Abstract
Transcription factors of the NFAT (nuclear factor of activated T cells) family are expressed in most immune system cells and in a range of other cell types. Signaling through NFAT is implicated in the regulation of transcription for the immune response and other processes, including differentiation and apoptosis. NFAT normally resides in the cytoplasm, and a key aspect of the NFAT activation pathway is the regulation of its nuclear import by the Ca2+/calmodulin-dependent phosphatase calcineurin. In a cell line stably expressing green fluorescent protein (GFP)-NFAT, this import can be triggered by elevation of intracellular calcium and visualized in live cells. Here we show that the inducible nuclear import of GFP-NFAT is efficiently blocked at early stages of herpes simplex virus (HSV) infection. This is a specific effect, since we observed abundant nuclear accumulation of a test viral protein and no impediment to general nuclear localization signal-dependent nuclear import and retention in infected cells. We show that virus binding at the cell surface is not itself sufficient to inhibit the signaling that induces NFAT nuclear translocation. Since the block occurs following infection in the presence of phosphonoacetic acid but not cycloheximide, we infer that the entry of the virion and early gene transcription are required but the effect is independent of DNA replication or late virus gene expression. A consequence of the block to GFP-NFAT import is a reduction in NFAT-dependent transcriptional activation from the interleukin-2 promoter in infected cells. This HSV-mediated repression of the NFAT pathway may constitute an immune evasion strategy or subversion of other NFAT-dependent cellular processes to promote viral replication.
Herpesviruses subvert a range of host cellular processes by interference with signal transduction pathways. For example, mitogenic kinase cascades are activated to establish a cellular environment favorable for virus replication (19, 33). Apoptotic pathways are repressed during infection to prevent cell death prior to virus release and possibly also to protect latently infected neuronal cells from destruction (9, 24, 30). Furthermore, to help evade host immune detection, infection disrupts cytokine-mediated signaling networks crucial for cell-mediated immunity (14), and apoptosis is induced in activated T lymphocytes and macrophages (16, 46, 54).
A key aspect of the regulated transcription response to immune stimuli is the Ca2+/calmodulin-dependent signal cascade which leads to the activation of the NFAT (nuclear factor of activated T cells) family of transcription factors (51). The family encompasses four closely related proteins, NFAT1 to -4, which share structural and functional homology and are expressed in numerous cell types of the immune system as well as in a range of other cells, including muscle, cardiac, and neuronal cells (7, 20, 37). These proteins have a conserved N-terminal transactivation domain and a central DNA binding domain with homology to the DNA binding domains of Rel/NF-κB factors. Between these regions lies a regulatory domain which contains phosphorylation sites, nuclear import and export signals, and a binding site for the cellular phosphatase calcineurin. For detailed reviews of NFAT structure and distribution, see references 51 and 48.
The NFAT signaling pathway has now been described in some detail and is conserved between NFAT1 to -4. Expression of NFAT target genes is regulated at the levels of NFAT nuclear import and of NFAT transcriptional activation status, both of which are determined by the phosphorylation state of the protein (41). The pathway was originally defined in T lymphocytes, in which NFAT is cytoplasmic when the cell is in its unstimulated state. An extracellular stimulus causing T-cell receptor activation leads to an increase in levels of intracellular Ca2+ and activation of calmodulin. Under these conditions the Ca2+/calmodulin-dependent cellular phosphatase calcineurin is activated. Calcineurin binds to NFAT at defined sites within its regulatory domain (1, 43), dephosphorylating NFAT at multiple sites and causing a conformational switch believed to unmask its nuclear localization signal (NLS) and allow nuclear import (41, 56). Thus, in activated T cells and other cell types stimulated to raise intracellular levels of Ca2+, NFAT is localized to the nucleus. Once inside the nucleus, and in its dephosphorylated state (41), NFAT is capable of stimulating the transcription of target genes containing NFAT response elements in their upstream enhancer sequences. Such elements are widely distributed (23) and are almost ubiquitous in cytokine promoters. The prototypic element is the distal NFAT binding site of the human and murine interleukin-2 (IL-2) promoters (21). NFAT can stimulate transcription alone (25, 32) but shows cooperative and synergistic binding with a number of other transcription factors, with the most widely documented being AP-1 (21, 51). NFAT nuclear accumulation is rapid, occurring within 5 to 10 min of stimulation, and reversible (22, 52). Export occurs following rephosphorylation of NFAT by kinases, including GSK-3, most likely by remasking the NLS and allowing a constitutively active nuclear export signal to dominate (2, 56). Thus, a balance between cellular phosphatase and kinase activities determines NFAT localization, with the outcome being dependent on levels of intracellular Ca2+.
The NFAT signaling pathway is the target of the immunosuppressive drugs cyclosporin A and FK506, which act at the level of calcineurin activation (15, 31). The pathway is also subject to interference by a number of viruses, including human immunodeficiency virus (26), hepatitis C virus (3), and African swine fever virus (ASFV) (35, 36), aiming either to induce a cellular state permissive for viral infection and replication or to suppress immune detection and clearance of the virus. These examples include both stimulatory and inhibitory interventions acting at various levels of the cascade. Beyond this immunomodulatory role, primarily in T-cell activation and differentiation, the range of NFAT target genes and dependent processes is expanding to include genes encoding cell surface receptors and ligands and to involvement in apoptosis and differentiation of nonimmune cell types (7, 12, 23, 38, 47).
Here we report on the effect of herpes simplex virus (HSV) infection on the NFAT signaling pathway. The experimental model used is a cell line stably expressing human NFAT2 with a green fluorescent protein (GFP) tag at its N terminus (GFP-NFAT). GFP-NFAT exhibits the same nuclear transport behavior and responses to inhibitors as described for endogenous NFAT in vivo (22) and also retains the transcriptional activation properties of the endogenous protein. Consequently, we have been able to study the ionomycin-inducible nuclear import and transcriptional activity of NFAT in the context of HSV infection.
MATERIALS AND METHODS
Cells, viruses, and Western blotting.
HeLa cells stably expressing GFP-human NFAT2 were generously provided by Ralph Kehlenbach, Scripps Research Institute, La Jolla, Calif. (22). These cells were grown in Dulbecco's modified Eagle medium containing 10% newborn calf serum. To induce high levels of GFP-NFAT expression, cells were treated with 250 nM trichostatin A (Sigma-Aldrich) overnight. Virus infections were performed using HSV type 1 (HSV-1) (strain 17), HSV-2 (strain G), or ΔgH HSV-1 (strain HFEM) (49). Infections were routinely performed at a multiplicity of infection (MOI) of 10 in serum-free medium. For infection with the gH-negative strain (kindly supplied by Helena Browne), virus was applied at 1,000 particles/cell on the basis of particle counts quantified by electron microscopy. After 1 h of incubation at 37°C, the inoculum was replaced with medium containing 2% newborn calf serum. For analysis of VP16 expression by Western blotting, infected cells were washed in cold phosphate-buffered saline (PBS) and total lysates were prepared by adding 250 μl of sodium dodecyl sulfate (SDS) loading buffer. Samples were briefly sonicated prior to electrophoresis. Equal amounts of total cell extracts were fractionated by SDS-polyacrylamide gel electrophoresis and transferred to Hybond-C membranes (Amersham). VP16 expression was detected using the anti-VP16 monoclonal antibody LP1 (1:4,000) and visualized by enhanced chemiluminescence (Pierce).
GFP-NFAT import assay and immunofluorescence.
Two days before the import assay, GFP-NFAT/HeLa cells were seeded at 2 × 105 cells/well into six-well cluster dishes containing 13-mm-diameter coverslips. One day before the assay, the culture medium was supplemented with 250 nM trichostatin A. On the day of the assay, cells were infected as described above or left uninfected as controls. At 5 h postinfection, the culture medium was supplemented with 1 μM ionomycin (Sigma-Aldrich) and 30 mM lithium acetate; this is referred to as the trigger. The cells were fixed 2 h after the trigger in ice-cold methanol (20 min), rinsed in PBS, and mounted in Vectashield mountant (Vector Laboratories). Quantitation for each condition was performed by counting cells in five randomly chosen fields (total of 50 to 120 cells per test). In some instances the import assay was carried out in the presence of 50 μg of cycloheximide per ml or 300 μg of phosphonoacetic acid (PAA) per ml. Cycloheximide was present in the cell culture medium from 30 min preinfection until the cells were fixed. PAA was added at 1 h postinfection and was present until the cells were fixed. For analysis of localization of ICP5 by indirect immunofluorescence, coverslips of infected cells were fixed in ice-cold methanol (20 min) and blocked with 10% newborn calf serum in PBS for 20 min. The coverslips were incubated (20 min) with monoclonal antibody to ICP5 (clone 3B6; Virusys) diluted 1:200 in blocking solution, rinsed in PBS, and then incubated with Alexa 488-labeled goat anti-mouse secondary antibody (Molecular Probes). Fluorescence images were acquired with a Zeiss LSM410 laser scanning confocal microscope using the 40× or 63× objective lenses.
NFAT-luc reporter assay.
The optimized assay conditions for transcriptional induction through the NFAT pathway were as follows. The day before transfection, GFP-NFAT/HeLa cells were seeded into 24-well dishes at 2 × 105 cells per well. Cells were transfected with 0.2 μg of pNFAT-Luc, which contains four direct repeats of the NFAT binding site (−286 to −257) from the IL-2 gene promoter upstream of a minimal promoter (Stratagene). Carrier DNA (0.8 μg of pUC19 per well) was included together with 10 μl of Lipofectamine (Life Technologies), and the cells were transfected according to the manufacturers' instructions. After 4 h, the transfection mix was supplemented with culture medium to contain a final concentration of 10% newborn calf serum and 250 nM trichostatin A. Cells were superinfected 18 h after transfection. At 5 h postinfection, cells were triggered by addition of 1.3 μM ionomycin and/or 100 nM phorbol myristate acetate (PMA) (Sigma-Aldrich). The cells were rinsed 5 h later in PBS and harvested in 150 μl of reporter lysis buffer (Promega). Lysates (10 μl) were incubated in 100 μl of luciferase substrate (Promega) and assayed for activity in a microplate luminometer (EG&G Berthold). Each experimental condition was assayed in triplicate.
Nucleo-cytoplasmic trafficking assay.
The day before transfection, HeLa cells were seeded into two-well chamber coverglasses (Nunc) at 2 × 105 cells per well. Cells were transfected with 0.2 μg of pSL28, which contains the NLS of the host cell protein HCF (KRPMSSPEHKSAPKKSK) fused to the C terminus of GFP in the commercial vector pEGFP.C1 (Clontech), constructed as previously described (27). Transfections included 0.8 μg of pUC19 carrier DNA per well and were performed using the calcium phosphate precipitation method modified by using BES [N,N-bis(2-hydroxethyl)-2-amino-ethanesulfonic acid]-buffered saline (pH 7.06) in the place of HEPES-buffered saline. Cells were superinfected 18 h after transfection. The subcellular localization of the pSL28 product GFP.HCF.NLS was visualized in live cells using a Zeiss LSM410 laser scanning confocal microscope with the 40× objective lens.
RESULTS
Inducible nuclear import of GFP-NFAT is blocked in HSV-1- and HSV-2-infected HeLa cells.
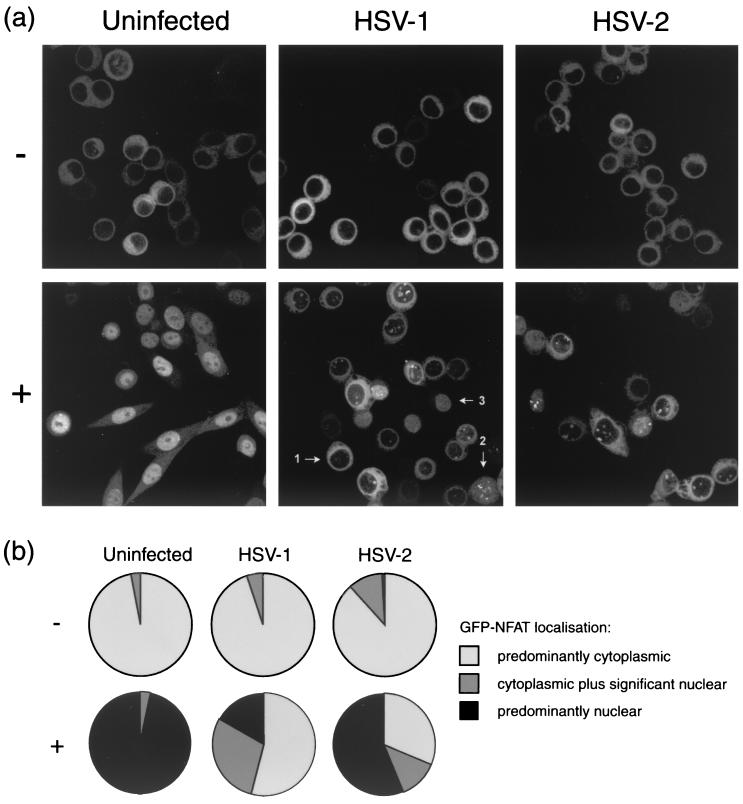
A HeLa cell line stably expressing GFP-NFAT has been established and characterized previously, and the GFP-NFAT has been shown to exhibit features of compartmentalization and activation similar to those of native NFAT (22). These cells were mock infected or infected with HSV-1 or HSV-2 (MOI of 10), and at 5 h postinfection the cells were treated with medium containing 1 μM ionomycin to raise intracellular calcium levels. Parallel cultures of infected and uninfected cells were left in normal medium as controls. Elevation of intracellular calcium is known to activate calcineurin, which results in the dephosphorylation of NFAT proteins and their subsequent nuclear import. The ionomycin treatment was carried out in the presence of 30 mM lithium acetate, which blocks export of NFAT (22). Cells were fixed 2 h after the trigger and analyzed by confocal microscopy. The subcellular localization of GFP-NFAT was scored for individual cells within five randomly selected fields of view from each experimental condition. Representative fields are shown in Fig. 1a, and the combined data from all fields are represented in Fig. 1b.
FIG. 1.
HeLa cells stably expressing GFP-NFAT were infected with either HSV-1 or HSV-2 or left uninfected. At 5 h, cells were treated with 1 μM ionomycin–30 mM lithium acetate (+) or left untreated (−). At 7 h, cells were fixed in methanol and examined by confocal microscopy. (a) Representative images of each experimental condition as described in the text. (b) Summary of GFP-NFAT localization, scored for every cell in five randomly chosen fields of view in each experimental condition. Cells were scored as having GFP-NFAT localization that was predominantly cytoplasmic, cytoplasmic plus significantly nuclear, or predominantly nuclear, and examples of each cell type (arrows 1, 2, and 3, respectively) are marked in the panel for treated, HSV-1-infected cells in panel a. Approximately 100 cells were counted for each of the test conditions.
Consistent with previous reports (22), GFP-NFAT in uninfected cells is predominantly cytoplasmic prior to the ionomycin trigger (Fig. 1a, −). As expected, after exposure to ionomycin in the presence of lithium acetate, virtually all cells in the population had responded and GFP-NFAT now exhibited a predominantly diffuse nuclear pattern, with a subpopulation containing NFAT in a speckled pattern underlying the diffuse pattern (Fig. 1, +). A significant amount of nuclear GFP-NFAT could be observed within 10 min of application of the ionomycin (data not shown), but nuclear accumulation to homogeneity across the cell population could take between 1 and 3 h, with some variation between experiments. For quantitative analysis in all cases, we chose to analyze the fields 2 h after treatment, at which point nuclear import was usually complete.
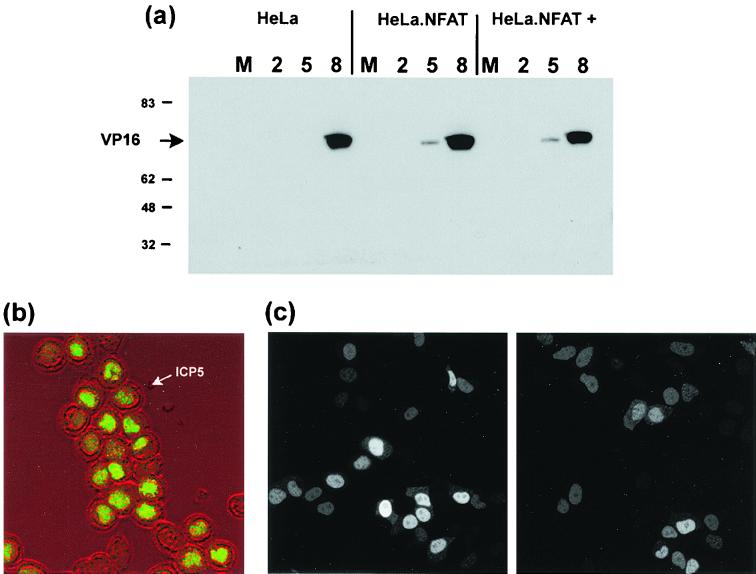
Like uninfected cells, HSV-infected cells show predominantly cytoplasmic GFP-NFAT prior to the ionomycin trigger (Fig. 1, HSV-1, −). However, infection of cells with HSV-1 had a very clear inhibitory effect on inducible GFP-NFAT import, typical examples of which are shown in Fig. 1a, with similar observations for cells infected with HSV-2. Some import remained, and over the course of the analysis approximately 17% of infected cells showed predominantly nuclear GFP-NFAT 2 h after the import trigger (Fig. 1b). However, this was in comparison to 97% of uninfected cells showing NFAT import. In addition, in a population of infected cells where nuclear NFAT was observed, it appeared to be exclusively in speckles rather than the normal diffuse pattern. We do not presently know the significance of this observation, although a speckled pattern was occasionally observed in uninfected cells underlying the more diffuse pattern (see above). What was clear was the dramatic reduction in infected cells in the pattern of normal nucleoplasmic NFAT. While the block for HSV-2 was less efficient, it was nonetheless also clear and significant. As a control to ensure that the cells were infected as normal, we compared accumulation of a test viral protein (VP16) in standard HeLa cells and in the GFP-NFAT/HeLa cell line. As expected, VP16 accumulated at similar rates and to similar levels in the two lines, and the trichostatin treatment used to induce NFAT had no effect on expression (Fig. 2a).
FIG. 2.
HeLa cells or HeLa cells stably expressing GFP-NFAT were mock infected (M) or infected with HSV-1 without or with (+) pretreatment with trichostatin. (a) At 2, 5, or 8 h, as indicated, infected cells were harvested and the accumulation of VP16 was measured by SDS-polyacrylamide gel electrophoresis followed by Western blotting with an anti-VP16 monoclonal antibody. Numbers on the left are molecular masses in kilodaltons. (b) HeLa cells stably expressing GFP-NFAT infected as for panel a in the presence of trichostatin were fixed 12 h after infection, and the accumulation of the late viral protein ICP5 was analyzed by immunofluorescence. The localization of ICP5 (green channel) is superimposed on the phase image of the infected cells to emphasize ICP5 nuclear accumulation. (c) HeLa cells transiently expressing GFP.HCF.NLS were infected with HSV-1 (right panel) or left uninfected (left panel), and localization in live cells was examined by confocal microscopy at 5 h postinfection.
The block to GFP-NFAT nuclear import by HSV-1 could be effected upstream of NFAT by specific interference with the calcineurin activation cascade or downstream via a broader effect on nuclear protein import. Clearly, infected cells accumulate significant amounts of newly synthesized proteins in the nucleus, and widespread cytoplasmic accumulation of cellular nuclear proteins has not been documented in HSV infection. Thus, a general block at the level of nuclear import seemed unlikely. However, to ensure that protein nuclear import was not generally affected in these cells, we first examined localization of a candidate viral protein (ICP5) which normally accumulates in the nuclei of infected cells. The results show that under conditions where import of NFAT was blocked, abundant nuclear import of the newly synthesized ICP5 could be readily observed (Fig. 2b). In a second assay we examined whether, as has been recently shown for poliovirus (18), HSV infection generally prevented the accumulation of a GFP containing an NLS. HeLa cells were transfected with a construct encoding GFP linked at its C terminus to the NLS from the host cell protein HCF (27). This protein is small enough to diffuse through the nuclear pore complex, but by virtue of the NLS it exhibits a predominantly nuclear localization (Fig. 2c, left panel). At 18 h posttransfection, cells were superinfected with HSV-1 and the subcellular localization of GFP.HCF.NLS was monitored in live cells by confocal microscopy. In the context of poliovirus infection, a similar GFP-NLS protein was redistributed to the cytoplasm by 4.5 h postinfection (18). In contrast, at 5 h postinfection with HSV-1, GFP.HCF.NLS remained predominantly nuclear (Fig. 2c, right panel), and this remained the case even at 20 h postinfection (data not shown). The experimental conditions were identical to those in which HSV-1 had blocked GFP-NFAT nuclear import. The location of the GFP-NLS probably reflects the combined activities of nuclear import and nuclear retention. However, the data, together with the results above demonstrating abundant nuclear accumulation of a test viral protein and the absence of any reports on general cytoplasmic accumulation of nuclear proteins, provide convincing evidence that HSV infection results in a marked and specific inhibition of NFAT nuclear accumulation. This would be predicted to have a profound effect on expression of NFAT target genes.
HSV-1 binding is insufficient to block GFP-NFAT import.
Previous results have shown that in the case of human cytomegalovirus, virion binding at the cell surface is sufficient to alter the activity of a cell signaling cascade (6). We therefore wished to address whether HSV binding might similarly be sufficient to block NFAT import by comparing the effect of wild-type (wt) virus with that of a gH-negative mutant (49) which is capable of binding to cells but not capable of fusing at the cell membrane (17).
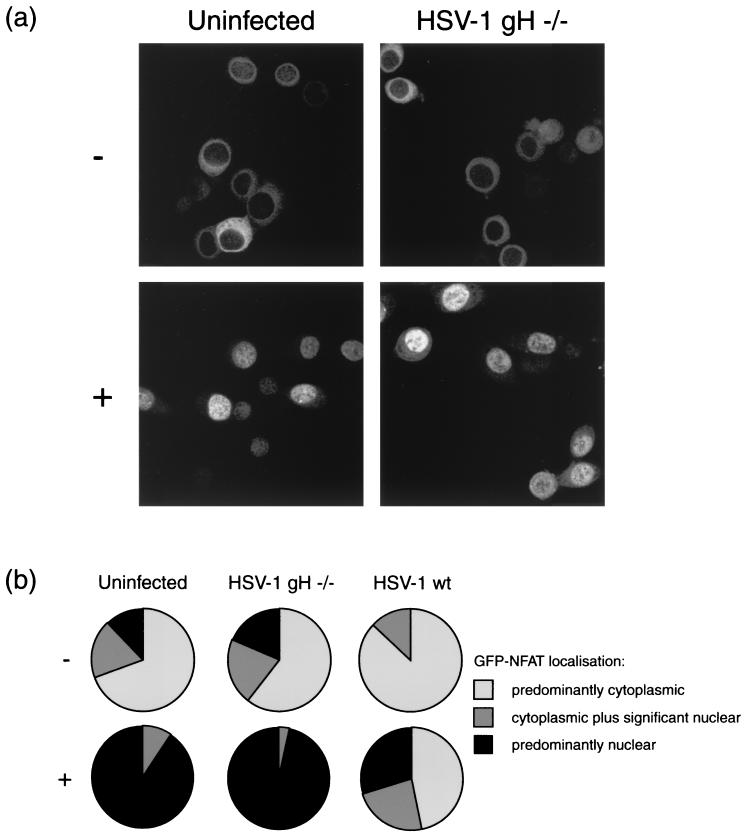
As before, the GFP-NFAT/HeLa cell line was infected with HSV-1 (wt or ΔgH) or left uninfected as a control. Cells were infected with 1,000 particles of the ΔgH strain per cell, which was estimated to be approximately equivalent to the MOI of 10 used in the previous experiment. At 5 h postinfection NFAT nuclear import was triggered with 1 μM ionomycin in the presence of lithium acetate, and cells were fixed after a further 2 h of incubation. GFP-NFAT localization was scored as before in five fields, and the combined data are presented in Fig. 3b.
FIG. 3.
HeLa cells stably expressing GFP-NFAT were infected with either HSV-1 or ΔgH HSV-1 or left uninfected. At 5 h, cells were treated with 1 μM ionomycin–30 mM lithium acetate (+) or left untreated (−). At 7 h, cells were fixed in methanol and examined by confocal microscopy. (a) Representative images of treated or untreated uninfected and ΔgH HSV-1-infected cells. (b) Summary of GFP-NFAT localization, scored for every cell in five randomly chosen fields of view in each experimental condition.
In the uninfected control cells, the ionomycin trigger efficiently promoted nuclear import of GFP-NFAT (Fig. 3a). Cells infected with ΔgH HSV-1 also showed efficient GFP-NFAT import (Fig. 3a), with 97% of cells showing predominantly nuclear staining (Fig. 3b). This was in contrast to cells infected with wt HSV-1, which, as before, significantly reduced the number of cells importing GFP-NFAT, with 47% retaining their predominantly cytoplasmic staining pattern (Fig. 3b). This suggests that HSV-1 binding at the cell surface is not sufficient to block nuclear import of NFAT.
Protein synthesis is required for HSV-1 to block GFP-NFAT import.
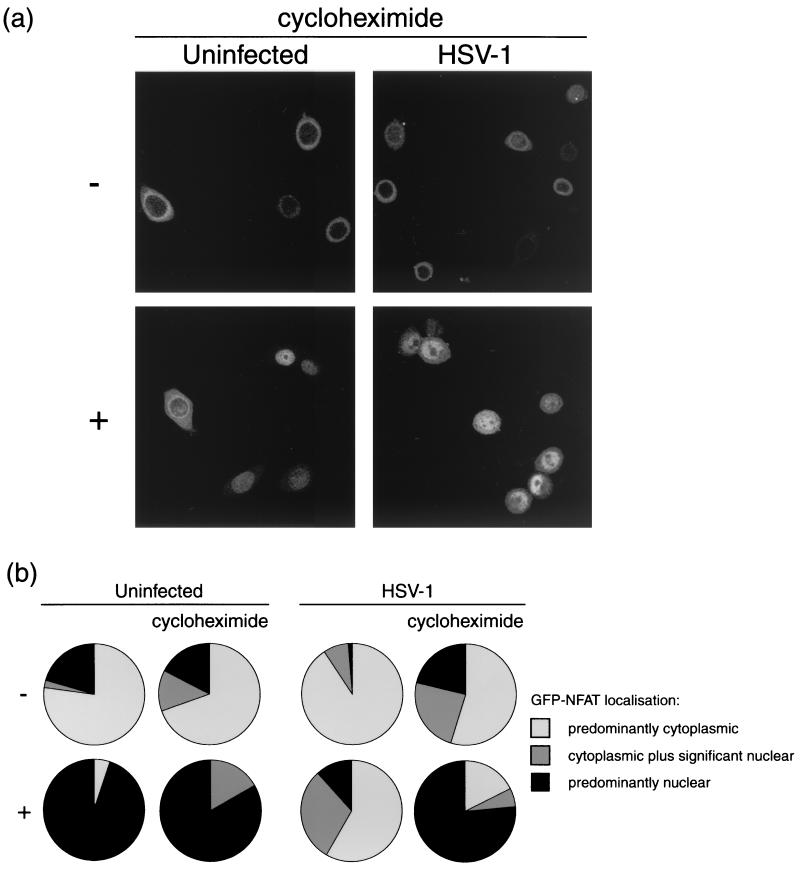
To establish the requirement for protein synthesis in viral inhibition of NFAT import, the experiment described above was repeated for wt HSV-1 infection in the presence of 50 μg of cycloheximide per ml. A parallel control procedure was carried out in the absence of cycloheximide for each experimental condition.
Figure 4a and Fig. 4b, left panel, show that the ionomycin trigger was successful in promoting nuclear import of GFP-NFAT in uninfected cells, even in the presence of cycloheximide. As before, HSV-1 infection significantly reduced the number of cells importing GFP-NFAT after the trigger, with approximately 60% of cells retaining NFAT within the cytoplasm in the absence of cycloheximide (Fig. 4b, right panel). However, in the presence of cycloheximide, the block normally induced by infection did not occur. Consequently, such infected cells were able to import GFP-NFAT almost as efficiently as uninfected cells (Fig. 4a), with 77% showing predominantly nuclear staining (Fig. 4b, right panel) after the trigger. Thus, in the absence of protein synthesis, HSV-1 infection is unable to block NFAT nuclear import.
FIG. 4.
HeLa cells stably expressing GFP-NFAT were infected with HSV-1 or left uninfected. At 5 h, cells were treated with 1 μM ionomycin–30 mM lithium acetate (+) or left untreated (−). Each assay was performed in duplicate, with one set of cells being examined in the presence of 50 μg of cycloheximide per ml, added at 30 min prior to infection. At 7 h, cells were fixed in methanol and examined by confocal microscopy. (a) Representative images of treated or untreated uninfected and infected cells in the presence of cycloheximide. (b) Summary of GFP-NFAT localization, scored for every cell in five randomly chosen fields of view in each experimental condition.
DNA synthesis-independent protein synthesis is sufficient for HSV-1 to block import of GFP-NFAT.
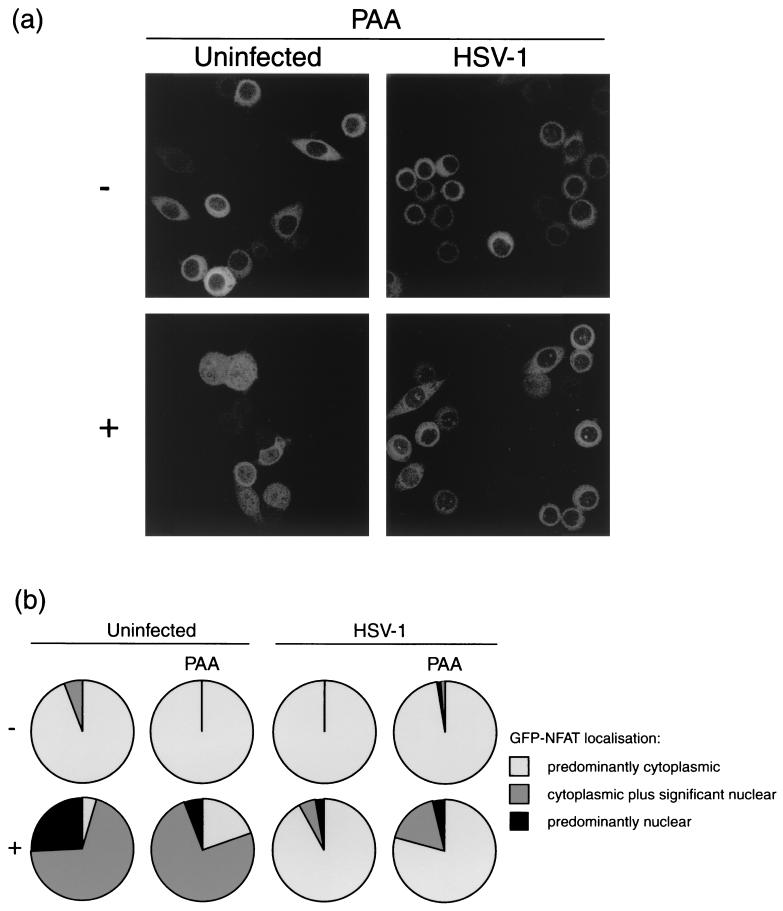
With the aim of refining the identification of the HSV factor(s) responsible for the block to NFAT nuclear import, the experiment was repeated in the presence of PAA, an inhibitor of virus DNA replication and thus of DNA synthesis-dependent protein synthesis. Note that on this occasion import in the control uninfected cells was not as efficient as before and was not as complete at the time the cells were fixed. The majority of triggered cells were scored as showing significant rather than predominantly nuclear staining (Fig. 5a). As expected, in uninfected cells PAA had little effect on NFAT import, with 95 and 80% of untreated and treated cells, respectively, showing nuclear staining (Fig. 5b, left panel).
FIG. 5.
HeLa cells stably expressing GFP-NFAT were infected with HSV-1 or left uninfected. At 5 h, cells were treated with 1 μM ionomycin–30 mM lithium acetate (+) or left untreated (−). Each assay was performed in duplicate, with one set analyzed in the presence of 300 μg of PAA per ml, added at 1 h postinfection. At 7 h, cells were fixed in methanol and examined by confocal microscopy. (a) Representative images of treated or untreated uninfected and infected cells in the presence of PAA. (b) Summary of GFP-NFAT localization, scored for every cell in five randomly chosen fields of view in each experimental condition.
As before, HSV-1-infected cells showed a block in NFAT import, where 92% of cells retained a predominantly cytoplasmic staining pattern 2 h after ionomycin treatment (Fig. 5b, right panel). This block remained in the presence of PAA (Fig. 5a), where 79% of triggered cells were still blocked for GFP-NFAT import (Fig. 5b, right panel). Thus, DNA synthesis-dependent protein synthesis is not required for HSV-1 to block NFAT import, suggesting that an immediate-early or early gene product is responsible.
HSV-1 reduces inducible reporter gene expression from the NFAT response element of the IL-2 promoter.
Our data suggest that by blocking import to the nucleus, HSV is likely to downregulate signaling through NFAT and the expression of NFAT-dependent target genes, whose products include immunoregulatory proteins such as IL-2. To investigate this further, we compared the expression of a reporter gene under the control of a basic promoter element plus four direct repeats of the distal NFAT binding site from the IL-2 promoter (pNFAT-luc) in infected and uninfected cells.
The GFP-NFAT/HeLa cell line was transfected with pNFAT-luc, and 18 h later the cells were infected with HSV-1 or mock infected. Uninfected and infected cells were then treated with ionomycin to trigger NFAT import. NFAT triggering was performed in the presence or absence of PMA, which, by activating the protein kinase C pathway and the transcription factor AP-1, is known to cooperate in the NFAT-mediated induction. The resulting reporter gene expression was quantified by measuring luciferase activity in cell lysates harvested 5 h after the trigger.
In line with published data, luciferase expression was stimulated when pNFAT-luc-transfected cells were treated with either ionomycin or PMA but was significantly higher when cells were treated with a combined ionomycin-PMA trigger (Fig. 6). This increase was specific and was not seen in cells transfected with a plasmid lacking the NFAT/AP-1 enhancer (data not shown). This enhanced luciferase activity was also dependent on the expression of GFP-NFAT, as endogenous NFAT levels in HeLa cells were insufficient to achieve this activation (data not shown).
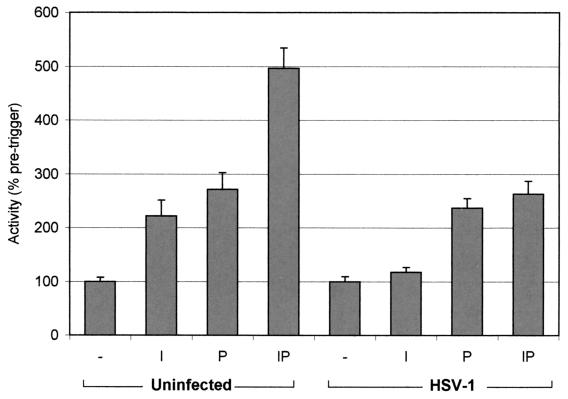
FIG. 6.
HeLa cells stably expressing GFP-NFAT were transiently transfected with pNFAT-luc and then infected with HSV-1 or left uninfected. At 5 h, cells were treated with either 1.3 μM ionomycin (I), 100 nM PMA (P), or both (IP) or were left untreated (−). At 10 h, cells were harvested and assayed for luciferase activity, which is expressed as a percentage of activity in uninfected or infected untreated cells. Each data point represents the mean from three samples prepared under identical conditions ± the standard error of the mean. Activation by the ionomycin-PMA over PMA is significant in mock-infected cells and suppressed so as to be insignificant in infected cells (P < 0.005 by the t test).
HSV-1 infection nonspecifically increased background expression from the reporter gene constructs, likely reflecting a general increase in transcriptional activity associated with the early stages of infection. Data from infected cells have been normalized accordingly. Nevertheless the specific effect of the ionomycin-PMA trigger was significantly suppressed compared to that observed in uninfected cells (Fig. 6). Thus, there was little significant effect of ionomycin alone and no ionomycin-induced enhancement of the PMA-induced effect. The effect of infection is somewhat limited by the fold enhancement of the inducers seen in the uninfected controls. However, the results were significant and reproducible. Thus, consistent with a virus-induced block in NFAT nuclear import, the transcriptional response of an NFAT enhancer element exhibits reduced activity in HSV-1-infected cells. Taken together, our results provide compelling evidence that NFAT signaling is blocked at early stages after virus infection by a virus-induced mechanism requiring immediate-early or early products.
DISCUSSION
A key level of regulation of the NFAT signaling pathway is the dephosphorylation of the NFAT transcription factor, resulting in a marked redistribution of the protein from the cytoplasm to the nucleus and rendering the protein transcriptionally active. This activation step can be conveniently monitored in a cell line expressing GFP-NFAT, which retains the functions of the endogenous protein. Using a HeLa cell line stably expressing GFP-NFAT2, we have examined the effect of HSV infection on NFAT activation. Since key features of NFAT structure and regulation are highly conserved, it is likely that our observations on NFAT2 reflect the behavior of all NFAT family members following HSV infection. We report that both HSV-1 and HSV-2 infection profoundly inhibit NFAT activation as reflected by ionomycin-inducible nuclear import. We have characterized this inhibitory activity for HSV-1 and find that virus penetration and early viral gene expression are required for the block to NFAT activation. An HSV-1 gH deletion virus capable of cell binding but not penetration failed to elicit the block, while wt HSV-1 infection in the presence of PAA, permitting only penetration and early gene expression, still inhibited NFAT import.
This is the first report of modulation of the NFAT pathway by an alpha-herpesvirus. Two closely related gamma-herpesviruses, Kaposi's sarcoma-associated herpesvirus (KSHV) and rhesus monkey rhadinovirus, affect the NFAT pathway, but in these cases infection results in activation (13, 28). These viruses infect B lymphocytes, and the homologous transmembrane proteins KSHV K1 and rhesus monkey rhadinovirus R1 exhibit cell transformation activity which may underlie the lymphoproliferative disorders associated with these infections. It seems that K1 and R1 act as constitutive signal transducers at the cell surface, raising intracellular Ca2+ levels and activating NFAT. The NFAT pathway has also been implicated in reactivation of KSHV from latency (57). Hepatitis C virus core protein also positively regulates the pathway; however, in this case the virus appears to act downstream of intracellular Ca2+ levels but still upstream of calcineurin activity (3). Hepatitis C virus is believed to infect T and B lymphocytes, and its upregulation of NFAT-dependent cytokine production is proposed to shift the balance between cell- and antibody-mediated immunity to favor persistent infection. In contrast, ASFV infection negatively regulates the NFAT pathway, as we report here for HSV. For ASFV the activity has been attributed to the viral protein A238L, which perturbs the pathway by competitively inhibiting the interaction of NFAT with calcineurin (35, 36). A238L contains a motif (PxIxITxC/S) which is necessary and sufficient for binding to calcineurin and which mimics the conserved calcineurin binding site of the NFAT proteins (35).
As outlined above, viral regulation of NFAT signaling can occur at many levels of the pathway. The level at which HSV inhibits import and the identity of the factor responsible remain to be determined. While the assay may reflect the combined activities of nuclear import and nuclear retention, we have shown that the distribution of a GFP molecule with an NLS was not perturbed by infection. The data, together with the results demonstrating abundant nuclear accumulation of a test viral protein, provide convincing evidence that HSV infection results in a specific inhibition of NFAT nuclear accumulation, upstream of import at or above the level of NFAT dephosphorylation. It is noteworthy that two HSV proteins, ICP32 tegument protein and the DNA polymerase subunit encoded by UL30, contain motifs with some limited homology to the calcineurin binding domains of NFAT proteins and ASFV A238L protein. Both proteins have a temporal expression profile that could be consistent with a PAA-insensitive factor interfering with NFAT activation, but the significance of the homology remains to be established (42, 50). We have observed an HSV-induced block to accumulation of NFAT in the nucleus and a consequent reduction in transcription from a target gene promoter. It remains possible, although less likely in our view, that NFAT import is maintained during infection but that its reexport rate is increased, with the net result being reduced residence time in the nucleus. The precise mechanism, including, e.g., whether an individual immediate-early protein is sufficient, will be the subject of future investigation.
A number of instances in which herpesviruses regulate cellular signaling relating to the immune response have been described, most notably affecting the interferon (IFN)-responsive pathway. Human cytomegalovirus activates the induction of IFN-responsive genes by surface binding of the viral glycoproteins gB with a cellular receptor (6). Similarly, an early event during HSV-1 infection is the induction of an antiviral state involving the upregulation of IFN-responsive genes (39, 40). The HSV-1 neurovirulence protein ICP34.5 overcomes aspects of the IFN-inducible antiviral response by circumventing the activity of the double-stranded-RNA-dependent protein kinase R (8, 29). Mossman et al. (39) have also described an HSV-1-encoded factor, proposed to consist of one or more of the immediate-early proteins, which disarms an aspect of the host antiviral response through an unknown mechanism. We have identified a specific effector molecule, NFAT, which is targeted by HSV. It is possible that the suppression of the cellular response observed by Mossman et al. and our observations of inhibition of NFAT translocation are in some way related.
Although HSV's most widely recognized target cells in vivo are epithelial cells at the point of infection and neuronal cells in which latent infections are established, several reports describe infection of T lymphocytes and other inflammatory cells by HSV (11, 16, 46, 54). Thus, an appealing rationale for HSV suppression of NFAT activation could be an immunosuppressive strategy in which the virus blocks NFAT-mediated cytokine gene expression in inflammatory cells, thereby disrupting the signaling networks of cell-mediated immunity and protecting the virus from immune clearance. This strategy is used by ASFV, which suppresses cytokine gene expression in infected macrophages, although these are the main cell types infected by ASFV in vivo. It is also possible that HSV does not infect immune cells to block NFAT activity but somehow achieves suppression of cytokine production in trans (55). However, the early stage of infection at which we observe the block to NFAT activation makes this less likely.
Alternatively, HSV may downregulate a less well-defined activity of NFAT in cells more commonly infected by the virus. NFAT pathway elements and/or NFAT activity has been reported in a range of nonlymphoid cell types, including arterial endothelial cells (10), smooth muscle cells (4), skeletal muscle cells (7), and neuronal and neuronal accessory cells (5, 20, 34, 38, 44). Although an extensive set of NFAT target genes have been confirmed in T lymphocytes (23), it is likely that additional targets in other cell types remain to be identified. For example, NFAT has been implicated as both a positive (53) and negative (38, 45) regulator of apoptosis. Herpesviruses have been reported to stimulate and suppress apoptotic signaling, and it is conceivable that this regulation could be achieved by interference with the NFAT pathway.
In summary, we have described an additional example of herpesvirus interference with cellular signaling, i.e., the disruption of the NFAT signaling pathway by a factor induced early after HSV infection. This results in a block in nuclear accumulation of NFAT and compromises transcription from a promoter containing prototypic NFAT response elements. We are currently investigating the precise mechanism of this block. A likely candidate for the signaling step targeted by the viral factor is the calcineurin-mediated dephosphorylation of NFAT to expose its NLS. We propose that these observations are likely to be of significance for modulation of the host immune response or an alternative cellular process controlled by NFAT signaling.
ACKNOWLEDGMENTS
We thank Ralph Kehlenbach, Scripps Research Institute, La Jolla, Calif., for the GFP-NFAT/HeLa cell line and Helena Browne, University of Cambridge, Cambridge, United Kingdom, for ΔgH HSV-1.
This work was funded by the Marie Curie Research Foundation.
REFERENCES
- 1.Aramburu J, Garcia-Cozar F, Raghavan A, Okamura H, Rao A, Hogan P G. Selective inhibition of NFAT activation by a peptide spanning the calcineurin targeting site of NFAT. Mol Cell. 1998;1:627–637. doi: 10.1016/s1097-2765(00)80063-5. [DOI] [PubMed] [Google Scholar]
- 2.Beals C R, Sheridan C M, Turck C W, Gardner P, Crabtree G R. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997;275:1930–1934. doi: 10.1126/science.275.5308.1930. [DOI] [PubMed] [Google Scholar]
- 3.Bergqvist A, Rice C M. Transcriptional activation of the interleukin-2 promoter by hepatitis C virus core protein. J Virol. 2001;75:772–781. doi: 10.1128/JVI.75.2.772-781.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boss V, Abbott K L, Wang X F, Pavlath G K, Murphy T J. The cyclosporin A-sensitive nuclear factor of activated T cells (NFAT) proteins are expressed in vascular smooth muscle cells. Differential localization of NFAT isoforms and induction of NFAT-mediated transcription by phospholipase C-coupled cell surface receptors. J Biol Chem. 1998;273:19664–19671. doi: 10.1074/jbc.273.31.19664. [DOI] [PubMed] [Google Scholar]
- 5.Boss V, Talpade D J, Murphy T J. Induction of NFAT-mediated transcription by Gq-coupled receptors in lymphoid and non-lymphoid cells. J Biol Chem. 1996;271:10429–10432. doi: 10.1074/jbc.271.18.10429. [DOI] [PubMed] [Google Scholar]
- 6.Boyle K A, Pietropaolo R L, Compton T. Engagement of the cellular receptor for glycoprotein B of human cytomegalovirus activates the interferon-responsive pathway. Mol Cell Biol. 1999;19:3607–3613. doi: 10.1128/mcb.19.5.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chin E R, Olson E N, Richardson J A, Yang Q, Humphries C, Shelton J M, Wu H, Zhu W, Bassel-Duby R, Williams R S. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 1998;12:2499–2509. doi: 10.1101/gad.12.16.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou J, Chen J J, Gross M, Roizman B. Association of a M(r) 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor eIF-2 alpha and premature shutoff of protein synthesis after infection with gamma 134.5- mutants of herpes simplex virus 1. Proc Natl Acad Sci USA. 1995;92:10516–10520. doi: 10.1073/pnas.92.23.10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou J, Roizman B. The gamma 1(34.5) gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programmed cell death in neuronal cells. Proc Natl Acad Sci USA. 1992;89:3266–3270. doi: 10.1073/pnas.89.8.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cockerill G W, Bert A G, Ryan G R, Gamble J R, Vadas M A, Cockerill P N. Regulation of granulocyte-macrophage colony-stimulating factor and E-selectin expression in endothelial cells by cyclosporin A and the T-cell transcription factor NFAT. Blood. 1995;86:2689–2698. [PubMed] [Google Scholar]
- 11.Contreras A, Zadeh H H, Nowzari H, Slots J. Herpesvirus infection of inflammatory cells in human periodontitis. Oral Microbiol Immunol. 1999;14:206–212. doi: 10.1034/j.1399-302x.1999.140402.x. [DOI] [PubMed] [Google Scholar]
- 12.Crabtree G R. Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell. 1999;96:611–614. doi: 10.1016/s0092-8674(00)80571-1. [DOI] [PubMed] [Google Scholar]
- 13.Damania B, DeMaria M, Jung J U, Desrosiers R C. Activation of lymphocyte signaling by the R1 protein of rhesus monkey rhadinovirus. J Virol. 2000;74:2721–2730. doi: 10.1128/jvi.74.6.2721-2730.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis-Poynter N J, Farrell H E. Masters of deception: a review of herpesvirus immune evasion strategies. Immunol Cell Biol. 1996;74:513–522. doi: 10.1038/icb.1996.84. [DOI] [PubMed] [Google Scholar]
- 15.Flanagan W M, Corthesy B, Bram R J, Crabtree G R. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991;352:803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- 16.Fleck M, Mountz J D, Hsu H C, Wu J, Edwards C K, 3rd, Kern E R. Herpes simplex virus type 2 infection induced apoptosis in peritoneal macrophages independent of Fas and tumor necrosis factor-receptor signaling. Viral Immunol. 1999;12:263–275. doi: 10.1089/vim.1999.12.263. [DOI] [PubMed] [Google Scholar]
- 17.Forrester A, Farrell H, Wilkinson G, Kaye J, Davis-Poynter N, Minson T. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J Virol. 1992;66:341–348. doi: 10.1128/jvi.66.1.341-348.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gustin K E, Sarnow P. Effects of poliovirus infection on nucleo-cytoplasmic trafficking and nuclear pore complex composition. EMBO J. 2001;20:240–249. doi: 10.1093/emboj/20.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halford W P, Carr D J. Subversion of intracellular signal transduction by herpes simplex virus type 1. Adv Neuroimmunol. 1995;5:327–334. doi: 10.1016/0960-5428(95)00017-v. [DOI] [PubMed] [Google Scholar]
- 20.Ho A M, Jain J, Rao A, Hogan P G. Expression of the transcription factor NFATp in a neuronal cell line and in the murine nervous system. J Biol Chem. 1994;269:28181–28186. [PubMed] [Google Scholar]
- 21.Jain J, McCaffrey P G, Miner Z, Kerppola T K, Lambert J N, Verdine G L, Curran T, Rao A. The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature. 1993;365:352–355. doi: 10.1038/365352a0. [DOI] [PubMed] [Google Scholar]
- 22.Kehlenbach R H, Dickmanns A, Gerace L. Nucleocytoplasmic shuttling factors including Ran and CRM1 mediate nuclear export of NFAT In vitro. J Cell Biol. 1998;141:863–874. doi: 10.1083/jcb.141.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kel A, Kel-Margoulis O, Babenko V, Wingender E. Recognition of NFATp/AP-1 composite elements within genes induced upon the activation of immune cells. J Mol Biol. 1999;288:353–376. doi: 10.1006/jmbi.1999.2684. [DOI] [PubMed] [Google Scholar]
- 24.Kieff E, Shenk T. Modulation of apoptosis by herpesviruses. Semin Virol. 1998;8:471–480. [Google Scholar]
- 25.Kim L J, Ferguson H A, Seto A G, Goodrich J A. Characterization of DNA binding, transcriptional activation, and regulated nuclear association of recombinant human NFATp. BMC Immunol. 2000;1:1. doi: 10.1186/1471-2172-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinoshita S, Chen B K, Kaneshima H, Nolan G P. Host control of HIV-1 parasitism in T cells by the nuclear factor of activated T cells. Cell. 1998;95:595–604. doi: 10.1016/s0092-8674(00)81630-x. [DOI] [PubMed] [Google Scholar]
- 27.LaBoissiere S, Hughes T, O'Hare P. HCF-dependent nuclear import of VP16. EMBO J. 1999;18:480–489. doi: 10.1093/emboj/18.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lagunoff M, Majeti R, Weiss A, Ganem D. Deregulated signal transduction by the K1 gene product of Kaposi's sarcoma-associated herpesvirus. Proc Natl Acad Sci USA. 1999;96:5704–5709. doi: 10.1073/pnas.96.10.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leib D A, Machalek M A, Williams B R, Silverman R H, Virgin H W. Specific phenotypic restoration of an attenuated virus by knockout of a host resistance gene. Proc Natl Acad Sci USA. 2000;97:6097–6101. doi: 10.1073/pnas.100415697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leopardi R, Van Sant C, Roizman B. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc Natl Acad Sci USA. 1997;94:7891–7896. doi: 10.1073/pnas.94.15.7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Farmer J D, Jr, Lane W S, Friedman J, Weissman I, Schreiber S L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 32.Macian F, Garcia-Rodriguez C, Rao A. Gene expression elicited by NFAT in the presence or absence of cooperative recruitment of fos and Jun. EMBO J. 2000;19:4783–4795. doi: 10.1093/emboj/19.17.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLean T I, Bachenheimer S L. Activation of c-Jun N-terminal kinase by herpes simplex virus type 1 enhances viral replication. J Virol. 1999;73:8415–8426. doi: 10.1128/jvi.73.10.8415-8426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minneman K P, Lee D, Zhong H, Berts A, Abbott K L, Murphy T J. Transcriptional responses to growth factor and G protein-coupled receptors in PC12 cells: comparison of alpha(1)-adrenergic receptor subtypes. J Neurochem. 2000;74:2392–2400. doi: 10.1046/j.1471-4159.2000.0742392.x. [DOI] [PubMed] [Google Scholar]
- 35.Miskin J E, Abrams C C, Dixon L K. African swine fever virus protein A238L interacts with the cellular phosphatase calcineurin via a binding domain similar to that of NFAT. J Virol. 2000;74:9412–9420. doi: 10.1128/jvi.74.20.9412-9420.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miskin J E, Abrams C C, Goatley L C, Dixon L K. A viral mechanism for inhibition of the cellular phosphatase calcineurin. Science. 1998;281:562–565. doi: 10.1126/science.281.5376.562. [DOI] [PubMed] [Google Scholar]
- 37.Molkentin J D, Lu J R, Antos C L, Markham B, Richardson J, Robbins J, Grant S R, Olson E N. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosieniak G, Pyrzynska B, Kaminska B. Nuclear factor of activated T cells (NFAT) as a new component of the signal transduction pathway in glioma cells. J Neurochem. 1998;71:134–141. doi: 10.1046/j.1471-4159.1998.71010134.x. [DOI] [PubMed] [Google Scholar]
- 39.Mossman K L, Macgregor P F, Rozmus J J, Goryachev A B, Edwards A M, Smiley J R. Herpes simplex virus triggers and then disarms a host antiviral response. J Virol. 2001;75:750–758. doi: 10.1128/JVI.75.2.750-758.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicholl M J, Robinson L H, Preston C M. Activation of cellular interferon-responsive genes after infection of human cells with herpes simplex virus type 1. J Gen Virol. 2000;81:2215–2218. doi: 10.1099/0022-1317-81-9-2215. [DOI] [PubMed] [Google Scholar]
- 41.Okamura H, Aramburu J, Garcia-Rodriguez C, Viola J P, Raghavan A, Tahiliani M, Zhang X, Qin J, Hogan P G, Rao A. Concerted dephosphorylation of the transcription factor NFAT1 induces a conformational switch that regulates transcriptional activity. Mol Cell. 2000;6:539–550. doi: 10.1016/s1097-2765(00)00053-8. [DOI] [PubMed] [Google Scholar]
- 42.Pande N T, Petroski M D, Wagner E K. Functional modules important for activated expression of early genes of herpes simplex virus type 1 are clustered upstream of the TATA box. Virology. 1998;246:145–157. doi: 10.1006/viro.1998.9189. [DOI] [PubMed] [Google Scholar]
- 43.Park S, Uesugi M, Verdine G L. A second calcineurin binding site on the NFAT regulatory domain. Proc Natl Acad Sci USA. 2000;97:7130–7135. doi: 10.1073/pnas.97.13.7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plyte S, Boncristiano M, Fattori E, Galvagni F, Rossi Paccani S, Majolini M B, Oliviero S, Ciliberto G, Telford J L, Baldari C T. Identification and characterization of a novel NFAT1 isoform expressed in mouse brain. J Biol Chem. 2001;276:14350–14358. doi: 10.1074/jbc.M007854200. [DOI] [PubMed] [Google Scholar]
- 45.Pyrzynska B, Lis A, Mosieniak G, Kaminska B. Cyclosporin A-sensitive signaling pathway involving calcineurin regulates survival of reactive astrocytes. Neurochem Int. 2001;38:409–415. doi: 10.1016/s0197-0186(00)00105-4. [DOI] [PubMed] [Google Scholar]
- 46.Raftery M J, Behrens C K, Muller A, Krammer P H, Walczak H, Schonrich G. Herpes simplex virus type 1 infection of activated cytotoxic T cells: induction of fratricide as a mechanism of viral immune evasion. J Exp Med. 1999;190:1103–1114. doi: 10.1084/jem.190.8.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ranger A M, Gerstenfeld L C, Wang J, Kon T, Bae H, Gravallese E M, Glimcher M J, Glimcher L H. The nuclear factor of activated T cells (NFAT) transcription factor NFATp (NFATc2) is a repressor of chondrogenesis. J Exp Med. 2000;191:9–22. doi: 10.1084/jem.191.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rao A, Luo C, Hogan P G. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 49.Rodger G, Boname J, Bell S, Minson T. Assembly and organization of glycoproteins B, C, D, and H in herpes simplex virus type 1 particles lacking individual glycoproteins: no evidence for the formation of a complex of these molecules. J Virol. 2001;75:710–716. doi: 10.1128/JVI.75.2.710-716.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roizman B. The function of herpes simplex virus genes: a primer for genetic engineering of novel vectors. Proc Natl Acad Sci USA. 1996;93:11307–11312. doi: 10.1073/pnas.93.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Serfling E, Berberich-Siebelt F, Chuvpilo S, Jankevics E, Klein-Hessling S, Twardzik T, Avots A. The role of NF-AT transcription factors in T cell activation and differentiation. Biochim Biophys Acta. 2000;1498:1–18. doi: 10.1016/s0167-4889(00)00082-3. [DOI] [PubMed] [Google Scholar]
- 52.Shibasaki F, Price E R, Milan D, McKeon F. Role of kinases and the phosphatase calcineurin in the nuclear shuttling of transcription factor NF-AT4. Nature. 1996;382:370–373. doi: 10.1038/382370a0. [DOI] [PubMed] [Google Scholar]
- 53.Srivastava R K, Sasaki C Y, Hardwick J M, Longo D L. Bcl-2-mediated drug resistance: inhibition of apoptosis by blocking nuclear factor of activated T lymphocytes (NFAT)-induced Fas ligand transcription. J Exp Med. 1999;190:253–265. doi: 10.1084/jem.190.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tropea F, Troiano L, Monti D, Lovato E, Malorni W, Rainaldi G, Mattana P, Viscomi G, Ingletti M C, Portolani M, et al. Sendai virus and herpes virus type 1 induce apoptosis in human peripheral blood mononuclear cells. Exp Cell Res. 1995;218:63–70. doi: 10.1006/excr.1995.1131. [DOI] [PubMed] [Google Scholar]
- 55.York I A, Johnson D C. Direct contact with herpes simplex virus-infected cells results in inhibition of lymphokine-activated killer cells because of cell-to-cell spread of virus. J Infect Dis. 1993;168:1127–1132. doi: 10.1093/infdis/168.5.1127. [DOI] [PubMed] [Google Scholar]
- 56.Zhu J, McKeon F. Nucleocytoplasmic shuttling and the control of NF-AT signaling. Cell Mol Life Sci. 2000;57:411–420. doi: 10.1007/PL00000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zoeteweij J P, Moses A V, Rinderknecht A S, Davis D A, Overwijk W W, Yarchoan R, Orenstein J M, Blauvelt A. Targeted inhibition of calcineurin signaling blocks calcium-dependent reactivation of Kaposi sarcoma-associated herpesvirus. Blood. 2001;97:2374–2380. doi: 10.1182/blood.v97.8.2374. [DOI] [PubMed] [Google Scholar]