Abstract
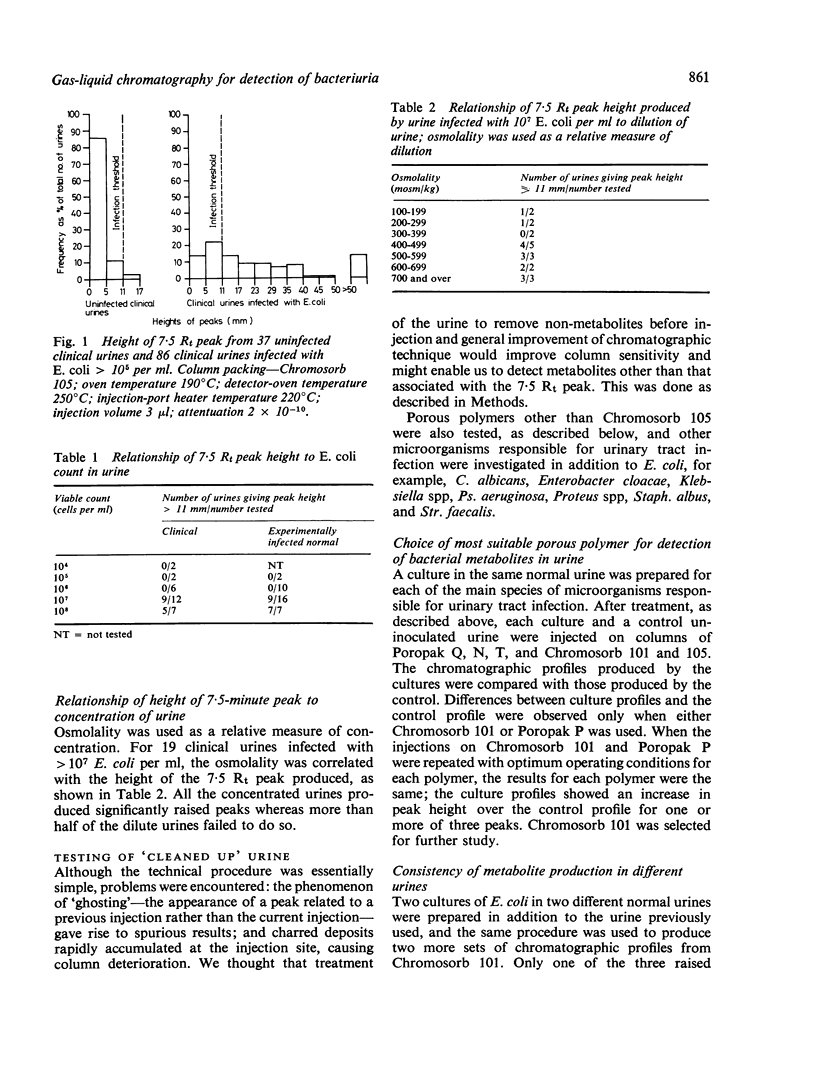
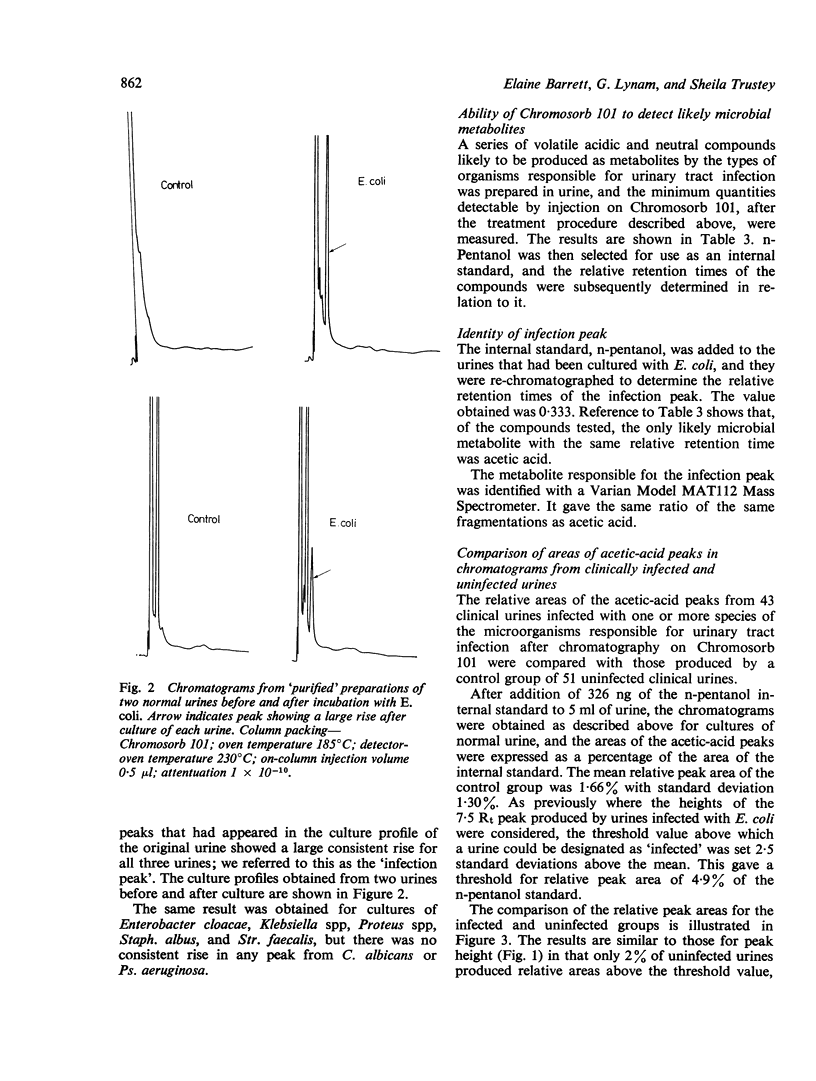
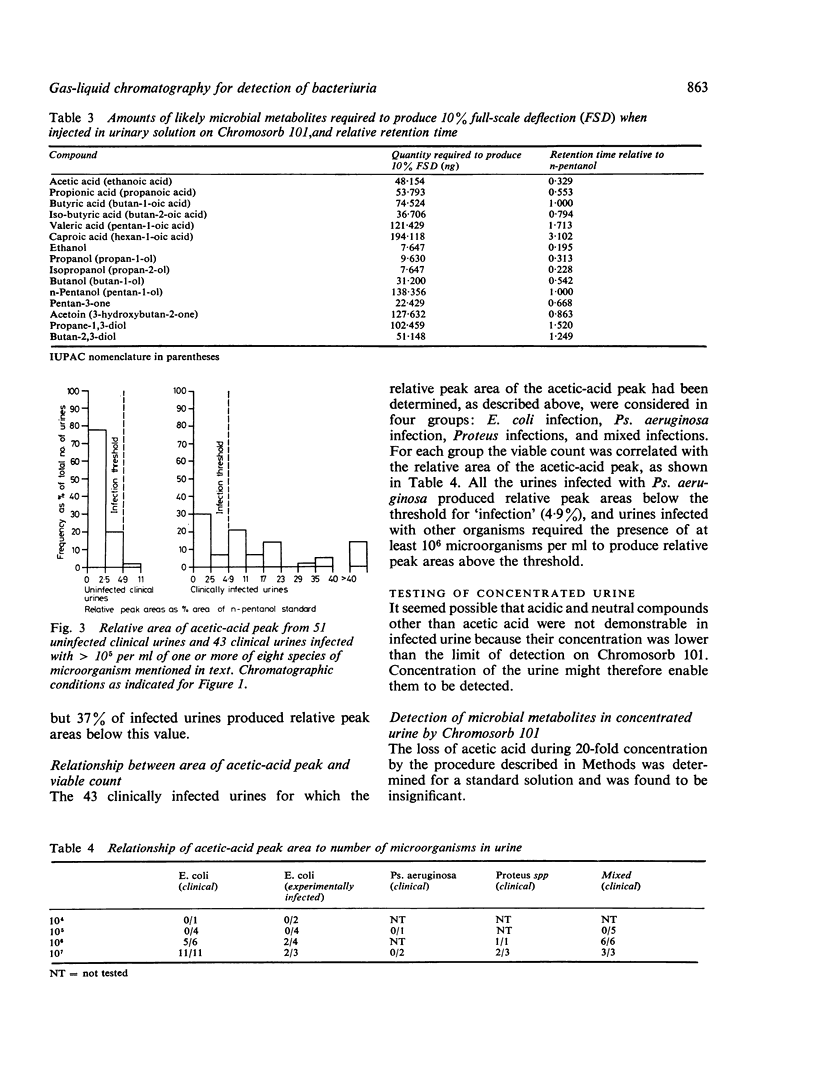
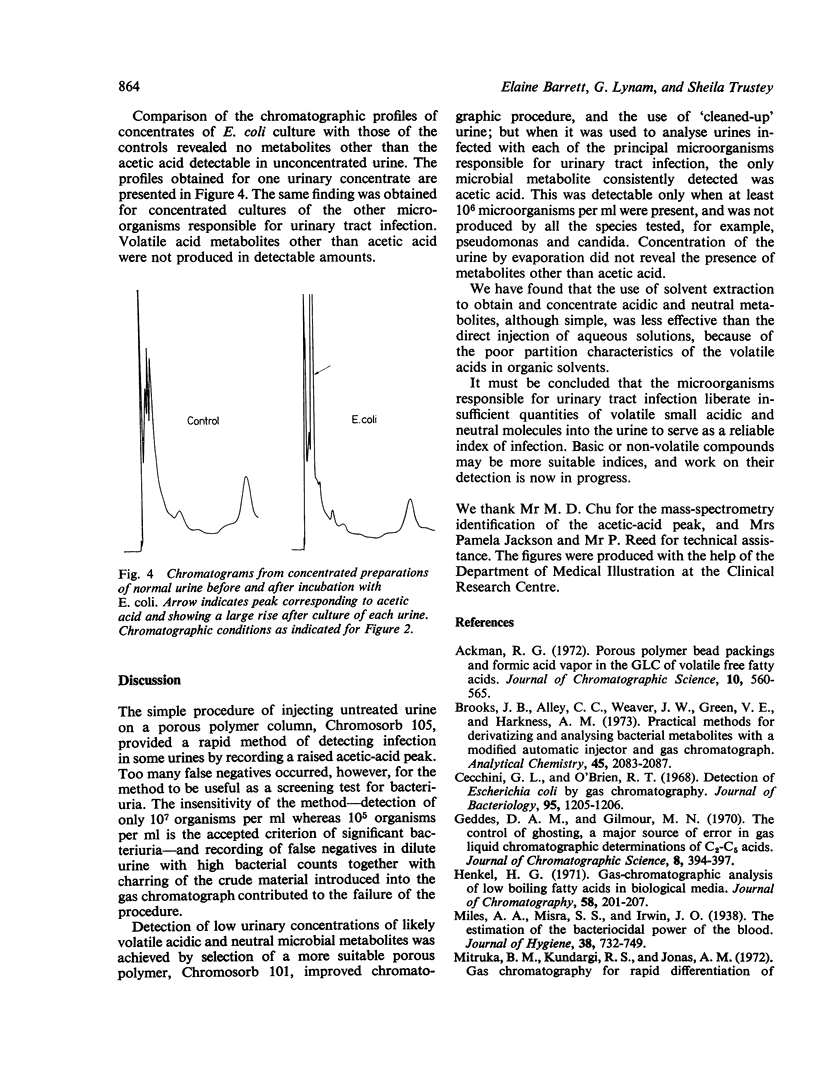
Direct injection of clinically infected urines on porous-polymer columns was investigated to determine which microbial metabolites were consistently detectable, and whether their presence could be used as a reliable index of infection. Chromosorb 101 was found to be the most suitable porous polymer for the detection of microbial metabolites; greater sensitivity of detection was achieved by partial purification of the urine before injection. Acetic acid was the only compound found consistently and it enabled 10(6) microorganisms per ml to be detected in urine. However, as urinary tract infection is diagnosed by the presence of 10(5) organisms or more per ml, our method is insufficiently sensitive for the detection of bacteriuria. Escherichia coli, Enterobacter cloacae, Klebsiella spp, Proteus spp, Stapyhlococcus albus, and Streptocococcus faecalis were detectable by our method but Pseudomonas aeruginosa and Candida albicans were not.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackman R. G. Porous polymer bead packings and formic acid vapor in the GLC of volatile free fatty acids. J Chromatogr Sci. 1972 Sep;10(9):560–565. doi: 10.1093/chromsci/10.9.560. [DOI] [PubMed] [Google Scholar]
- Brooks J. B., Alley C. C., Weaver J. W., Green V. E., Harkness A. M. Practical methods for derivatizing and analyzing bacterial metabolites with a modified automatic injector and gas chromatograph. Anal Chem. 1973 Oct;45(12):2083–2087. doi: 10.1021/ac60334a022. [DOI] [PubMed] [Google Scholar]
- Cecchini G. L., O'Brien R. T. Detection of Escherichia coli by gas chromatography. J Bacteriol. 1968 Mar;95(3):1205–1206. doi: 10.1128/jb.95.3.1205-1206.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel H. G. Gas chromatographic analysis of low boiling fatty acids in biological media. J Chromatogr. 1971 Jun 24;58(2):201–207. doi: 10.1016/s0021-9673(00)96612-3. [DOI] [PubMed] [Google Scholar]
- Mitruka M., Kundargi R. S., Jonas A. M. Gas chromatography for rapid differentiation of bacterial infections in man. Med Res Eng. 1972;11(2):7–11. [PubMed] [Google Scholar]
- Rogosa M., Love L. L. Direct quantitative gas chromatographic separation of C2-C6 fatty acids, methanol, and ethyl alcohol in aqueous microbial fermentation media. Appl Microbiol. 1968 Feb;16(2):285–290. doi: 10.1128/am.16.2.285-290.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eenaeme C., Bienfait J. M., Lambot O., Pondant A. Studies on ghosting, an important source of error in the quantitative estimation of free volatile fatty acids by GLC. I. Occurrence of ghosting and factors influencing it. J Chromatogr Sci. 1974 Jul;12(7):398–403. doi: 10.1093/chromsci/12.7.398. [DOI] [PubMed] [Google Scholar]
- Yoshioka, Kitamura M., Tamura Z. Rapid gas chromatographic analysis of microbial volatile metabolites. Jpn J Microbiol. 1969 Mar;13(1):87–93. doi: 10.1111/j.1348-0421.1969.tb00438.x. [DOI] [PubMed] [Google Scholar]


