intramuscular injection as described previously (32).
Enumeration of IFN-γ-secreting cells.
Single-cell suspensions of splenocytes were prepared before and 3, 5, 9, 17, and 28 days after vaccination. Threefold dilutions of the splenocytes were suspended in CM (see above) containing 10 IU of recombinant mouse interleukin-2 (R&D Systems, Minneapolis, Minn.) either with or without the immunodominant peptide of gp120, P18MN (RIHIGPGRAFYTTKN) (10 μg/ml). The cell suspensions were used immediately (i.e., without in vitro expansion) in an IFN-γ-specific enzyme-linked immune spot (IFN-γ-ELISPOT) assay to enumerate the gp120-specific IFN-γ-ELISPOTs by the method of Versteegen et al. (30) as modified by Miyahira et al. (20).
CD4+ or CD8+ cells were depleted by negative selection from splenic cells using CD4+ cell- or CD8+ cell-specific Dynabeads, respectively, according to the manufacturer's protocols (Dynal, Lake Success, N.Y.). The unfractionated cells and CD4- and CD8-depleted cells were used immediately in the IFN-γ-ELISPOT assays.
Vaccinia-env challenge.
The level of antiviral protection induced by each DNA vaccine modality was determined using a vaccinia-env challenge model as described previously (6). Briefly, inocula of vP1174 were prepared by culturing the recombinant vaccinia virus on BSC-1 cells until 90% of the cells were lysed. The lysed cells were removed from the culture supernatants by centrifugation at 4,000 × g for 10 min, and aliquots of the supernatants were stored in liquid nitrogen until used. The culture supernatants typically yielded about 5 × 109 vP1174 PFU/ml, as determined by a direct plaque assay on BSC-1 cells. Mice were inoculated with 108 PFU of vP1174 via intraperitoneal injection 28 days after vaccination. Six days after the challenge, the ovaries of the mice were harvested and homogenized with a mechanical tissue grinder. The homogenates were clarified by centrifugation at 4,000 × g for 10 min, and the number of vP1174 PFU in the resultant supernatants was enumerated by infecting BSC-1 cell monolayers with 10-fold serial dilutions of these fluids and counting plaques after 2 days in culture at 37°C in a 5% CO2 environment.
RESULTS
Induction of Env-specific CD8+ T cells.
The antigen-specific IFN-γ-ELISPOT assay is a rapid, reproducible, and sensitive method for monitoring the immunogenicity of vaccine modalities for CD8+ T-cell response (20, 30). Therefore, this assay was utilized to assess the magnitude and kinetics of the gp120-specific IFN-γ-CD8+ T-cell responses that transpired after vaccination of mice with prototype Shigella gp120 DNA vaccine vector strain H1012.
A dose of 104 H1012 CFU was determined in a series of preliminary experiments to be optimal for the induction of CD8+ T-cell responses to gp120. Doses lower or greater than 104 CFU of H1012 were found to be significantly less immunogenic for this response (data not shown). Thus, mice were vaccinated intranasally with a single dose containing 104 CFU of strain H1012 or of negative-control strain H1016. Splenocytes were harvested 3, 5, 9, 17, and 28 days after vaccination, and Env-specific IFN-γ-ELISPOTs were enumerated without in vitro expansion (Fig. 1). Only a low number of nonspecific IFN-γ-ELISPOTs were detected 3 days after vaccination; however, significant numbers of Env-specific IFN-γ-ELISPOTs were detected 5 days after vaccination with strain H1012 (Fig. 1).
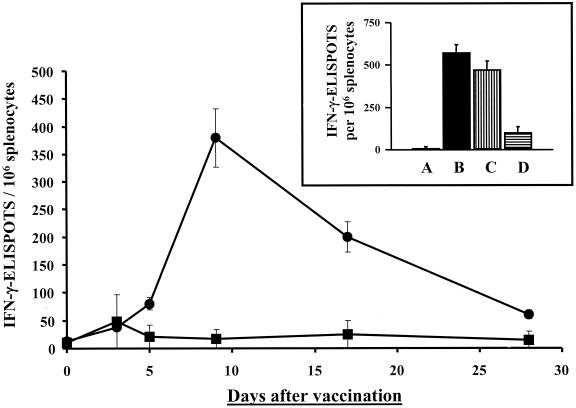
FIG. 1.
Kinetics of the Env-specific IFN-γ-ELISPOT response. BALB/c mice were vaccinated intranasally with 104 CFU of Shigella gp120 DNA vaccine vector strain H1012 (●) or control strain H1016 (■). Env-specific IFN-γ-ELISPOTs were enumerated before and 3, 5, 9, 17, and 28 days after vaccination. (Insert) Number of Env-specific IFN-γ-ELISPOTs in unfractionated splenocytes 9 days after vaccination with strain H1016 (A) or H1012 (B) or in CD4+-cell-depleted (C) or CD8+ cell-depleted (D) splenocytes harvested 9 days after vaccination with H1012. The results are expressed as the mean numbers of gp120-specific IFN-γ-ELISPOTs per 106 splenocytes ± standard deviations and are representative of three independent experiments.
The response elicited by the Shigella gp120 DNA vaccine vector to gp120 in unexpanded splenocytes displayed the kinetics of a primary effector T-cell response, peaking 9 days after vaccination and almost undetectable 28 days after vaccination (Fig. 1). Furthermore, depletion of CD8+ cells by negative selection demonstrated that the gp120-specific IFN-γ-ELISPOT response was due to the presence of CD8+ T cells, whereas depletion of CD4+ T cells only marginally altered the number of gp120-specific IFN-γ-ELISPOTs (Fig. 1, insert).
Immunoblot analysis showed that the Shigella gp120 DNA vaccine vector did not express detectable levels of gp120 (i.e., <10 pg per 106 vector bacilli; data not shown). Thus, the gp120-specific CD8+ T-cell responses that developed after vaccination with the Shigella vector were most likely due to expression of the gp120 DNA vaccine by host cells. Together, these data confirm that Shigella vectors deliver passenger DNA vaccines to host cells (7), resulting in the stimulation CD8+ T-cell responses to the antigens expressed by host cells harboring vector-delivered DNA vaccines.
Comparison of vaccine modalities.
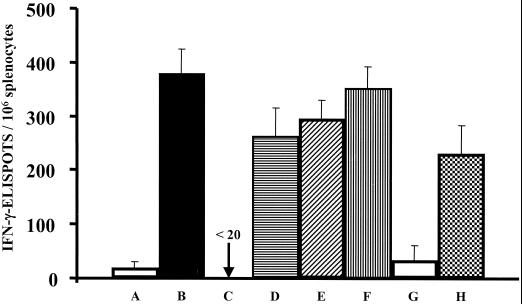
In light of the above results, we conducted a side-by-side comparison of strain H1012 with gp120 DNA vaccine pOGL1 and vaccinia-env vector vP1174. Parallel groups of mice were vaccinated as follows: intranasally with 104 CFU of strain H1012; intraperitoneally with 107, 3 × 107, or 108 PFU of vaccinia-env vector vP1174; or intramuscularly with 20 μg of endotoxin-free (<0.5 EU/mg) pOGL1 DNA. Negative-control mice were vaccinated with Shigella strain H1016, control vaccinia-lacZ vector vSC8, or pcDNA3.1ZEO DNA. Nine days after vaccination, the gp120-specific IFN-γ-ELISPOTs were enumerated, revealing that the magnitude of the Env-specific IFN-γ-ELISPOT response elicited by H1012 was comparable to the analogous responses elicited by vaccinia-env vector strain vP1174 or gp120 DNA vaccine pOGL1 (Fig. 2). We concluded that these vaccination modalities display similar capacities to prime gp120-specific IFN-γ-ELISPOT responses.
FIG. 2.
Immunogenicity of a Shigella DNA vaccine vector versus a vaccinia virus vector. To compare priming vaccine modalities, groups of BALB/c mice were vaccinated intranasally with H1016 (A) or H1012 (B). In parallel, mice were vaccinated intraperitoneally with 108 PFU of vaccinia-lacZ vector vSC8 (C) or with 107 (D), 3 × 107 (E). or 108 (F) PFU of vaccinia-env vector vP1174. Additional groups of mice were vaccinated intramuscularly with 20 μg of pcDNA3.1ZEO (G) or pOGL1 (H) DNA. Nine days after vaccination, splenocytes were harvested, and the number of Env-specific IFN-γ-ELISPOTs was enumerated. The results are expressed as the mean numbers of gp120-specific IFN-γ-ELISPOTs per 106 splenocytes ± standard deviations and are representative of two independent experiments.
Measurement of antiviral protection.
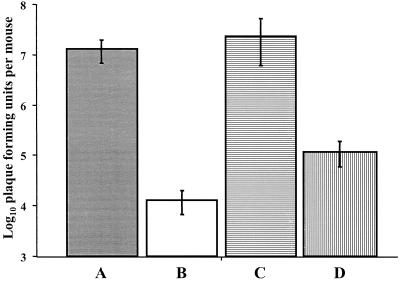
Recently, Belyakov et al. (6) reported a vaccinia-env challenge model that measures HIV-specific effector CD8+ T-cell responses in mice. Therefore, we utilized this model to qualitatively assess the gp120-specific CD8+ T-cell responses that developed in mice vaccinated with the prototype Shigella gp120 DNA vaccine vector. Thus, groups of BALB/c mice were vaccinated intranasally with a single dose containing 104 CFU of strain H1012 or H1016. For comparative purposes, two additional groups of mice were vaccinated intramuscularly with 20 μg of pOGL1 or pcDNA3.1ZEO DNA. Twenty-eight days after primary vaccination, the mice were challenged with 108 CFU of vaccinia-env vector vP1174. Six days after inoculation with the challenge virus, the numbers of vP1174 PFU in the vaccinated and control mice were enumerated. The results showed that intranasal vaccination with the Shigella gp120 DNA vaccine vector afforded a significant level of protection against the challenge virus (Fig. 3). Notably, the number of vaccinia-env PFU recovered in mice vaccinated intranasally with the Shigella vector was about fivefold less than the number in mice vaccinated intramuscularly with the gp120 DNA vaccine alone (Fig. 3).
FIG. 3.
Antiviral immunity against a vaccinia-env challenge. Groups of BALB/c mice were vaccinated intranasally with 104 CFU of H1016 (A) or H1012 (B). Additional mice were vaccinated intramuscularly with 20 μg of pcDNA3.1ZEO (C) or pOGL1 (D) DNA. The mice were challenged intraperitoneally with 108 PFU of vaccinia-env vector vP1174 28 days after vaccination. Six days after the challenge the number of PFU in the ovaries of the mice was enumerated as described in Materials and Methods. The results are expressed as the mean numbers of vP1174 PFU ± standard deviations and are representative of two independent experiments.
Comparison of prime-boost vaccination protocols.
The above findings demonstrated that Shigella HIV-1 DNA vaccine vectors have the potential to function as the priming component in a prime-boost HIV-1 vaccine, such as those currently being prepared for phase 1 volunteer trials (10, 15, 25). In this regard, strategies that incorporate a DNA vaccine as a priming vaccine and a viral vector as a boosting vaccine are believe to be highly effective at eliciting CD8+ T-cell responses to HIV-1 antigens (10, 15, 25) and in two instances have been shown to afford antiviral protection against HIV-1 (15) and SHIV (25) in nonhuman primates. For this reason, we compared the priming capacity of the Shigella DNA vaccine vector to that of the DNA vaccine alone in a prime-boost vaccination protocol that used a vaccinia-env vector as the boosting vaccine.
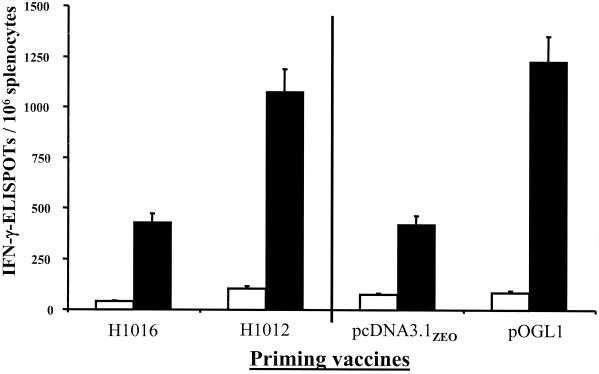
Accordingly, mice were vaccinated once intranasally with 104 CFU of strain H1016 or intramuscularly with of 20 μg of pOGL1 DNA. Control mice were vaccinated once intranasally with 104 CFU of strain H1012 or intramuscularly with 20 μg of pcDNA3.1ZEO DNA. Twenty-eight days after primary vaccination, the mice were given an intraperitoneal booster vaccination of 108 PFU of vP1174, and nine days after the boost, Env-specific IFN-γ-ELISPOTs were enumerated (Fig. 4). Since mice vaccinated with H1016 or pcDNA3.1ZEO were not primed with gp120, the Env-specific IFN-γ-ELISPOTs that arose in these mice were solely due to the vaccinia-env booster vaccine. Using this number as the denominator, both DNA vaccine modalities were effective priming vaccines, as the number of gp120-specific IFN-γ-ELISPOTs in mice primed with either H1012 or pOGL1 was significantly elevated than the number in mice primed with the respective mock controls (Fig. 4). Furthermore, the magnitude of the Env-specific IFN-γ-ELISPOT response in mice primed with Shigella gp120 DNA vaccine vector was comparable to the analogous response in mice vaccinated with the gp120 DNA vaccine (Fig. 4).
FIG. 4.
Comparison of prime-boost vaccination strategies. Groups of BALB/c mice were primed intranasally with 104 CFU of H1016 or H1012. For comparative purposes, two additional groups of mice were primed intramuscularly with 20 μg of pcDNA3.1ZEO or pOGL1 DNA. Twenty-eight days after the primary vaccination, the mice were given booster vaccinations of 108 PFU of vaccinia-env vector vP1074 intraperitoneally. Nine days after the booster vaccination, the numbers of IFN-γ-ELISPOTs were enumerated in unstimulated splenocytes (white bars) or splenocytes stimulated with 5 μg of peptide P18 per ml (black bars). The results are expressed as the mean numbers of IFN-γ-ELISPOTs per 106 splenocytes ± standard deviations and are representative of two independent experiments.
DISCUSSION
The results in this report confirm that Shigella vectors are capable of delivering passenger DNA vaccines to host cells (24, 26) and inducing robust CD8+ T-cell responses to antigens expressed by the DNA vaccines (7). We extended these earlier findings by showing that vaccination of mice with a single dose of a prototype Shigella gp120 DNA vaccine vector afforded significant protection against a vaccinia-env challenge. To our knowledge, this is the first documentation of antigen-specific antiviral protective immunity following vaccination with a live Shigella DNA vaccine vector. This finding has important ramifications, in light of mounting evidence indicating that CD8+ T-cell responses are capable of affording antiviral protection against HIV-1, as highlighted in recent studies that used parentally administered HIV-1 DNA vaccines to prime CD8+ T cells to HIV-1 antigens (5, 15, 25).
However, we believe that the live oral Shigella HIV-1 DNA vaccine vector system presents advantages over parentally administered HIV-1 DNA vaccines. In support of this notion, there is an emerging consensus that mucosal immunity to HIV-1 provides an important barrier against sexually acquired HIV-1 (6, 19). Moreover, attenuated oral Shigella strains retain the ability to enter colonic mucosal lymphoid tissues and induce both mucosal and systemic immune responses (13, 14, 16). Unfortunately, due to the restricted host specificity of Shigella, we could not directly assess the capacity of our prototype Shigella HIV-1 DNA vaccine vector to induce mucosal responses following oral administration. This question can be addressed only in nonhuman primates or in volunteers.
Nonetheless, the results presented in this report demonstrate that this vector has the capacity to function as the priming component of a prime-boost HIV-1 vaccine. We showed that the primary CD8+ T-cell response to gp120 following vaccination with the Shigella gp120 DNA vaccine vector was comparable to the magnitude of this response after vaccination with two commonly used vaccine modalities, a vaccinia-env vector and a gp120 DNA vaccine. In addition, the magnitudes of the secondary CD8+ T-cell responses to gp120 following a booster vaccination with the vaccinia-env vector were similar in mice primed with the Shigella gp120 DNA vaccine vector to those primed with the analogous gp120 DNA vaccine.
In summary, therefore, the data in this report indicate that Shigella vectors provide an effective and inexpensive method to deliver HIV-1 DNA vaccines to inductive lymphoid tissues. Given that the potency of the Shigella gp120 DNA vaccine vector was comparable to that of an analogous gp120 DNA vaccine, we believe this vector system holds promise for use as a public health tool in developed and developing countries.
ACKNOWLEDGMENTS
We thank Robert Powel, who laid the essential groundwork to these studies, and Michael Boysun and Christine Obriecht for providing technical support throughout these studies. We also thank George Lewis for his guidance and encouragement throughout the studies in this report.
This work was supported in part by NIAID grants AI41914, AI42603, and AI43756.
REFERENCES
- 1.Abacioglu Y H, Fouts T R, Laman J D, Claassen E, Pincus S H, Moore J P, Roby C A, Kamin-Lewis R, Lewis G K. Epitope mapping and topology of baculovirus-expressed HIV-1 gp160 determined with a panel of murine monoclonal antibodies. AIDS Res Hum Retrovir. 1994;10:371–381. doi: 10.1089/aid.1994.10.371. [DOI] [PubMed] [Google Scholar]
- 2.Andre S, Seed B, Eberle J, Schraut W, Bultmann A, Haas J. Increased immune response elicited by DNA vaccination with a synthetic gp120 sequence with optimized codon usage. J Virol. 1998;72:1497–1503. doi: 10.1128/jvi.72.2.1497-1503.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appay V, Nixon D F, Donahoe S M, Gillespie G M, Dong T, King A, Ogg G S, Spiegel H M, Conlon C, Spina C A, Havlir D V, Richman D D, Waters A, Easterbrook P, McMichael A J, Rowland-Jones S L. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K E. The polymerase chain reaction. New York, N.Y: John Wiley & Sons; 1990. [Google Scholar]
- 5.Barouch D H, Santra S, Schmitz J E, Kuroda M J, Fu T M, Wagner W, Bilska M, Craiu A, Zheng X X, Krivulka G R, Beaudry K, Lifton M A, Nickerson C E, Trigona W L, Punt K, Freed D C, Guan L, Dubey S, Casimiro D, Simon A, Davies M E, Chastain M, Strom T B, Gelman R S, Montefiori D C, Lewis M G, Emini E A, Shiver J W, Letvin N L. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290:486–492. doi: 10.1126/science.290.5491.486. [DOI] [PubMed] [Google Scholar]
- 6.Belyakov I M, Derby M A, Ahlers J D, Kelsall B L, Earl P, Moss B, Strober W, Berzofsky J A. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc Natl Acad Sci USA. 1998;95:1709–1714. doi: 10.1073/pnas.95.4.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fennelly G J, Khan S A, Abadi M A, Wild T F, Bloom B R. Mucosal DNA vaccine immunization against measles with a highly attenuated Shigella flexneri vector. J Immunol. 1999;162:1603–1610. [PubMed] [Google Scholar]
- 8.Fouts T R, Tuskan R G, Chada S, Hone D M, Lewis G K. Construction and immunogenicity of Salmonella typhimurium vaccine vectors that express HIV-1 gp120. Vaccine. 1995;13:1697–1705. doi: 10.1016/0264-410x(95)00106-b. [DOI] [PubMed] [Google Scholar]
- 9.Franchini G, Robert-Guroff M, Tartaglia J, Aggarwal A, Abimiku A, Benson J, Markham P, Limbach K, Hurteau G, Fullen J, et al. Highly attenuated HIV type 2 recombinant poxviruses, but not HIV-2 recombinant Salmonella vaccines, induce long-lasting protection in rhesus macaques. AIDS Res Hum Retrovir. 1995;11:909–920. doi: 10.1089/aid.1995.11.909. [DOI] [PubMed] [Google Scholar]
- 10.Hanke T, McMichael A. Pre-clinical development of a multi-CTL epitope-based DNA prime MVA boost vaccine for AIDS. Immunol Lett. 1999;66:177–181. doi: 10.1016/s0165-2478(98)00164-3. [DOI] [PubMed] [Google Scholar]
- 11.Hanke T, Samuel R V, Blanchard T J, Neumann V C, Allen T M, Boyson J E, Sharpe S A, Cook N, Smith G L, Watkins D I, Cranage M P, McMichael A J. Effective induction of simian immunodeficiency virus-specific cytotoxic T lymphocytes in macaques by using a multiepitope gene and DNA prime-modified vaccinia virus Ankara boost vaccination regimen. J Virol. 1999;73:7524–7532. doi: 10.1128/jvi.73.9.7524-7532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herremans T M, Reimerink J H, Buisman A M, Kimman T G, Koopmans M P. Induction of mucosal immunity by inactivated poliovirus vaccine is dependent on previous mucosal contact with live virus. J Immunol. 1999;162:5011–5018. [PubMed] [Google Scholar]
- 13.Karnell A, Li A, Zhao C R, Karlsson K, Nguyen B M, Lindberg A A. Safety and immunogenicity study of the auxotrophic Shigella flexneri 2a vaccine SFL1070 with a deleted aroD gene in adult Swedish volunteers. Vaccine. 1995;13:88–99. doi: 10.1016/0264-410x(95)80017-8. [DOI] [PubMed] [Google Scholar]
- 14.Karnell A, Sweiha H, Lindberg A A. Auxotrophic live oral Shigella flexneri vaccine protects monkeys against challenge with S. flexneri of different serotypes. Vaccine. 1992;10:167–174. doi: 10.1016/0264-410x(92)90007-7. [DOI] [PubMed] [Google Scholar]
- 15.Kent S J, Zhao A, Best S J, Chandler J D, Boyle D B, Ramshaw I A. Enhanced T-cell immunogenicity and protective efficacy of a human immunodeficiency virus type 1 vaccine regimen consisting of consecutive priming with DNA and boosting with recombinant fowlpox virus. J Virol. 1998;72:10180–8. doi: 10.1128/jvi.72.12.10180-10188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotloff K L, Losonsky G A, Nataro J P, Wasserman S S, Hale T L, Taylor D N, Newland J W, Sadoff J C, Formal S B, Levine M M. Evaluation of the safety, immunogenicity, and efficacy in healthy adults of four doses of live oral hybrid Escherichia coli-Shigella flexneri 2a vaccine strain EcSf2a-2. Vaccine. 1995;13:495–502. doi: 10.1016/0264-410x(94)00011-b. [DOI] [PubMed] [Google Scholar]
- 17.Kotloff K L, Noriega F, Losonsky G A, Sztein M B, Wasserman S S, Nataro J P, Levine M M. Safety, immunogenicity, and transmissibility in humans of CVD 1203, a live oral Shigella flexneri 2a vaccine candidate attenuated by deletions in aroA and virG. Infect Immun. 1996;64:4542–4548. doi: 10.1128/iai.64.11.4542-4548.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehner T, Wang Y, Cranage M, Bergmeier L A, Mitchell E, Tao L, Hall G, Dennis M, Cook N, Brookes R, Klavinskis L, Jones I, Doyle C, Ward R. Protective mucosal immunity elicited by targeted iliac lymph node immunization with a subunit SIV envelope and core vaccine in macaques. Nat Med. 1996;2:767–775. doi: 10.1038/nm0796-767. [DOI] [PubMed] [Google Scholar]
- 20.Miyahira Y, Murata K, Rodriguez D, Rodriguez J R, Esteban M, Rodrigues M M, Zavala F. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J Immunol Methods. 1995;181:45–54. doi: 10.1016/0022-1759(94)00327-s. [DOI] [PubMed] [Google Scholar]
- 20a.National Institutes of Health. National Institutes of Health guide for the care and use of laboratory animals. NIH publication no. 85-23. Bethesda, Md: National Institutes of Health; 1985. [Google Scholar]
- 21.Noriega F R, Wang J Y, Losonsky G, Maneval D R, Hone D M, Levine M M. Construction and characterization of attenuated ΔaroA ΔvirG Shigella flexneri 2a strain CVD 1203, a prototype live oral vaccine. Infect Immun. 1994;62:5168–5172. doi: 10.1128/iai.62.11.5168-5172.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 23.Perkus M E, Limbach K, Paoletti E. Cloning and expression of foreign genes in vaccinia virus, using a host range selection system. J Virol. 1989;63:3829–3836. doi: 10.1128/jvi.63.9.3829-3836.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powell R J, Lewis G K, Hone D M. Introduction of eukaryotic expression cassettes into animal cells using a bacterial vector delivery system. In: Brown F, Norrby E, Burton D, Mekalanos J, editors. Vaccine 96: molecular approaches to the control of infectious disease. New York, N.Y: Cold Spring Harbor Press; 1996. pp. 183–187. [Google Scholar]
- 25.Robinson H L, Montefiori D C, Johnson R P, Manson K H, Kalish M L, Lifson J D, Rizvi T A, Lu S, Hu S L, Mazzara G P, Panicali D L, Herndon J G, Glickman R, Candido M A, Lydy S L, Wyand M S, McClure H M. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nat Med. 1999;5:526–534. doi: 10.1038/8406. [DOI] [PubMed] [Google Scholar]
- 26.Sizemore D R, Branstrom A A, Sadoff J C. Attenuated Shigella as a DNA delivery vehicle for DNA-mediated immunization. Science. 1995;270:299–302. doi: 10.1126/science.270.5234.299. [DOI] [PubMed] [Google Scholar]
- 27.Steger K K, Waterman P M, Pauza C D. Acute effects of pathogenic simian-human immunodeficiency virus challenge on vaccine-induced cellular and humoral immune responses to Gag in rhesus macaques. J Virol. 1999;73:1853–1859. doi: 10.1128/jvi.73.3.1853-1859.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valentine P J, Meyer K, Rivera M M, Lipps C, Pauza D, Maziarz R T, So M, Heffron F. Induction of SIV capsid-specific CTL and mucosal sIgA in mice immunized with a recombinant S. typhimurium aroA mutant. Vaccine. 1996;14:138–146. doi: 10.1016/0264-410x(95)00130-s. [DOI] [PubMed] [Google Scholar]
- 29.van de Verg L L, Mallett C P, Collins H H, Larsen T, Hammack C, Hale T L. Antibody and cytokine responses in a mouse pulmonary model of Shigella flexneri serotype 2a infection. Infect Immun. 1995;63:1947–1954. doi: 10.1128/iai.63.5.1947-1954.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Versteegen J M, Logtenberg T, Ballieux R E. Enumeration of IFN-gamma-producing human lymphocytes by spot-ELISA. A method to detect lymphokine-producing lymphocytes at the single-cell level. J Immunol Methods. 1988;111:25–29. doi: 10.1016/0022-1759(88)90055-5. [DOI] [PubMed] [Google Scholar]
- 31.Walker C M, Moody D J, Stites D P, Levy J A. CD8+ T lymphocyte control of HIV replication in cultured CD4+ cells varies among infected individuals. Cell Immunol. 1989;119:470–475. doi: 10.1016/0008-8749(89)90259-1. [DOI] [PubMed] [Google Scholar]
- 32.Wolff J A, Malone R W, Williams P, Chong W, Acsadi G, Jani A, Felgner P L. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 33.Wu S, Pascual D W, Lewis G K, Hone D M. Induction of mucosal and systemic responses against human immunodeficiency virus type 1 glycoprotein 120 in mice after oral immunization with a single dose of a Salmonella-HIV vector. AIDS Res Hum Retrovir. 1997;13:1187–1194. doi: 10.1089/aid.1997.13.1187. [DOI] [PubMed] [Google Scholar]